Abstract
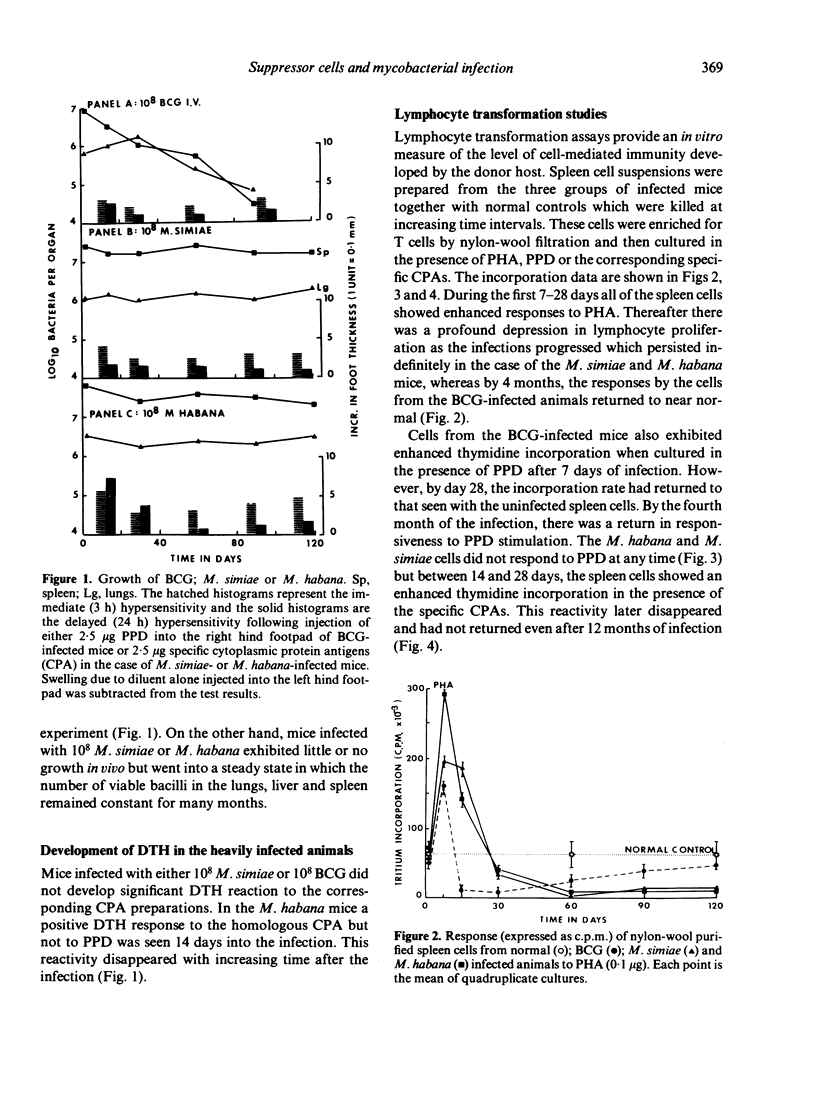
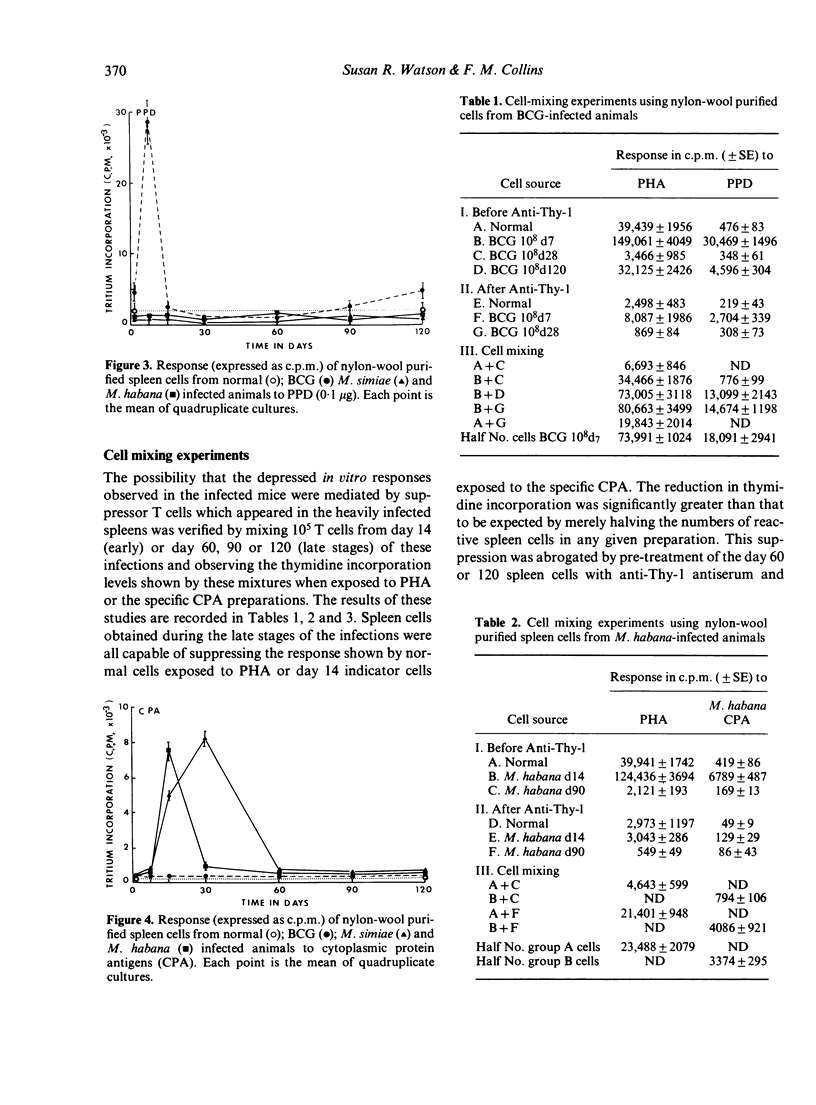
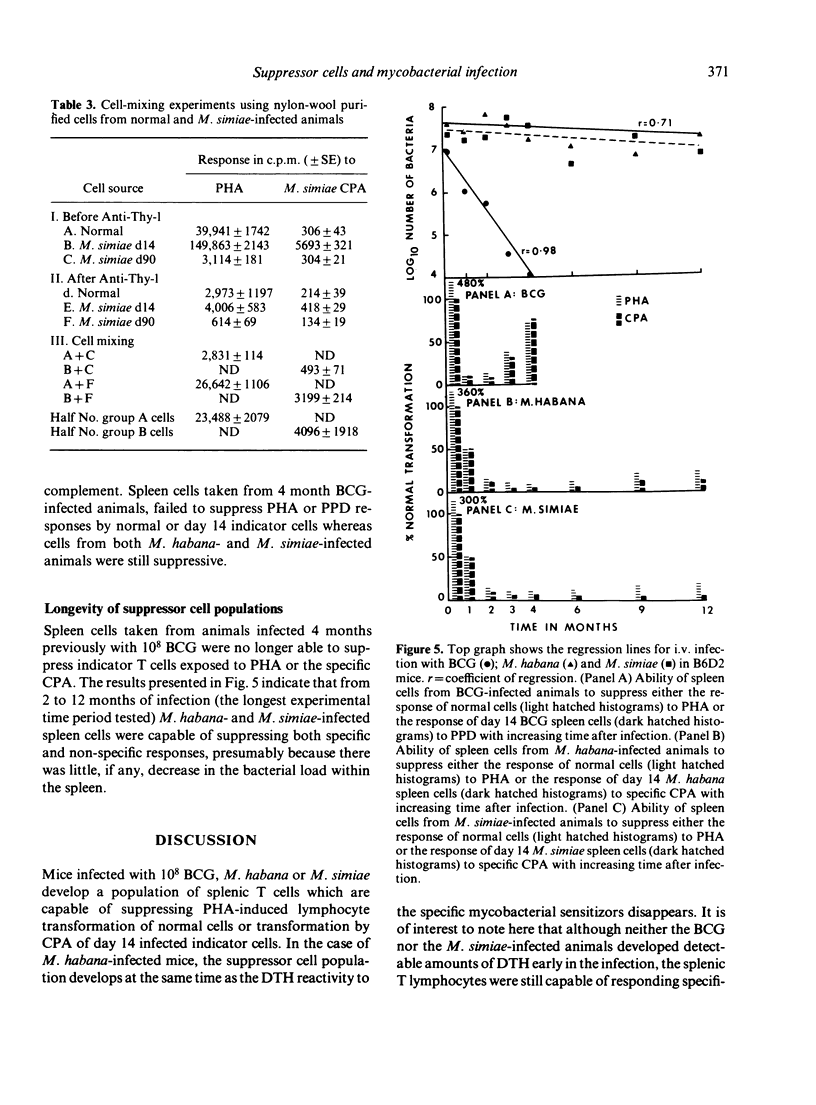
Specific pathogen-free B6D2 mice were infected intravenously with 10(8) viable BCG, M. habana or M. simiae and the level of tuberculin hypersensitivity to 2.5 micrograms PPD or cytoplasmic protein antigens (CPA) prepared from the other organisms was determined using the footpad swelling test with increasing time after infection. This was correlated with the growth or persistence of mycobacterial populations within the liver. Spleen cells were removed from these infected mice and the level of blast transformation following exposure to PHA, PPD or M. habana or M. simiae CPA was measured in vitro. Early in the mycobacterial infections (day 14) thymidine incorporation by the spleen cells was significantly enchanced followed by a profound depression in incorporation rates as the infection progressed. The mechanism of this depressed response involved the production of suppressor T cells in the spleen. In the case of the M. simiae or M. habana infection, cells capable of mediating suppression were still present even after 12 months of infection. In the BCG infection, suppressor T cells declined with time so that by 4 months incorporation rates were back to normal and suppressor cells were no longer detectable in the spleens of the infected animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Bast B. S. Critical review of previously reported animal studies of tumor immunotherapy with nonspecific immunostimulants. Ann N Y Acad Sci. 1976;277(00):60–93. doi: 10.1111/j.1749-6632.1976.tb41692.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E. Anergy and infection. Adv Intern Med. 1976;21:149–173. [PubMed] [Google Scholar]
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Volkman A., McGregor D. D. Transfer of delayed and Arthus sensitivity with blood plasma from x-irradiated guinea-pigs. Immunology. 1970 Sep;19(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Orbach-Arbouys S., Poupon M. F. Active suppression of in vitro reactivity of spleen cells after BCG treatment. Immunology. 1978 Mar;34(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Stoner G. L. Ir genes and leprosy. Int J Lepr Other Mycobact Dis. 1978 Apr-Jun;46(2):217–220. [PubMed] [Google Scholar]
- Turcotte R. Suppressor cells in experimental murine leprosy. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):358–363. [PubMed] [Google Scholar]
- Watson S. R., Morrison N. E., Collins F. M. Delayed hypersensitivity responses in mice and guinea pigs to Mycobacterium leprae, Mycobacterium vaccae, and Mycobacterium nonchromogenicum cytoplasmic proteins. Infect Immun. 1979 Jul;25(1):229–236. doi: 10.1128/iai.25.1.229-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



