Abstract
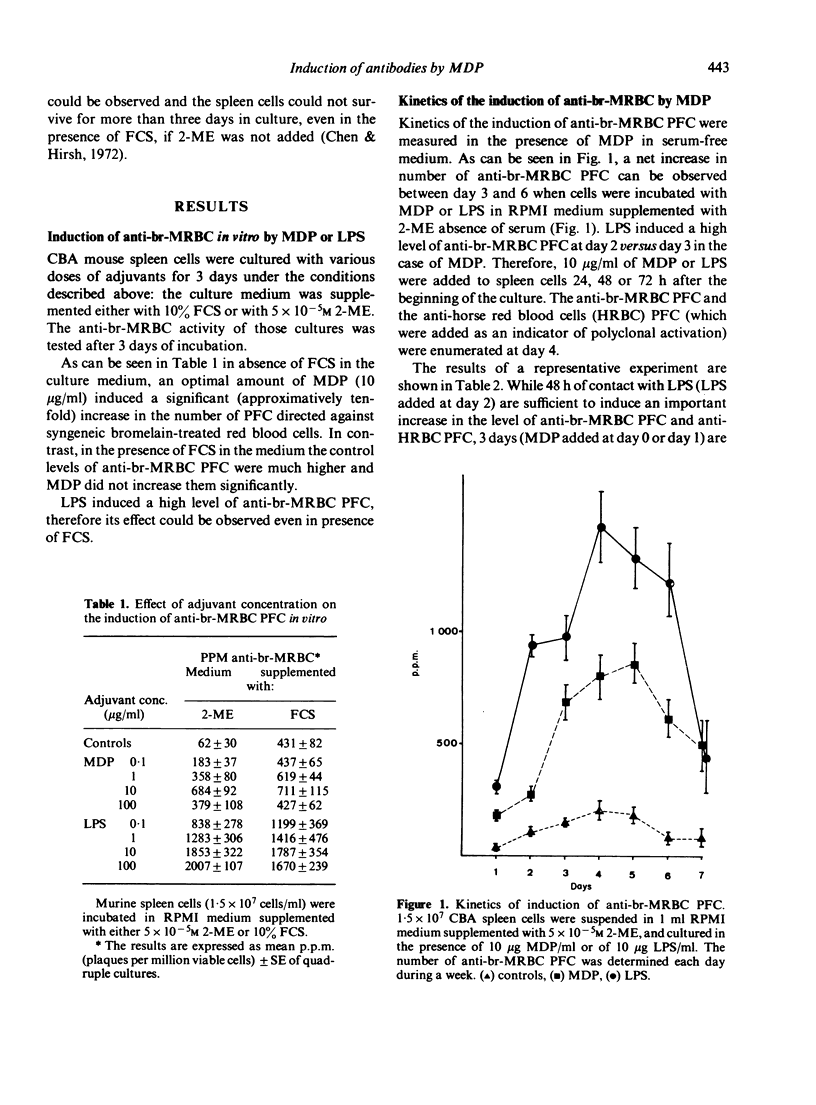
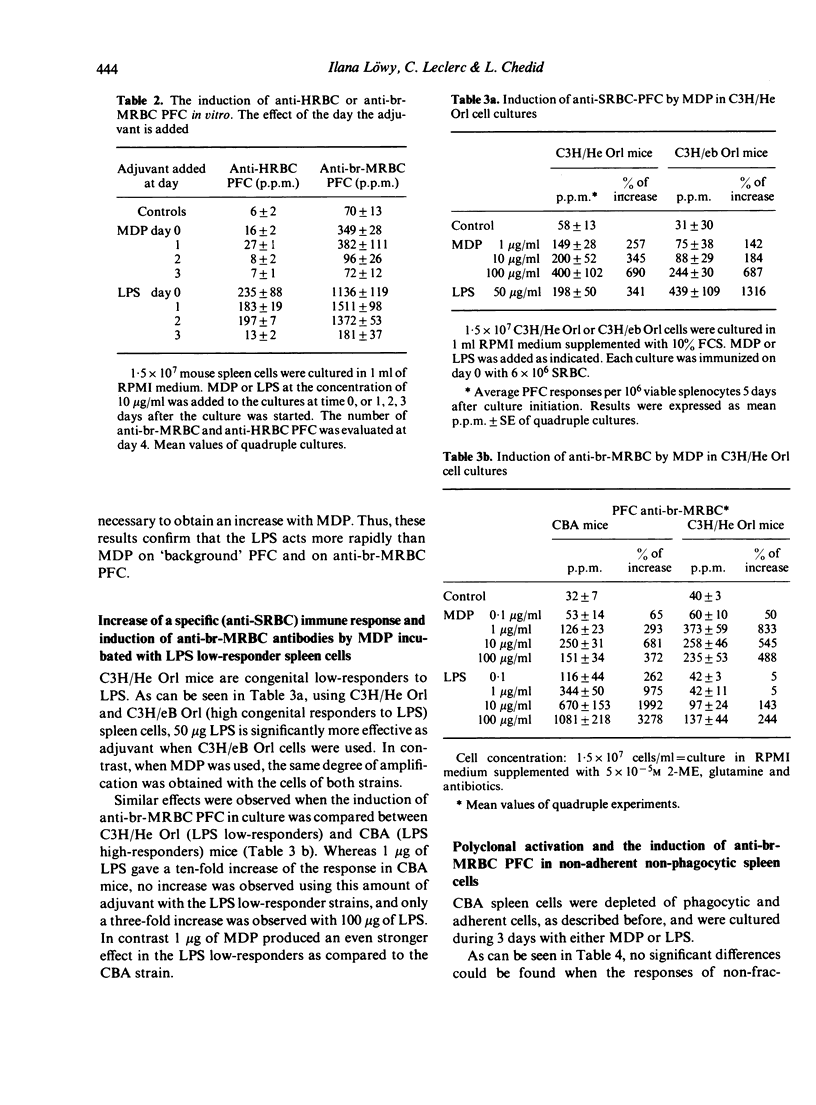
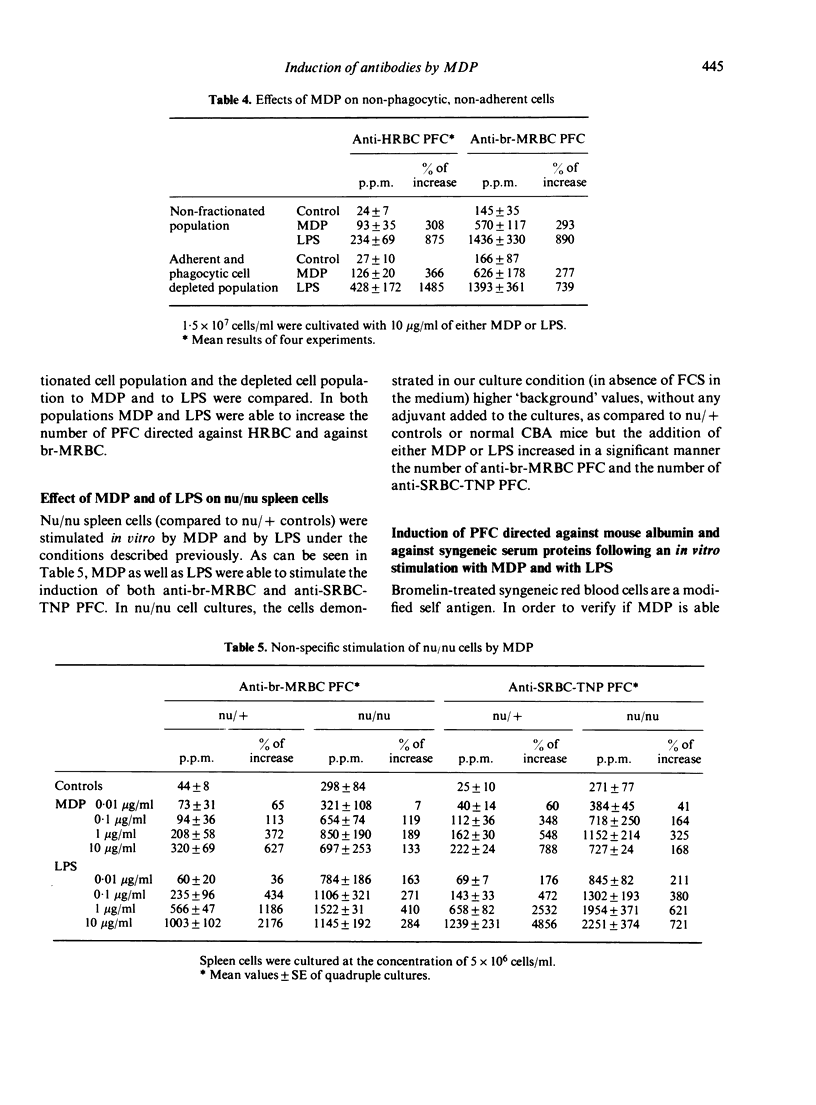
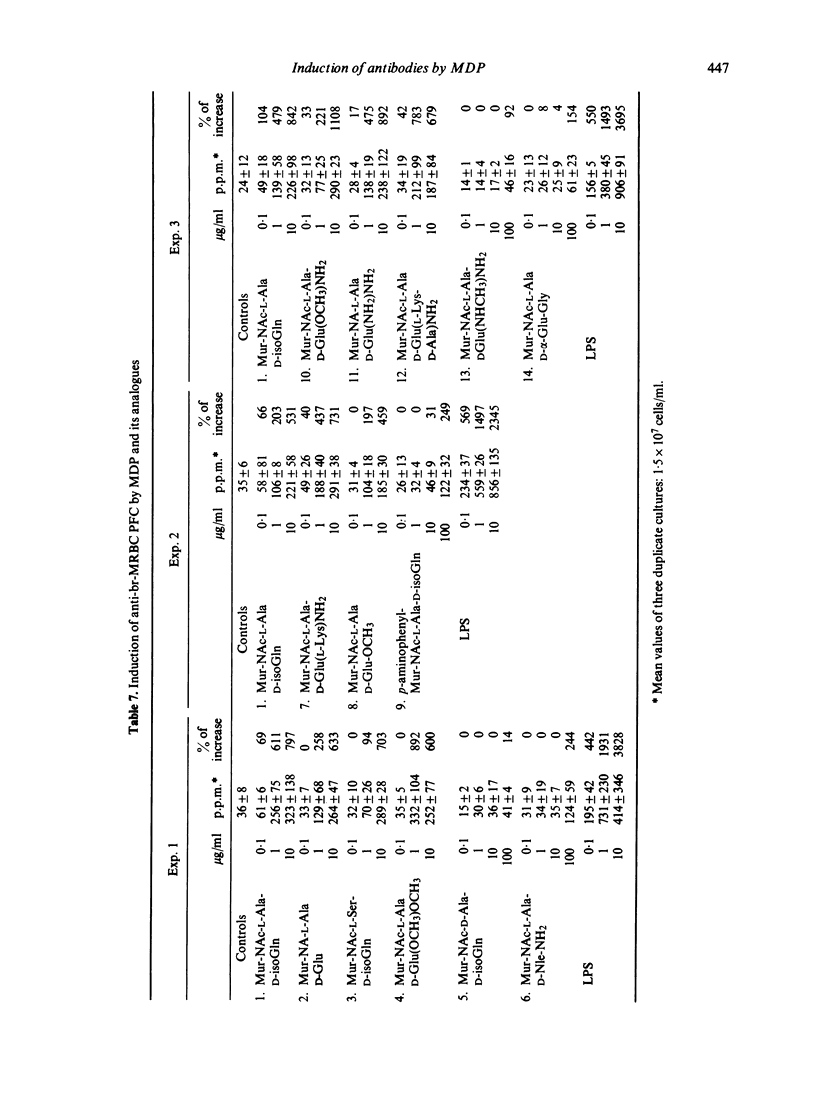
In a serum free, 2-mercaptoethanol supplemented culture medium muramyl dipeptide (MDP) is able to increase the number of plaque-forming cells (PFC) directed against syngeneic, bromelain-treated red blood cells (br-MRBC) and against an autoantigen, mouse albumin. The non-specific stimulation of anti-br-MRBC PFC by MDP, as by bacterial lipopolysaccharide (LPS), can be observed in spleen cell populations depleted of adherent and phagocytic cells, and in nu/nu spleen cell cultures. However, the kinetics of the induction of anti-br-MRBC PFC in murine spleen cell cultures in presence of LPS or of MDP are not identical. Moreover, MDP is able to stimulate C3H/He Orl (LPS low-responder strain) cells. Thus, the mechanisms of non-specific stimulation by MDP or by LPS could be different. Experiments done with thirteen structural analogues of MDP showed that there exists a good correlation between the adjuvant activity and the ability to induce anti-br-MRBC PFC.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Souvannavong V., Lederer E. Non-specific MIF-like activity induced by the synthetic immunoadjuvant: N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP). Biochem Biophys Res Commun. 1978 Nov 29;85(2):684–690. doi: 10.1016/0006-291x(78)91216-0. [DOI] [PubMed] [Google Scholar]
- Audibert F., Chédid L., Lefrancier P., Choay J. Distinctive adjuvanticity of synthetic analogs of mycobacterial water-soluble components. Cell Immunol. 1976 Feb;21(2):243–249. doi: 10.1016/0008-8749(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. Active suppressor mechanism maintaining tolerance to some self components. Nature. 1975 Mar 13;254(5496):143–144. doi: 10.1038/254143a0. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J. Large numbers of cells in normal mice produce antibody components of isologous erythrocytes. Nature. 1974 Dec 20;252(5485):749–751. doi: 10.1038/252749a0. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J. Self-tolerance maintained by active suppressor mechanisms. Transplant Rev. 1976;31:23–43. doi: 10.1111/j.1600-065x.1976.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L. Nonspecific activation of murine spleen cells in vitro by a synthetic immunoadjuvant (N-acetyl-muramyl-L-alanyl-D-isoglutamine). Cell Immunol. 1977 Nov;34(1):49–56. doi: 10.1016/0008-8749(77)90228-3. [DOI] [PubMed] [Google Scholar]
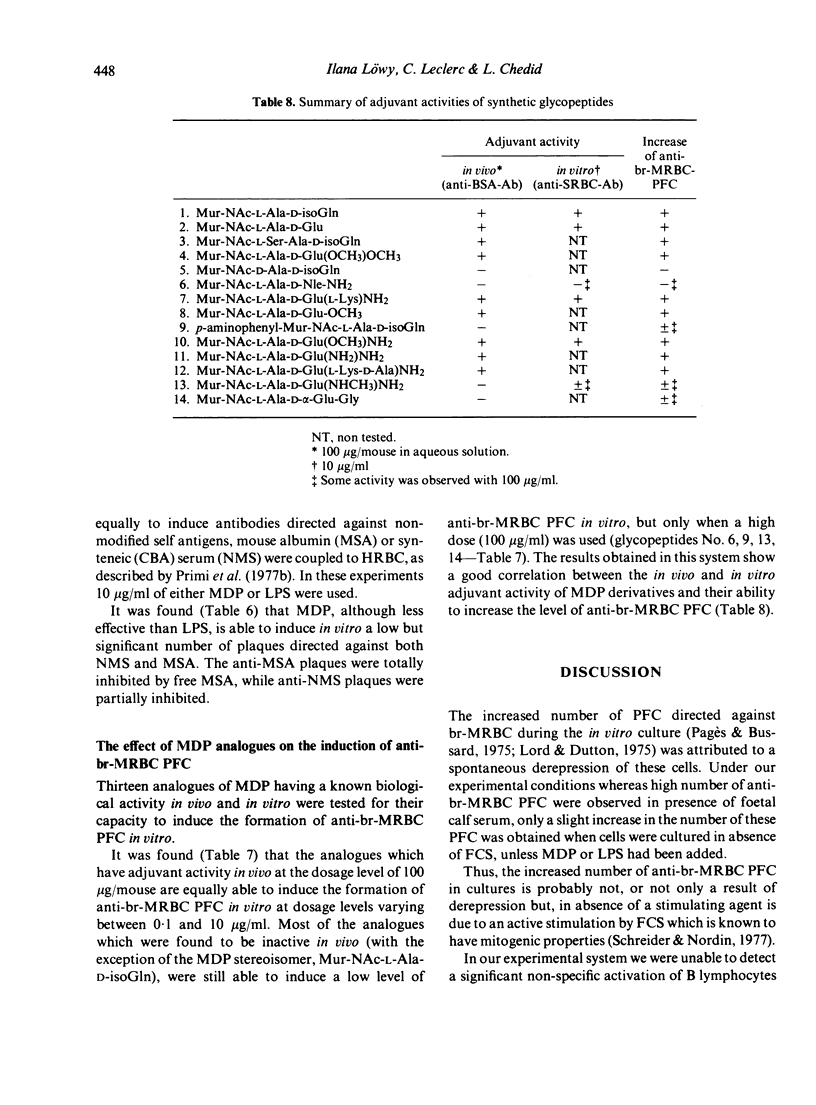
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O. Nonspecific activation of murine lymphocytes. I. Proliferation and polyclonal activation induced by 2-mercaptoethanol and alpha-thioglycerol. J Exp Med. 1977 Mar 1;145(3):473–489. doi: 10.1084/jem.145.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'age-Stehr J., Diamanstein T. Induction of autoreactive T lymphocytes and their suppressor cells by cyclophosphamide. Nature. 1978 Feb 16;271(5646):663–665. doi: 10.1038/271663a0. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Bourgeois E., Chedid L. Enhancement by muramyl dipeptide of in vitro nude mice responses to a T-dependent antigen. Immunol Commun. 1979;8(1):55–64. doi: 10.3109/08820137909044706. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Löwy I., Chedid L. Influence of MDP and of some analogous synthetic glycopeptides on the in vitro mouse spleen cell viability and immune response to sheep erythrocytes. Cell Immunol. 1978 Jul;38(2):286–293. doi: 10.1016/0008-8749(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Lefrancier P., Choay J., Derrien M., Lederman I. Synthesis of N-acetyl-muramyl-L-alanyl-D-isoglutamine, an adjuvant of the immune response, and of some n-acetyl-muramyl-peptide analogs. Int J Pept Protein Res. 1977;9(4):249–257. doi: 10.1111/j.1399-3011.1977.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Lefrancier P., Derrien M., Lederman I., Nief F., Choay J., Lederer E. Synthesis of some new analogs of the immunoadjuvant glycopeptide MDP (N-acetyl-muramyl-L-alanyl-D-isoglutamine). Int J Pept Protein Res. 1978 Apr;11(4):289–296. doi: 10.1111/j.1399-3011.1978.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Ling N. R., Bishop S., Jefferis Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods. 1977;15(3):279–289. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- Lord E. M., Dutton R. W. The properties of plaque-forming cells from autoimmune and normal strains of mice with specificity for autologous erythrocyte antigens. J Immunol. 1975 Nov;115(5):1199–1205. [PubMed] [Google Scholar]
- Löwy I., Bona C., Chedid L. Target cells for the activity of a synthetic adjuvant: muramyl dipeptide. Cell Immunol. 1977 Mar 1;29(1):195–199. doi: 10.1016/0008-8749(77)90288-x. [DOI] [PubMed] [Google Scholar]
- Merser C., Sinay P., Adam A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1316–1322. doi: 10.1016/0006-291x(75)90503-3. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages J., Bussard A. E. Precommitment of normal mouse peritoneal cells by erythrocyte antigens in relation to auto-antibody production. Nature. 1975 Sep 25;257(5524):316–317. doi: 10.1038/257316a0. [DOI] [PubMed] [Google Scholar]
- Primi D., Hammarström L., Smith C. I., Möller G. Characterization of self-reactive B cells by polyclonal B-cell activators. J Exp Med. 1977 Jan 1;145(1):21–30. doi: 10.1084/jem.145.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primi D., Smith C. I., Hammarström L., Möller G. Polyclonal B-cell activators induce immunological response to autologous serum proteins. Cell Immunol. 1977 Dec;34(2):367–375. doi: 10.1016/0008-8749(77)90258-1. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Specter S., Cimprich R., Friedman H., Chedid L. Stimulation of an enhanced in vitro immune response by a synthetic adjuvant, muramyl dipeptide. J Immunol. 1978 Feb;120(2):487–491. [PubMed] [Google Scholar]
- Sugimoto M., Germain R. N., Chedid L., Benacerraf B. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant, N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP). J Immunol. 1978 Mar;120(3):980–982. [PubMed] [Google Scholar]
- Watson J., Whitlock C. Effect of a synthetic adjuvant on the induction of primary immune responses in T cell-depleted spleen cultures. J Immunol. 1978 Jul;121(1):383–389. [PubMed] [Google Scholar]


