Abstract
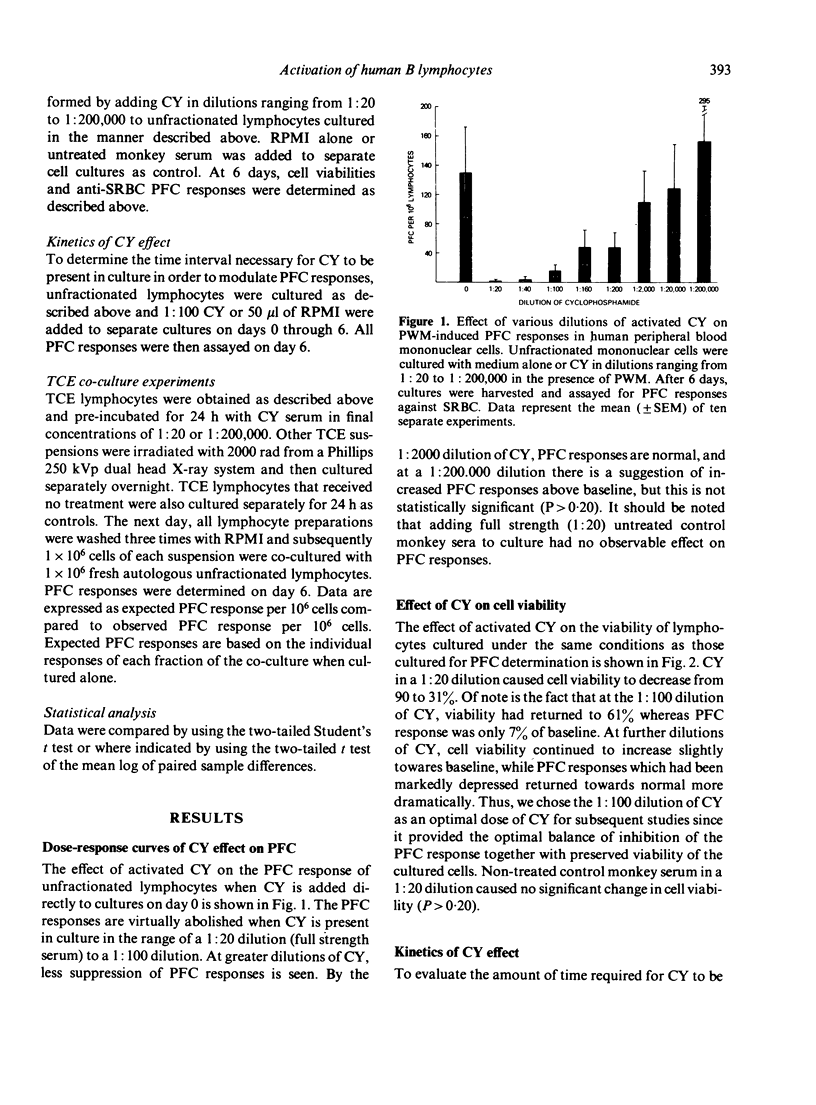
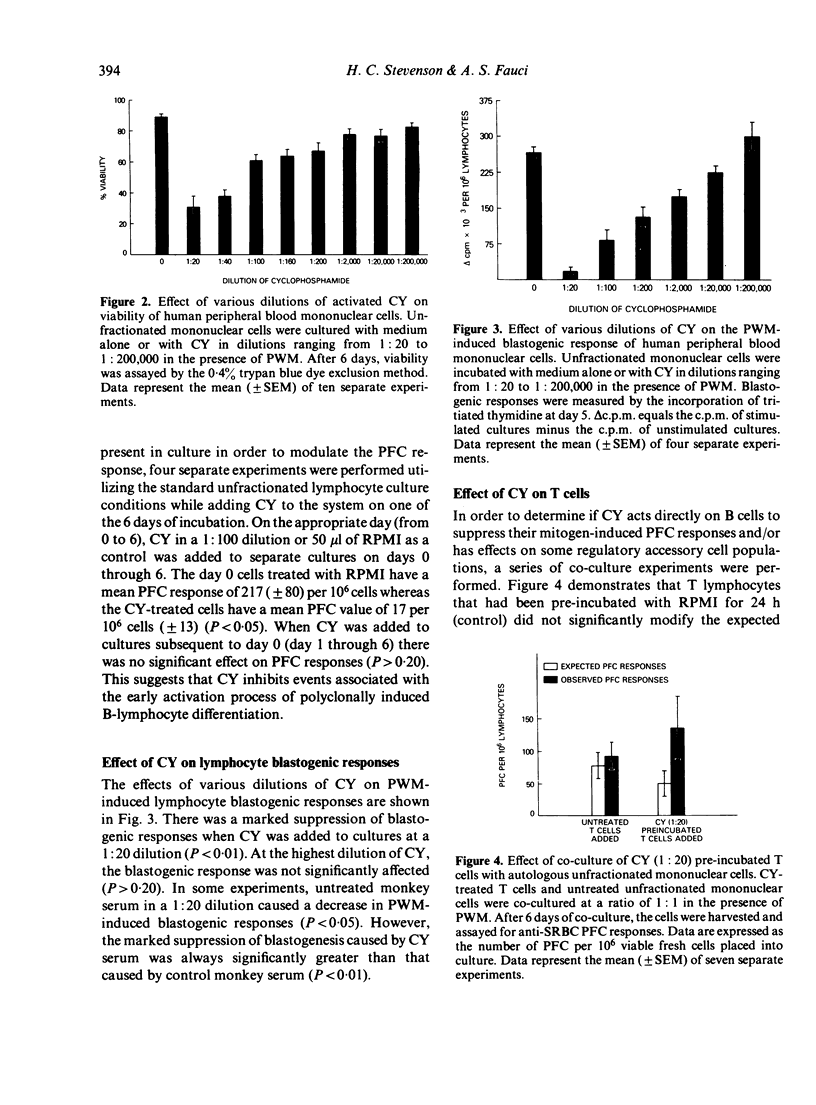
The differential effects of in vitro cyclophosphamide (CY) on subpopulations of normal human peripheral blood lymphocytes involved in the pokeweed mitogen-induced plaque-forming cell (PFC) response against sheep red blood cells were examined. It was found that the plaque-forming B cells in this system are sensitive to CY over a wide concentration range including concentrations which have a minimal effect on overall cell viability. Kinetic experiments revealed that CY exerts its inhibitory effect on the PFC response only if added very early in culture. Thus, it appears that in vitro CY must exert its inhibitory influence on an early phase of polyclonal B-cell activation. When T-cell enriched (TCE) populations were incubated overnight with high concentration CY and then added back in co-culture to fresh autologous B cells, significant enhancement of PFC responses was observed suggesting a selective inhibition or elimination of a regulatory suppressor cell population found in TCE lymphocyte preparations. Helper T cells are relatively resistant to the inhibitory actions of CY. Thus, human B cells appear to be most sensitive to CY, followed in sensitivity by the suppressor cell populations in the T-cell fraction with relative resistance of the helper T cells. These observations have direct relevance in understanding the mechanisms of selective action of CY on normal human lymphocyte subpopulations with possible application to disease states in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balow J. E., Hurley D. L., Fauci A. S. Cyclophosphamide suppression of established cell-mediated immunity. Quantitative vs. qualitative changes in lymphocyte populations. J Clin Invest. 1975 Jul;56(1):65–70. doi: 10.1172/JCI108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavida B. Antigen-induced cyclophosphamide-resistant suppressor T cells inhibit the in vitro generation of cytotoxic cells from one-way mixed leukocyte reactions. J Immunol. 1977 Oct;119(4):1530–1533. [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Cohen J. L., Jao J. Y. Enzymatic basis of cyclophosphamide activation by hepatic microsomes of the rat. J Pharmacol Exp Ther. 1970 Aug;174(2):206–210. [PubMed] [Google Scholar]
- Curtis J. E., Sharp J. T., Lidsky M. D., Hersh E. M. Immune response of patients with rheumatoid arthritis during cyclophosphamide treatment. Arthritis Rheum. 1973 Jan-Feb;16(1):34–42. doi: 10.1002/art.1780160106. [DOI] [PubMed] [Google Scholar]
- Dimitriu A., Fauci A. S. Activation of human B lymphocytes. XI. Differential effects of azathioprine on B lymphocytes and lymphocyte subpopulations regulating B cell function. J Immunol. 1978 Dec;121(6):2335–2339. [PubMed] [Google Scholar]
- Dimitriu A., Haynes B. F., Fauci A. S. Activation of human B lymphocytes. XIV. Characterization of the precursor of the pokeweed mitogen-induced anti-sheep red blood cell plaque-forming cell. J Immunol. 1979 Aug;123(2):890–895. [PubMed] [Google Scholar]
- Duclos H., Galanaud P., Devinsky O., Maillot M. C., Dormont J. Enhancing effect of low dose cyclophosphamide treatment on the in vitro antibody response. Eur J Immunol. 1977 Oct;7(10):679–684. doi: 10.1002/eji.1830071005. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Wolff S. M. Cyclophosphamide and lymphocyte subpopulations in Wegener's granulomatosis. Arthritis Rheum. 1974 Jul-Aug;17(4):355–361. doi: 10.1002/art.1780170404. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Human bone marrow lymphocytes. I. Distribution of lymphocyte subpopulations in the bone marrow of normal individuals. J Clin Invest. 1975 Jul;56(1):98–110. doi: 10.1172/JCI108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Polyclonal activation of bone-marrow-derived lymphocytes from human peripheral blood measured by a direct plaque-forming cell assay. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3676–3679. doi: 10.1073/pnas.73.10.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. IV. Regulatory effects of corticosteroids on the triggering signal in the plaque-forming cell response of human peripheral blood B lymphocytes to polyclonal activation. J Immunol. 1977 Aug;119(2):598–603. [PubMed] [Google Scholar]
- Fauci A. S., Wolff S. M., Johnson J. S. Effect of cyclophosphamide upon the immune response in Wegener's granulomatosis. N Engl J Med. 1971 Dec 30;285(27):1493–1496. doi: 10.1056/NEJM197112302852701. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Wolff S. M. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. Medicine (Baltimore) 1973 Nov;52(6):535–561. doi: 10.1097/00005792-197311000-00002. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Goetzl E. J., Steinberg A. D. Cyclophosphamide: use in practice. Ann Intern Med. 1974 Apr;80(4):531–540. doi: 10.7326/0003-4819-80-4-531. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. VI. Immunoregulation of antibody production by mitogen-induced and naturally occurring cells in normal individuals. Cell Immunol. 1978 Mar 15;36(2):294–302. doi: 10.1016/0008-8749(78)90273-3. [DOI] [PubMed] [Google Scholar]
- Noble B., Parker D., Scheper R. J., Turk J. L. The relation between B-cell stimulation and delayed hypersensitivity. The effect of cyclophosphamide pretreatment on antibody production. Immunology. 1977 Jun;32(6):885–891. [PMC free article] [PubMed] [Google Scholar]
- Polak L., Turk J. L. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974 Jun 14;249(458):654–656. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- Revell P. A. Studies on the effect of cyclophosphamide on T and B lymphocytes in the blood, lymph nodes, and thymus of normal guinea pigs. Int Arch Allergy Appl Immunol. 1974;47(6):864–874. doi: 10.1159/000231277. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Parker D., Noble B., Turk J. L. The relation of immune depression and B-cell stimulation during the development of delayed hypersensitivity to soluble antigens. Immunology. 1977 Feb;32(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- Shand F. L., Howard J. G. Cyclophosphamide inhibited B cell receptor regeneration as a basis for drug-induced tolerance;. Nature. 1978 Jan 19;271(5642):255–257. doi: 10.1038/271255a0. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Claman H. N. Immune suppression with supraoptimal doses of antigen in contact sensitivity. I. Demonstration of suppressor cells and their sensitivity to cyclophosphamide. J Immunol. 1977 Jul;119(1):240–244. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]


