Abstract
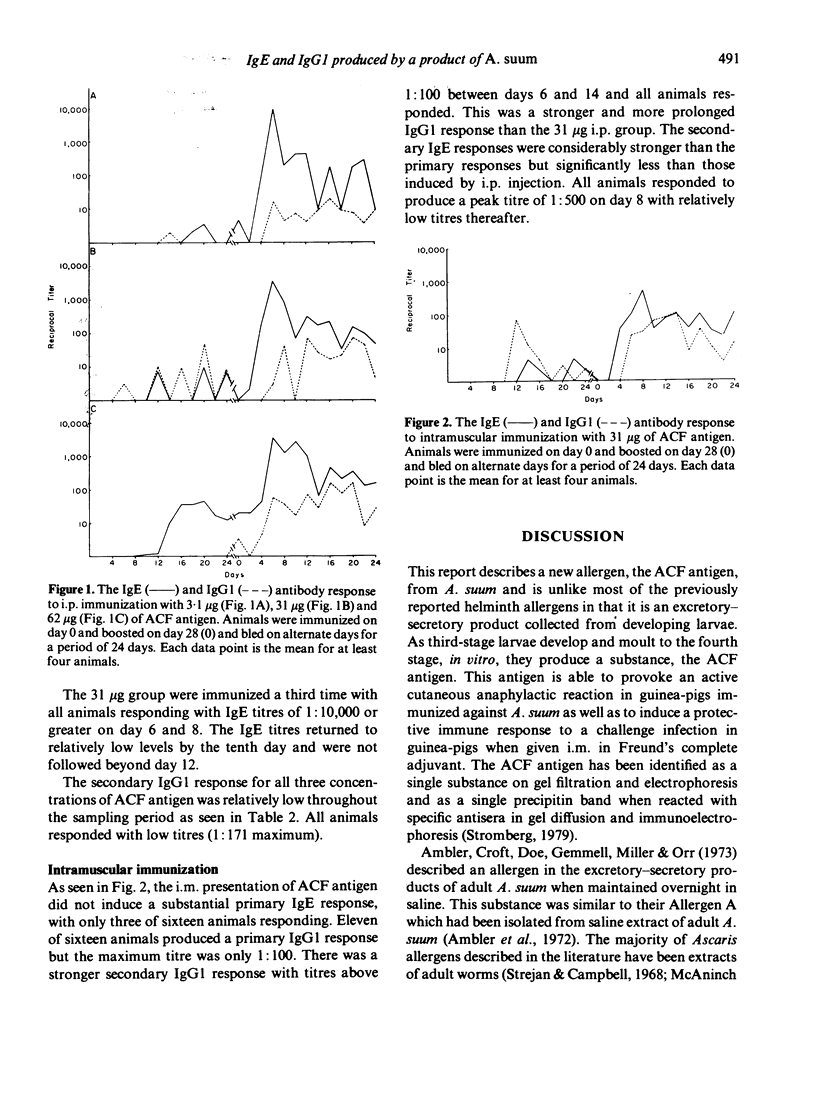
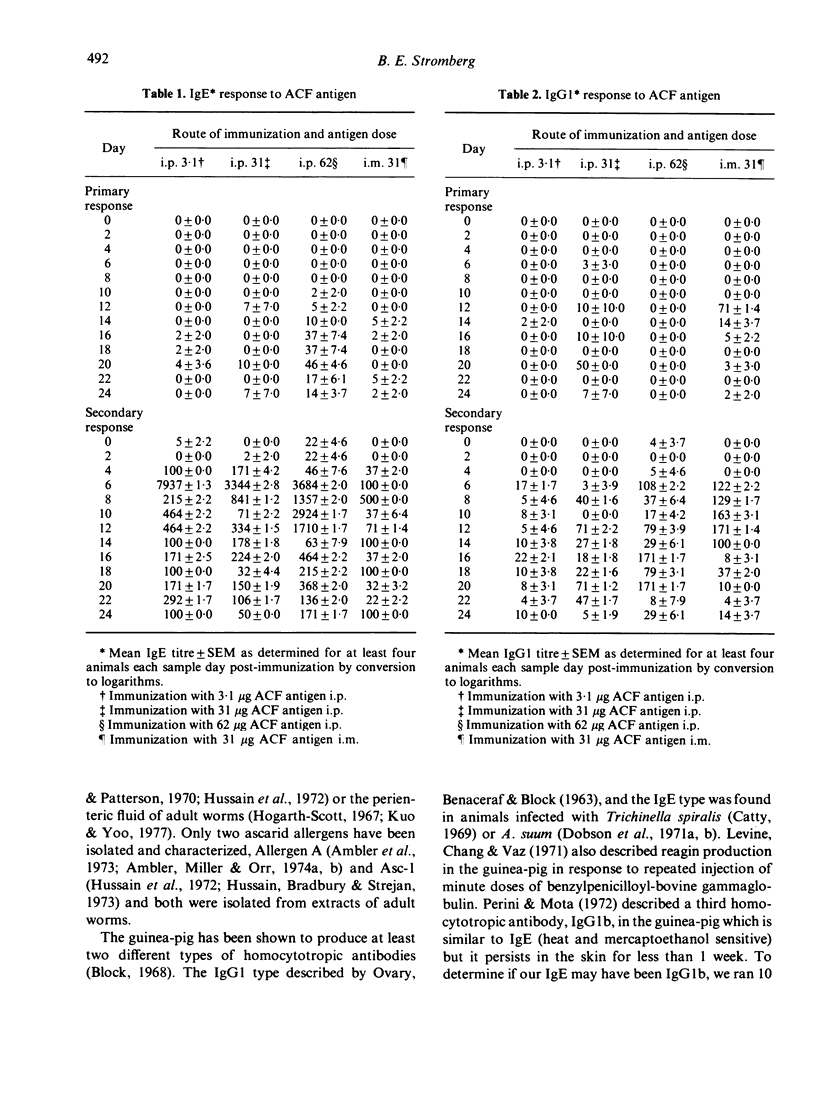
Third-stage larvae of Ascaris suum cultured to the fourth stage in a chemically defined culture medium produced a substance, the 'ACF antigen', which was allergenic in the guinea-pig. When three different concentrations (3.1, 31 and 62 micrograms) of the ACF antigen were given intraperitoneally, only the highest concentration induced a primary IgE specific antibody response (1:100 titre) as determined with the passive cutaneous anaphylaxis reaction. Upon secondary exposure all concentrations demonstrated a strong IgE response (1:50,000 peak titre) with very little IgG1 activity (1:100). The secondary IgE responses began to rise on the fourth day, peaked on the sixth day and returned to relatively low levels by the fourteenth day (1:100). The intramuscular administration of the ACF antigen did not induce the extremely high titres of IgE as found with the intraperitoneal injection, but rather a low level response (1:500 peak) which did not differ greatly from the IgG1 response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler J., Croft A. R., Doe J. E., Gemmell D. K., Miller J. N., Orr T. S. Biological techniques for studying the allergenic components of nematodes. II. The characterisation of the allergen released by Ascaris suum maintaind in saline. J Immunol Methods. 1973 Apr;2(3):315–323. doi: 10.1016/0022-1759(73)90058-6. [DOI] [PubMed] [Google Scholar]
- Ambler J., Doe J. E., Gemmell D. K., Roberts J. A., Orr T. S. Biological techniques for studying the allergenic components of nematodes. I. Detection of allergenic components in Ascaris suum extracts. J Immunol Methods. 1972 Aug;1(4):317–328. doi: 10.1016/0022-1759(72)90025-7. [DOI] [PubMed] [Google Scholar]
- Ambler J., Miller J. N., Johnson P., Orr T. S. Characterisation of an allergen extracted from Ascaris suum. Determination of the molecular weight, isoelectric point, amino acid and carbohydrate content of the native allergen. Immunochemistry. 1973 Dec;10(12):815–820. doi: 10.1016/0019-2791(73)90185-7. [DOI] [PubMed] [Google Scholar]
- Ambler J., Miller J. N., Orr T. S. Some properties of Ascaris suum allergen A. Int Arch Allergy Appl Immunol. 1974;46(3):427–437. doi: 10.1159/000231146. [DOI] [PubMed] [Google Scholar]
- André C., Heremans J. F., Vaerman J. P., Cambiaso C. L. A mechanism for the induction of immunological tolerance by antigen feeding: antigen-antibody complexes. J Exp Med. 1975 Dec 1;142(6):1509–1519. doi: 10.1084/jem.142.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C. R., Munoz J., Bergman R. K. A reaginic type of antibody stimulated by extracts of Bordetella pertussis in inbred strains of mice. J Immunol. 1970 Feb;104(2):312–319. [PubMed] [Google Scholar]
- Dobson C., Morseth D. J., Soulsby J. L. Immunoglobulin E-type antibodies induced by Ascaris suum infections in guinea pigs. J Immunol. 1971 Jan;106(1):128–133. [PubMed] [Google Scholar]
- Dobson C., Rockey J. H., Soulsby E. J. Immunoglobulin E antibodies in guinea pigs: characterization of monomeric and polymeric components. J Immunol. 1971 Nov;107(5):1431–1439. [PubMed] [Google Scholar]
- Hogarth-Scott R. S. The molecular weight range of nematode allergens. Immunology. 1967 Nov;13(5):535–537. [PMC free article] [PubMed] [Google Scholar]
- Hussain R., Bradbury S. M., Strejan G. Hypersensitivity to Ascaris antigens. 8. Characterization of a highly purified allergen. J Immunol. 1973 Jul;111(1):260–268. [PubMed] [Google Scholar]
- Hussain R., Strejan G., Campbell D. H. Hypersensitivity to ascaris antigens. VII. Isolation and partial characterization of an allergen. J Immunol. 1972 Sep;109(3):638–647. [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M., McDougall W., McNulty E. Rat IgE production. II. Primary and booster reaginic antibody responses following intradermal or oral immunization. Immunology. 1976 May;30(5):671–677. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M. Time course studies on rat IgE production in N. Brasiliensis infection. Clin Exp Immunol. 1976 May;24(2):346–351. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Stewart D. C. Rat IgE production. I. Effect of dose of antigen on primary and secondary reaginic antibody responses. Immunology. 1974 Sep;27(3):365–381. [PMC free article] [PubMed] [Google Scholar]
- Khoury P. B., Stromberg B. E., Soulsby E. J. Immune mechanisms to Ascaris suum in inbred guinea-pigs. I. Passive transfer of immunity by cells or serum. Immunology. 1977 Apr;32(4):405–411. [PMC free article] [PubMed] [Google Scholar]
- Kuo C. Y., Yoo T. J. A new allergen from the perienteric fluid of Ascaris suum with respect to charges. Int Arch Allergy Appl Immunol. 1977;54(4):308–314. doi: 10.1159/000231842. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine B. B., Chang H., Jr, Vaz N. M. The production of hapten-specific reaginic antibodies in the guinea pig. J Immunol. 1971 Jan;106(1):29–33. [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Mota I., Perini A. A heat labile mercaptoethanol susceptible homocytotropic antibody in the guinea pig. Life Sci II. 1970 Aug 22;9(16):923–930. doi: 10.1016/0024-3205(70)90063-9. [DOI] [PubMed] [Google Scholar]
- OVARY Z., BENACERRAF B., BLOCH K. J. Properties of guinea pig 7S antibodies. II. Identification of antibodies involved in passive cutaneous and systemic anaphylaxis. J Exp Med. 1963 Jun 1;117:951–964. doi: 10.1084/jem.117.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini A., Mota I. Heterogeneity of guinea-pig homocytotropic antibodies. Immunology. 1972 May;22(5):915–923. [PMC free article] [PubMed] [Google Scholar]
- Strejan G. H., Surlan D. Reaginic antibody production to Ascaris suum allergen, ASC-1. I. The function of glutaraldehyde-polymerized antigen in the induction of reaginic (IgE) antibodies in the rat. Int Arch Allergy Appl Immunol. 1977;54(6):487–501. [PubMed] [Google Scholar]
- Strejan G., Campbell D. H. Hypersensitivity to Ascaris antigens. IV. Production of homocytotropic antibodies in the rat. J Immunol. 1968 Oct;101(4):628–637. [PubMed] [Google Scholar]
- Tada T., Okumura K., Ochiai T., Iwasa S. Effect of lymphocytosis-promoting factor of Bordetella pertussis on the immune response. II. Adjuvant effect for the production of reaginic antibody in the rat. Int Arch Allergy Appl Immunol. 1972;43(2):207–216. doi: 10.1159/000230838. [DOI] [PubMed] [Google Scholar]
- Vaz N. M., Maia L. C., Hanson D. G., Lynch J. M. Inhibition of homocytotropic antibody responses in adult inbred mice by previous feeding of the specific antigen. J Allergy Clin Immunol. 1977 Aug;60(2):110–115. doi: 10.1016/0091-6749(77)90035-5. [DOI] [PubMed] [Google Scholar]


