Abstract
By comparing the amounts of precipitate formed by the reaction between human serum albumin (HSA) and isomolar solutions of rabbit anti-HSA F(ab')2 fragments and of the corresponding intact rabbit anti-HSA IgG, respectively, it was found that the Fc portion of IgG was of great importance for the precipitin reaction. In the present antigen-antibody system, about half of the antigen was precipitated by this mechanism. This effect was called Fc-mediated precipitation and was most clearly expressed in the biologically important zone of low antigen excess and the zone of equivalence. It was found that the pepsin digestion did not change the ability of the F(ab')2 fragments to bind the antigen in comparison to intact IgG. Furthermore, turbidimetric analyses indicated that the Fc portion was involved in the overall mechanism of the precipitin reaction throughout the range of the precipitin curve.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMIRAIAN K., LEIKHIM E. J. Interaction of fragment III of rabbit gamma globulin and guinea pig complement. Proc Soc Exp Biol Med. 1961 Nov;108:454–457. doi: 10.3181/00379727-108-26963. [DOI] [PubMed] [Google Scholar]
- Aladjem F., Palmiter M. T., Chang F. W. On the mechanism of the quantitative precipitin reaction. Immunochemistry. 1966 Sep;3(5):419–424. doi: 10.1016/0019-2791(66)90179-0. [DOI] [PubMed] [Google Scholar]
- Amiraian K., Leikhim E. J. Properties of fragments of human Wassermann antibodies. Immunology. 1966 Apr;10(4):349–353. [PMC free article] [PubMed] [Google Scholar]
- Ansari A. A., Salahuddin A. Effects of pH, ionic strength and temperature on the ovalbuminanti-ovalbumin precipitin reaction. Immunology. 1973 Sep;25(3):377–383. [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. Determination of soluble immune complex molar composition and antibody association constants by ammonium sulfate precipitation. J Immunol. 1974 Feb;112(2):451–461. [PubMed] [Google Scholar]
- Czop J., Nussenzweig V. Studies on the mechanism of solubilization of immune precipitates by serum. J Exp Med. 1976 Mar 1;143(3):615–630. doi: 10.1084/jem.143.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E. The use of a sensitive micronephelometer in the estimation of antigens and precipitating antibodies. Immunology. 1971 May;20(5):779–787. [PMC free article] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Fritz R. B., Lassiter S., Day E. D. The effect of iodination on antifibrinogen antibodies with respect to precipitating and adsorption activities. Immunochemistry. 1967 Sep;4(5):283–293. doi: 10.1016/0019-2791(67)90111-5. [DOI] [PubMed] [Google Scholar]
- Fudenberg H., Mandy W. J., Nisonoff A. SEROLOGIC STUDIES OF PROTEOLYTIC FRAGMENTS OF RABBIT AGGLUTINATING ANTIBODIES. J Clin Invest. 1962 Dec;41(12):2123–2134. doi: 10.1172/JCI104670. [DOI] [PMC free article] [PubMed] [Google Scholar]
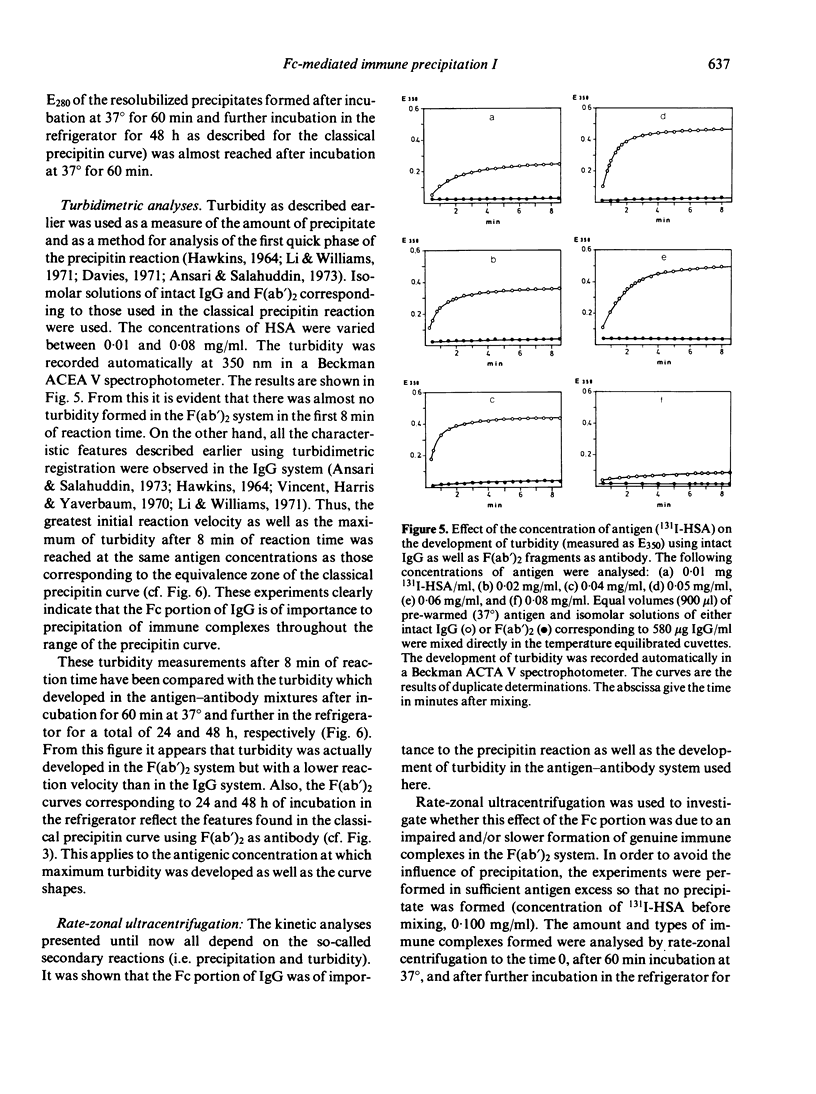
- HAWKINS J. D. SOME STUDIES ON THE PRECIPITIN REACTION USING A TURBIDIMETRIC METHOD. Immunology. 1964 May;7:229–238. [PMC free article] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
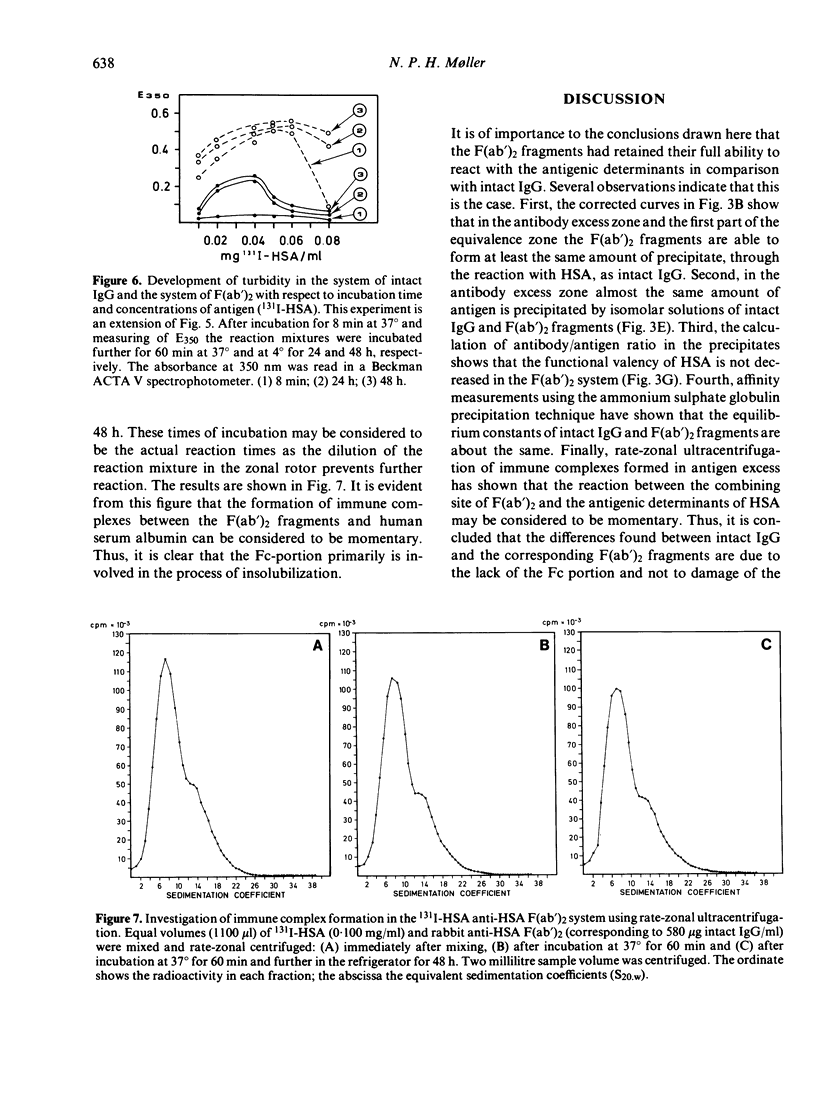
- JAQUET H., CEBRA J. J. COMPARISON OF TWO PRECIPITATING DERIVATIVES OF RABBIT ANTIBODY: FRAGMENT I DIMER AND THE PRODUCT OF PEPSIN DIGESTION. Biochemistry. 1965 May;4:954–963. doi: 10.1021/bi00881a024. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Møller N. P., Steensgaard J. Fc-mediated immune precipitation. II. Analysis of precipitating immune complexes by rate-zonal ultracentrifugation. Immunology. 1979 Nov;38(3):641–648. [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A. ENZYMATIC DIGESTION OF RABBIT GAMMA GLOBULIN AND ANTIBODY AND CHROMATOGRAPHY OF DIGESTION PRODUCTS. Methods Med Res. 1964;10:134–141. [PubMed] [Google Scholar]
- NISONOFF A., PRESSMAN D. Loss of precipitating activity of antibody without destruction of binding sites. Science. 1958 Sep 19;128(3325):659–660. doi: 10.1126/science.128.3325.659. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960 Dec 9;132(3441):1770–1771. doi: 10.1126/science.132.3441.1770. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Steensgaard J., Johansen H. K., Moller N. P. Computer simulation of immunochemical interactions. Immunology. 1975 Sep;29(3):571–579. [PMC free article] [PubMed] [Google Scholar]
- Steensgaard J., Liu B. M., Cline G. B., Møller N. P. The properties of immune complex-forming systems. A new theoretical approach. Immunology. 1977 Apr;32(4):445–456. [PMC free article] [PubMed] [Google Scholar]
- Steensgaard J., Moller N. P., Funding L. The effects of overloading in density-gradient centrifugation. Eur J Biochem. 1975 Feb 21;51(2):483–493. doi: 10.1111/j.1432-1033.1975.tb03948.x. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The use of ammonium sulphate globulin precipitation for determination of affinity of anti-protein antibodies in mouse serum. Immunology. 1972 May;22(5):747–756. [PMC free article] [PubMed] [Google Scholar]
- Vincent W. F., Harris E. W., Yaverbaum S. The estimation of precipitating antibody using a turbidimetric technique. Immunology. 1970 Feb;18(2):143–147. [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L. Immunoglobulin structure and effector functions. Immunochemistry. 1978 Sep;15(9):695–714. doi: 10.1016/0161-5890(78)90044-5. [DOI] [PubMed] [Google Scholar]


