Abstract
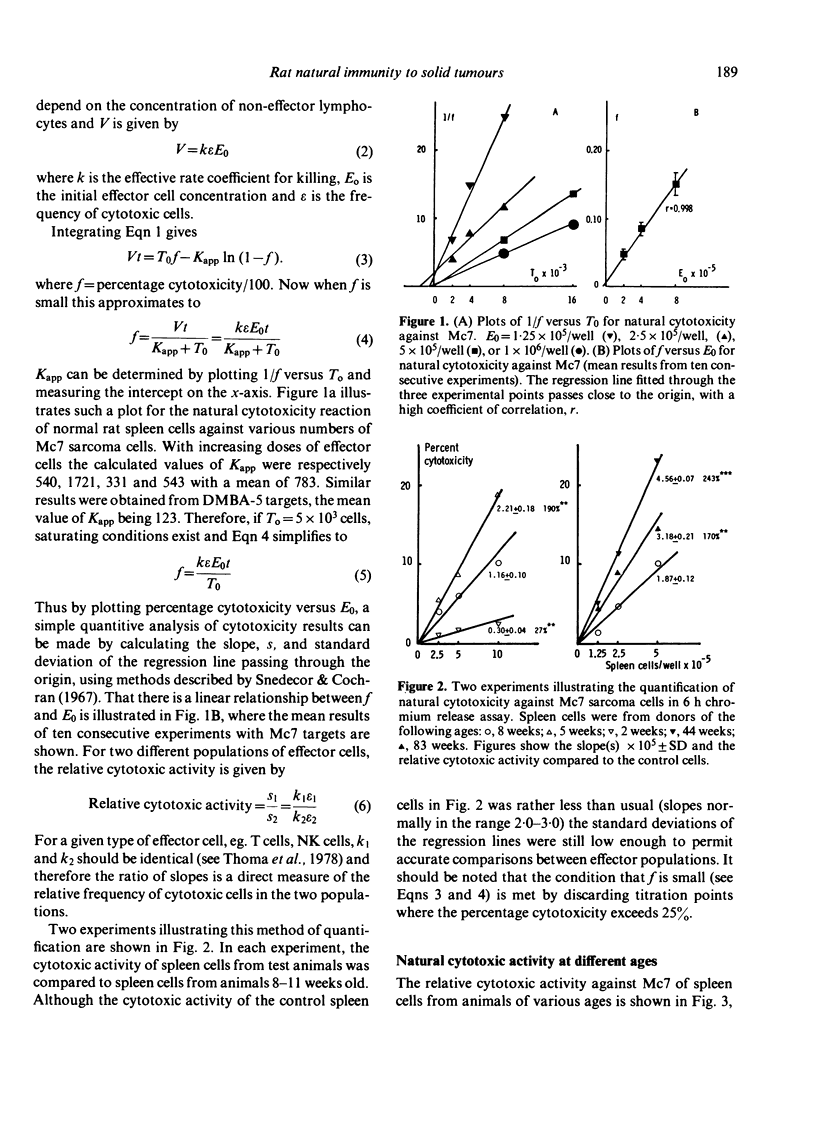
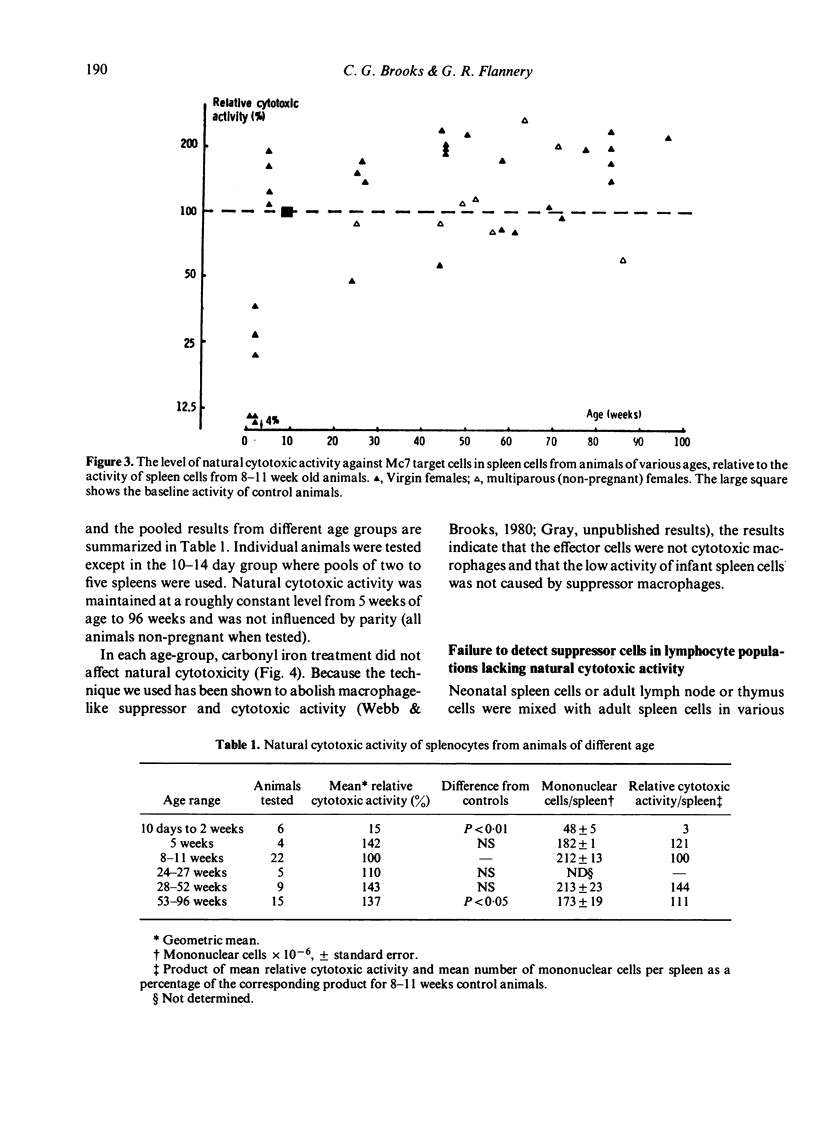
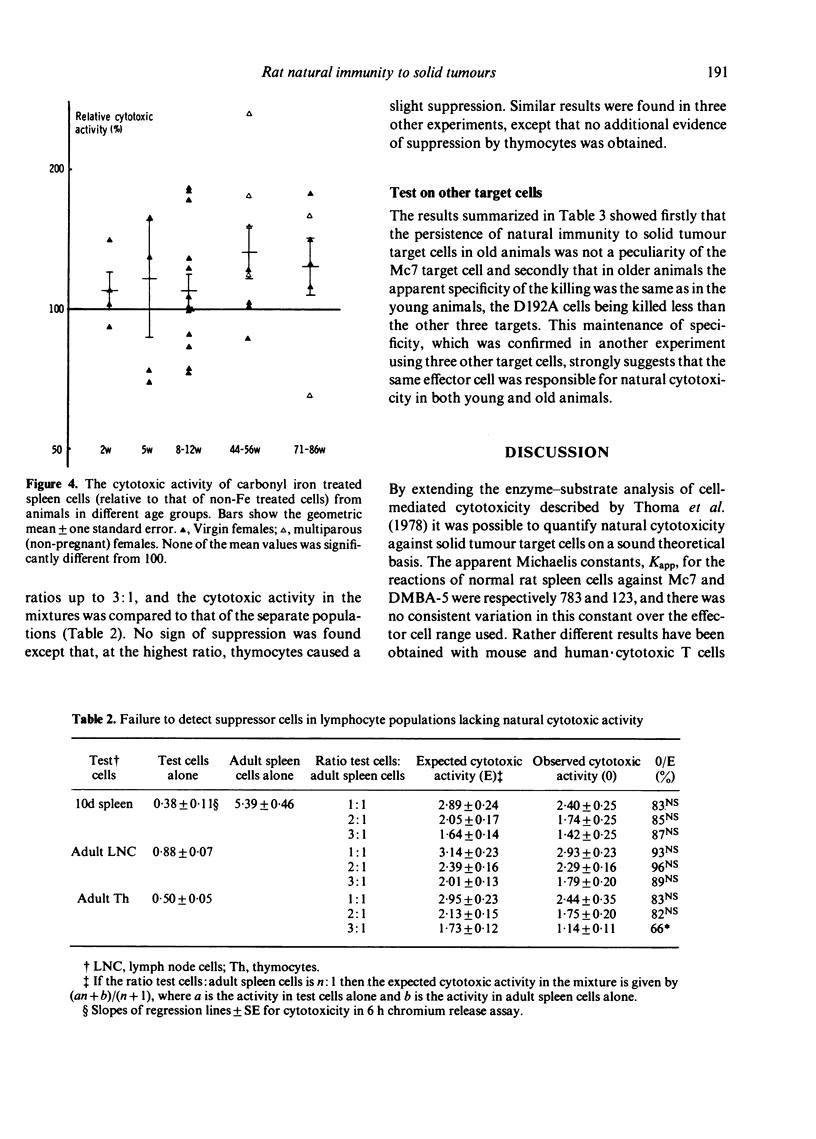
Consideration of cell-mediated cytotoxicity as an enzyme-substrate reaction provided a theoretical and practical basis for the quantification of natural immunity to solid tumours. The natural cytotoxic activity of spleen cells from normal rats towards cultured target cells from solid tumours, measured in a 6 h chromium release assay, developed between 2 and 5 weeks after birth and, in contrast to previous reports of natural cytotoxicity against lymphoid tumour cells, showed no sign of decline up till at least 22 months of age. Both virgin and breeder (multiparous) females showed equally good maintenance of natural immunity, and the apparent specificity of this natural immunity on a panel of four target cells did not change between 8 weeks and 18 months of age. The low level of natural immunity in infant spleen, and also in adult lymph nodes and thymus, was not caused by suppressor cells but rather by an absence of appropriate effector cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Rees R. C., Leach R. H. High nonspecific reactivity of normal lymphocytes against mycoplasma-infected target cells in cytotoxicity assays. Eur J Immunol. 1979 Feb;9(2):159–165. doi: 10.1002/eji.1830090213. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Rees R. C., Robins R. A. Studies on the microcytotoxicity test. II. The uptake of amino acids ([3H]leucine or [75Se]methionine) but not nucleosides ([3H]thymidine or [125I]IUdR) or 51CrO24-provides a direct and quantitative measure of target cell survival in the presence of lymphoid cells. J Immunol Methods. 1978;21(1-2):111–124. doi: 10.1016/0022-1759(78)90228-4. [DOI] [PubMed] [Google Scholar]
- Callewaert D. M., Johnson D. F., Kearney J. Spontaneous cytotoxicity of cultured human cell lines mediated by normal peripheral blood lymphocytes. III. Kinetic parameters. J Immunol. 1978 Aug;121(2):710–717. [PubMed] [Google Scholar]
- Datta S. K., Gallagher M. T., Trentin J. J. Natural cell-mediated cytotoxicity in hamsters. Int J Cancer. 1979 May 15;23(5):728–734. doi: 10.1002/ijc.2910230522. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Haller O., Kiessling R., Orn A., Wigzell H. Generation of natural killer cells: an autonomous function of the bone marrow. J Exp Med. 1977 May 1;145(5):1411–1416. doi: 10.1084/jem.145.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herrick M. V., Pollack S. B. Kinetic analysis of antibody-dependent cellular cytotoxicity: evidence for noncompetitive inhibition by autologous lymphoid cells. J Immunol. 1978 Oct;121(4):1348–1352. [PubMed] [Google Scholar]
- Kiessling R., Haller O. Natural killer cells in the mouse: an alternative immune surveillance mechanism? Contemp Top Immunobiol. 1978;8:171–201. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Lindsay L. R., Nunn M. E., Herberman R. B. Natural cell-mediated cytotoxicity in rats. I. Tissue and strain distribution, and demonstration of a membrance receptor for the Fc portion of IgG. Int J Cancer. 1978 Feb 15;21(2):204–209. doi: 10.1002/ijc.2910210212. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Lindsay L. R., Nunn M. E., Holden H. T., Herberman R. B. Natural cell-mediated cytotoxicity in rats. II. In vivo augmentation of NK-cell activity. Int J Cancer. 1978 Feb 15;21(2):210–220. doi: 10.1002/ijc.2910210213. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Figarella E. F., Cuttito M. J., Cahan A., Stutman O. Natural cytotoxic cells against solid tumors in mice. II. Some characteristics of the effector cells. J Immunol. 1978 Nov;121(5):1827–1835. [PubMed] [Google Scholar]
- Savary C. A., Lotzová E. Suppression of natural killer cell cytotoxicity by splenocytes from Corynebacterium parvum-injected, bone marrow-tolerant, and infant mice. J Immunol. 1978 Jan;120(1):239–243. [PubMed] [Google Scholar]
- Sendo F., Aoki T., Boyse E. A., Buafo C. K. Natural occurrence of lymphocytes showing cytotoxic activity to BALB/c radiation-induced leukemia RL male 1 cells. J Natl Cancer Inst. 1975 Sep;55(3):603–609. doi: 10.1093/jnci/55.3.603. [DOI] [PubMed] [Google Scholar]
- Shellam G. R. Gross-virus-induced lymphoma in the rat. V. Natural cytotoxic cells are non-T cells. Int J Cancer. 1977 Feb 15;19(2):225–235. doi: 10.1002/ijc.2910190212. [DOI] [PubMed] [Google Scholar]
- Shellam G. R., Hogg N. Gross-virus-induced lymphoma in the rat. IV. Cytotoxic cells in normal rats. Int J Cancer. 1977 Feb 15;19(2):212–224. doi: 10.1002/ijc.2910190211. [DOI] [PubMed] [Google Scholar]
- Stutman O., Paige C. J., Figarella E. F. Natural cytotoxic cells against solid tumors in mice. I. Strain and age distribution and target cell susceptibility. J Immunol. 1978 Nov;121(5):1819–1826. [PubMed] [Google Scholar]
- Takasugi M., Mickey M. R., Terasaki P. I. Reactivity of lymphocytes from normal persons on cultured tumor cells. Cancer Res. 1973 Nov;33(11):2898–2902. [PubMed] [Google Scholar]
- Thoma J. A., Touton M. H., Clark W. R. Interpretation of 51Cr-release data: a kinetic analysis. J Immunol. 1978 Mar;120(3):991–997. [PubMed] [Google Scholar]
- Thorn R. M., Henney C. S. Kinetic analysis of target cell destruction by effector T cells. I. Delineation of parameters related to the frequency and lytic efficiency of killer cells. J Immunol. 1976 Dec;117(6):2213–2219. [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. Introduction of "natural" killer' cells by BCG. Nature. 1976 Aug 12;262(5569):584–586. doi: 10.1038/262584a0. [DOI] [PubMed] [Google Scholar]
- Zeijlemaker W. P., van Oers R. H., de Goede R. E., Schellekens P. T. Cytotoxic activity of human lymphocytes: quanitative analysis of T cell and K cell cytotoxicity, revealing enzyme-like kinetics. J Immunol. 1977 Oct;119(4):1507–1514. [PubMed] [Google Scholar]


