Abstract
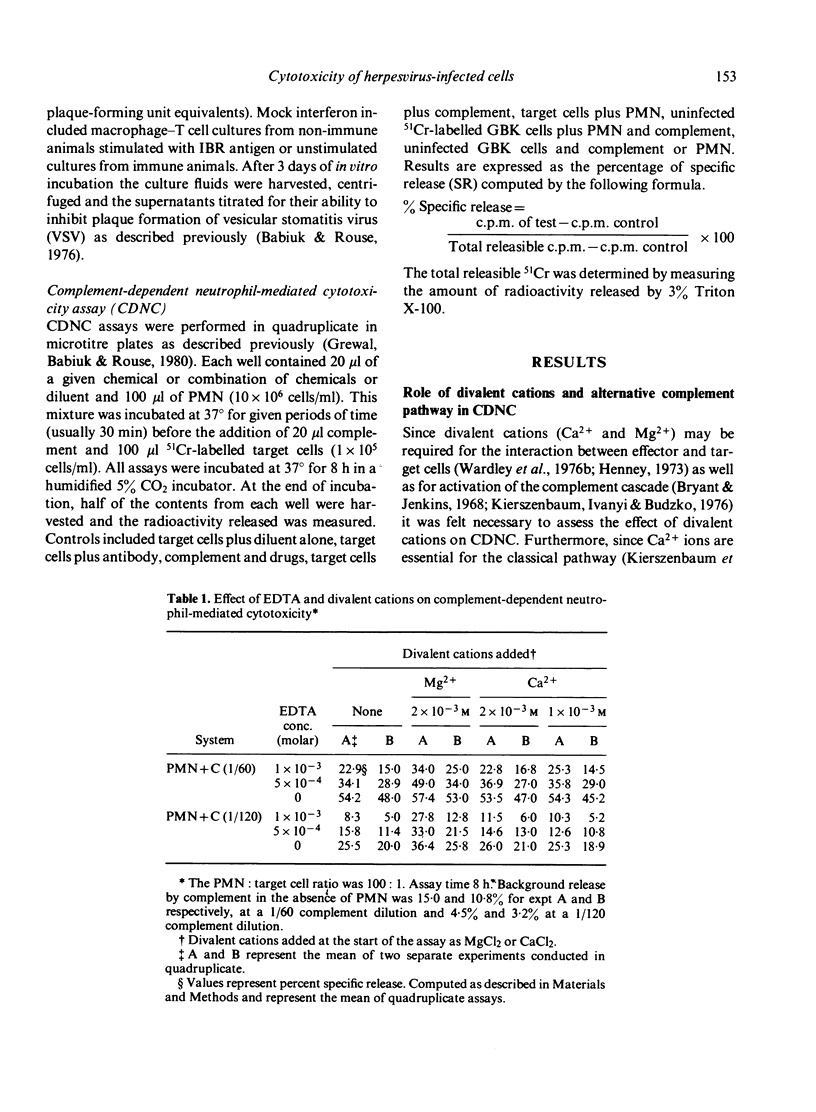
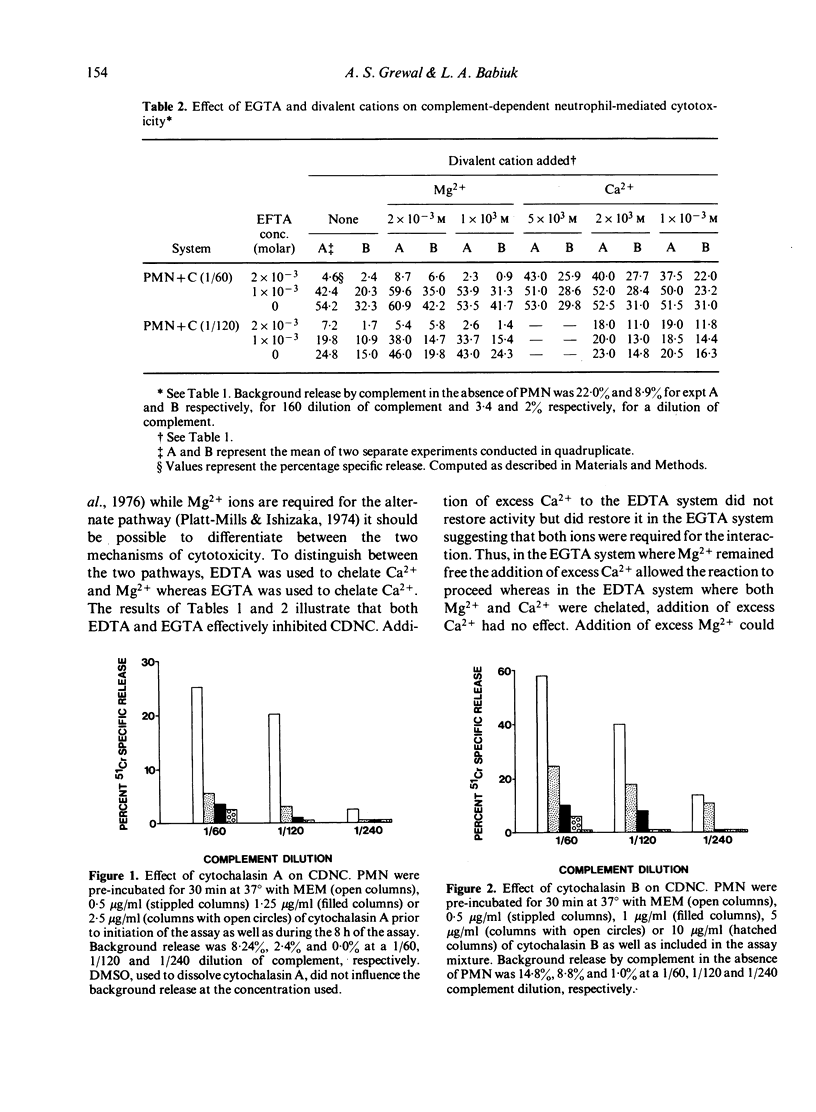
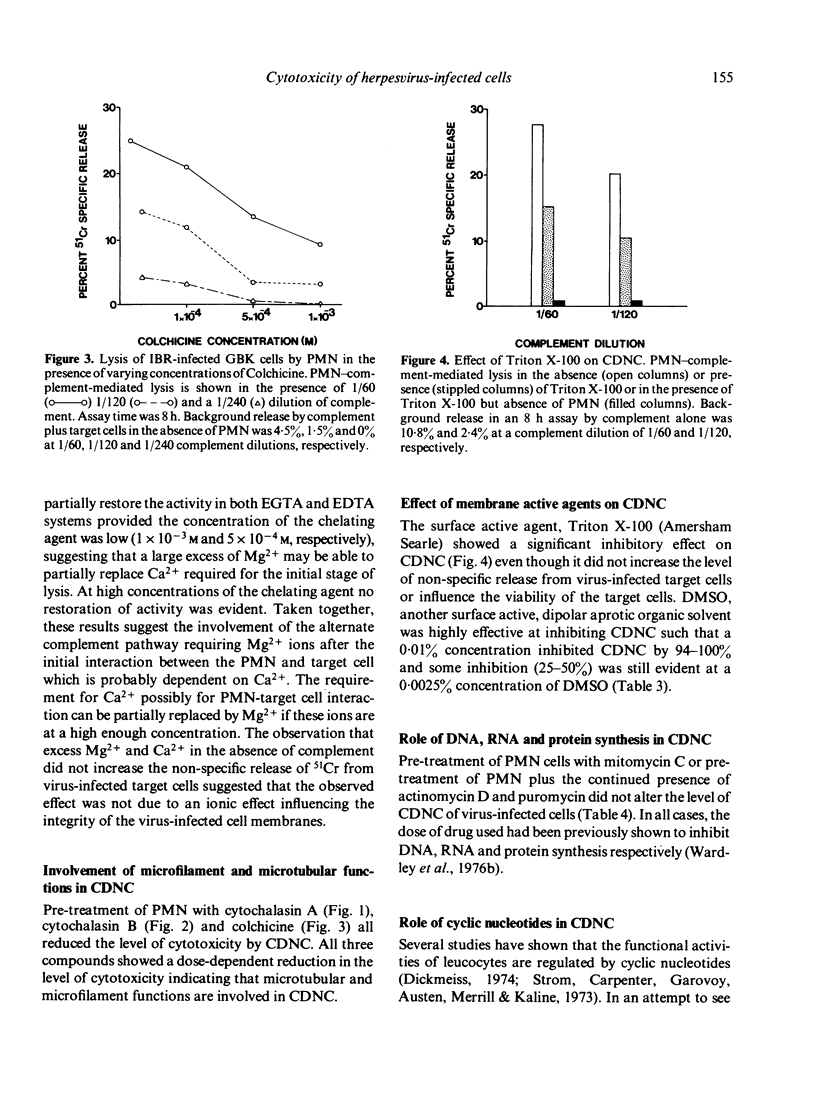
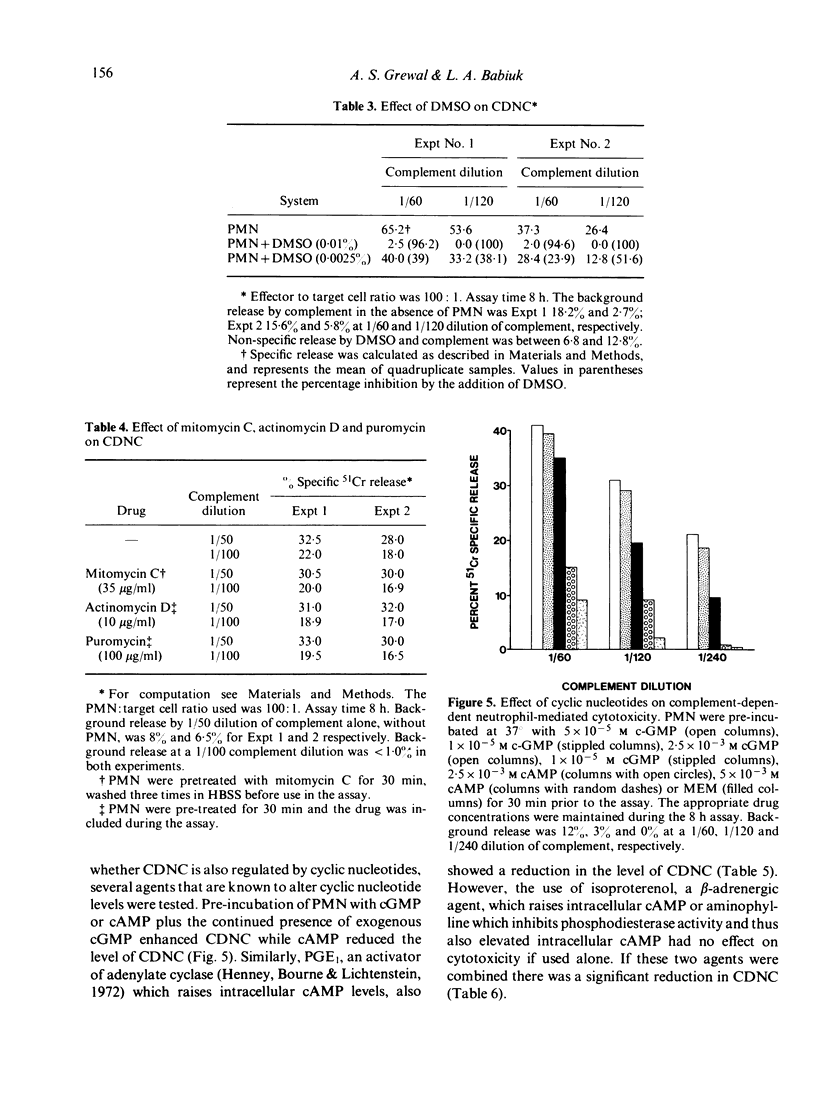
Highly enriched bovine polymorphonuclear neutrophils (PMN) were able to destroy herpesvirus-infected cells in the presence of complement. Our results suggest that some viral antigens are capable of activating the alternate complement pathway directly which results in cytotoxicity however, the addition of PMN plus complement resulted in high levels of cytotoxicity. Chelation of Ca2+ or Mg2+ indicated that Ca2+ was involved in PMN--target cell interactions but Mg2+ was involved in activation of the alternate complement pathway. Cytotoxicity in the presence of PMNs was shown to be under bidirectional control of cyclic nucleotides. Thus agents that elevated cAMP reduced cytotoxicity whereas agents that elevated GMP increased it. If the PMN is secreting some product during the cytotoxic reaction it must be performed since chemical shown to inhibit DNA, RNA and protein synthesis had no influence on CDNC. Our results are discussed in terms of the mechanism of CDNC lysis as well as in the vivo significance of such a defence mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
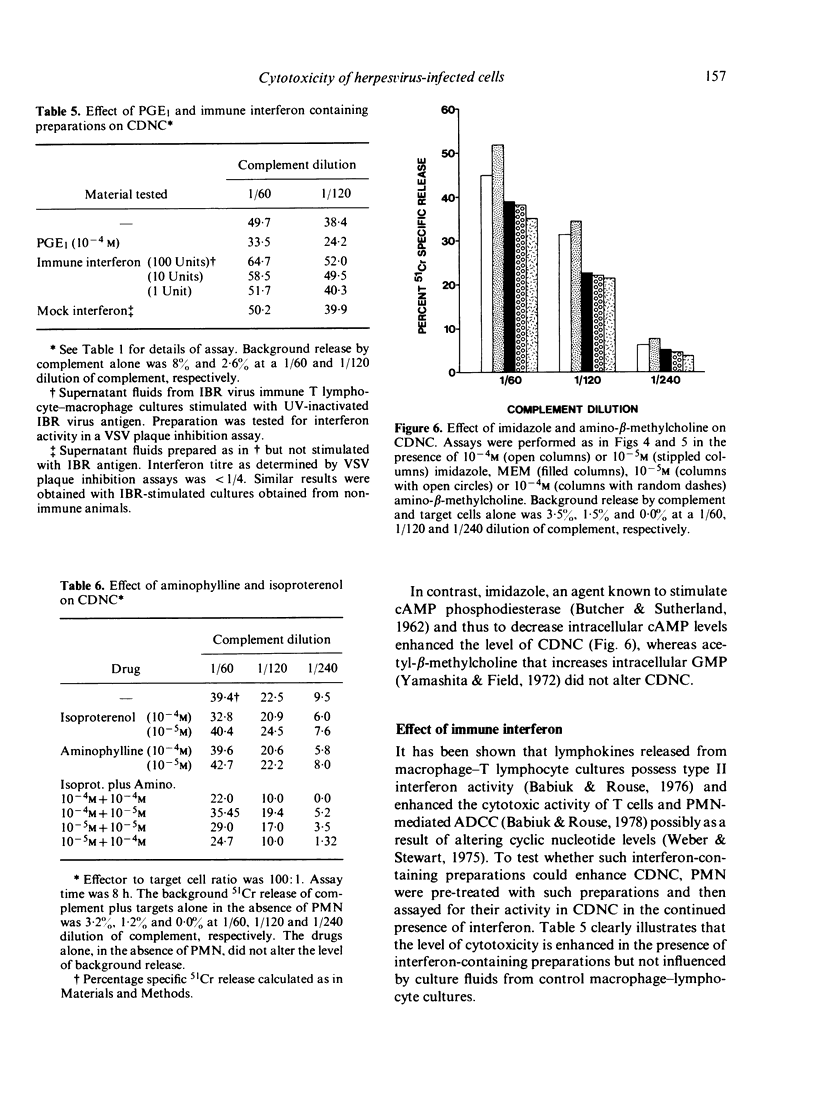
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Babiuk L. A., Rouse B. T. Immune interferon production by lymphoid cells: role in the inhibition of herpesviruses. Infect Immun. 1976 Jun;13(6):1567–1578. doi: 10.1128/iai.13.6.1567-1578.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., Rouse B. T. Interactions between effector cell activity and lymphokines: implications for recovery from herpesvirus infections. Int Arch Allergy Appl Immunol. 1978;57(1):62–73. doi: 10.1159/000232085. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Suppressive effect of interferon on the humoral immune response to sheep red blood cells in mice. J Immunol. 1974 Oct;113(4):1319–1325. [PubMed] [Google Scholar]
- Bryant R. E., Jenkins D. E., Jr Calcium requirements for complement dependent hemolytic reactions. J Immunol. 1968 Oct;101(4):664–668. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Reversible inhibition of lymphocyte-mediated cytotoxicity by cytochalasin B. Nat New Biol. 1972 Jun 28;237(78):272–273. doi: 10.1038/newbio237272a0. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Studies on the mechanism of antibody-dependent polymorphonuclear leukocyte-mediated cytotoxicity. J Immunol. 1977 Oct;119(4):1413–1418. [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeiss E. Comparative study of antibody-dependent and direct lymphocyte-mediated cytotoxicity in vitro after alloimmunization in the human. II. Chemical inhibitors. Scand J Immunol. 1974;3(6):817–821. doi: 10.1111/j.1365-3083.1974.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Modulation of polymorphonuclear leukocyte-mediated antibody-dependent cellular cytotoxicity. J Immunol. 1974 Dec;113(6):1793–1800. [PubMed] [Google Scholar]
- Gelfand E. W., Morris S. A., Resch K. Antibody-dependent cytotoxicity: modulation by the cytochalasins and microtubule-disruptive agents. J Immunol. 1975 Mar;114(3):919–924. [PubMed] [Google Scholar]
- Gresser I. On the varied biologic effects of interferon. Cell Immunol. 1977 Dec;34(2):406–415. doi: 10.1016/0008-8749(77)90262-3. [DOI] [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Characterization of surface receptors on bovine leukocytes. Int Arch Allergy Appl Immunol. 1978;56(4):289–300. doi: 10.1159/000232034. [DOI] [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Mechanisms of recovery from viral infections: destruction of infected cells by neutrophils and complement. J Immunol. 1980 Jan;124(1):312–319. [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Henney C. S. On the mechanism of T-cell mediated cytolysis. Transplant Rev. 1973;17(0):37–70. doi: 10.1111/j.1600-065x.1973.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Henney C. S. T cell mediated cytolysis: consideration of the role of a soluble mediator. J Reticuloendothel Soc. 1975 Apr;17(4):231–235. [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Bukovic J. A., Baron S. Interferon inhibition of the primary in vitro antibody response to a thymus-independent antigen. Cell Immunol. 1975 Nov;20(1):104–109. doi: 10.1016/0008-8749(75)90089-1. [DOI] [PubMed] [Google Scholar]
- Juy D., Billecocq A., Faure M., Bona C. Damage of liposomes in antibody-dependent cell-mediated cytotoxicity. Scand J Immunol. 1977;6(6-7):607–614. doi: 10.1111/j.1365-3083.1977.tb02140.x. [DOI] [PubMed] [Google Scholar]
- KIRK J. M. The mode of action of actinomycin D. Biochim Biophys Acta. 1960 Jul 29;42:167–169. doi: 10.1016/0006-3002(60)90769-1. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the specific cytotoxicity of sensitized lymphocytes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):721–725. doi: 10.1073/pnas.69.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. Presidential address to the American Association of Immunologists, delivered in Chicago, Illinois, April 6, 1977. Mechanism of cytolysis by lymphocytes: A comparison with complement. J Immunol. 1977 Oct;119(4):1195–1203. [PubMed] [Google Scholar]
- McConnell I., Klein G., Lint T. F., Lachmann P. J. Activation of the alternative complement pathway by human B cell lymphoma lines is associated with Epstein-Barr virus transformation of the cells. Eur J Immunol. 1978 Jul;8(7):453–458. doi: 10.1002/eji.1830080702. [DOI] [PubMed] [Google Scholar]
- Mills B. J., Cooper N. R. Antibody-independent neutralization of vesicular stomatitis virus by human complement. I. Complement requirements. J Immunol. 1978 Oct;121(4):1549–1557. [PubMed] [Google Scholar]
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. 3. The role of microfilaments and microtubules. J Immunol. 1973 Mar;110(3):771–780. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host responses to infectious bovine rhinotracheitis virus. III. Isolation and immunologic activities of bovine T lymphocytes. J Immunol. 1974 Nov;113(5):1391–1398. [PubMed] [Google Scholar]
- Rouse B. T., Grewal A. S., Babiuk L. A. Complement enhances antiviral antibody-dependent cell cytotoxicity. Nature. 1977 Mar 31;266(5601):456–458. doi: 10.1038/266456a0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. The role of antibody dependent cytotoxicity in recovery from herpesvirus infections. Cell Immunol. 1976 Mar 1;22(1):182–186. doi: 10.1016/0008-8749(76)90019-8. [DOI] [PubMed] [Google Scholar]
- Sellin D., Wallach D. F., Fischer H. Intercellular communication in cell-mediated cytotoxicity. Fluorescein transfer between H-2 d target cells and H-2 b lymphocytes in vitro. Eur J Immunol. 1971 Dec;1(6):453–458. doi: 10.1002/eji.1830010609. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Schur P. H. Lectin-dependent neutrophil-mediated cytotoxicity. I. Characteristics. Immunology. 1976 Aug;31(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Schur P. H. Lectin-dependent neutrophil-mediated cytotoxicity. II. Possible mechanisms. Immunology. 1976 Aug;31(2):313–322. [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley R. C., Babiuk L. A., Rouse B. T. Polymorph-mediated antibody-dependent cytoxicity--modulation of activity by drugs and immune interferon. Can J Microbiol. 1976 Sep;22(9):1222–1228. doi: 10.1139/m76-181. [DOI] [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. The mammary gland of the ox: a convenient source for the repeated collection of neutrophils and macrophages. J Reticuloendothel Soc. 1976 Jan;19(1):29–36. [PubMed] [Google Scholar]
- Weber J. M., Stewart R. B. Cyclic AMP potentiation of interferon antiviral activity and effect of interferon on cellular cyclic AMP levels. J Gen Virol. 1975 Sep;28(3):363–372. doi: 10.1099/0022-1317-28-3-363. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic AMP modulates microvillus formation and agglutinability in transformed and normal mouse fibroblasts. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1263–1267. doi: 10.1073/pnas.72.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Elevation of cyclic guanosine 3',5'-monophosphate levels in dog thyroid slices caused by acetylcholine and sodium fluoride. J Biol Chem. 1972 Nov 10;247(21):7062–7066. [PubMed] [Google Scholar]


