Abstract
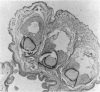
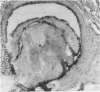
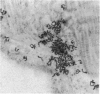
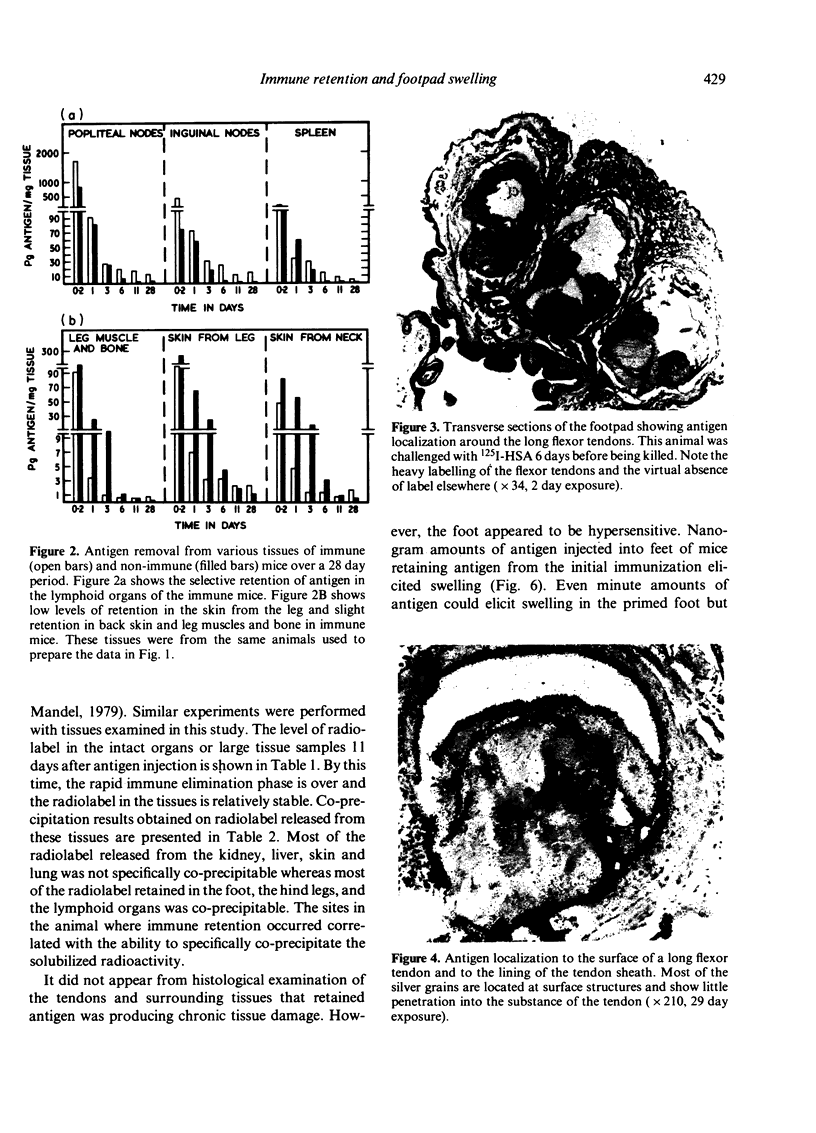
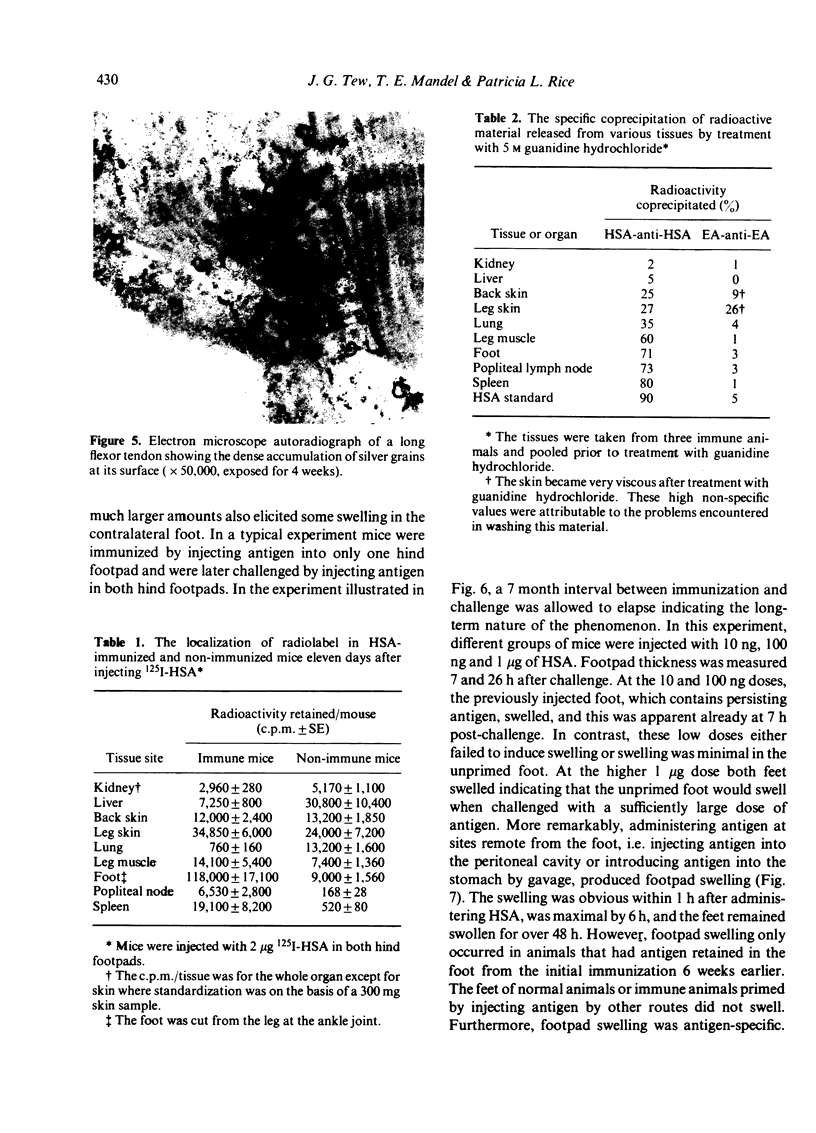
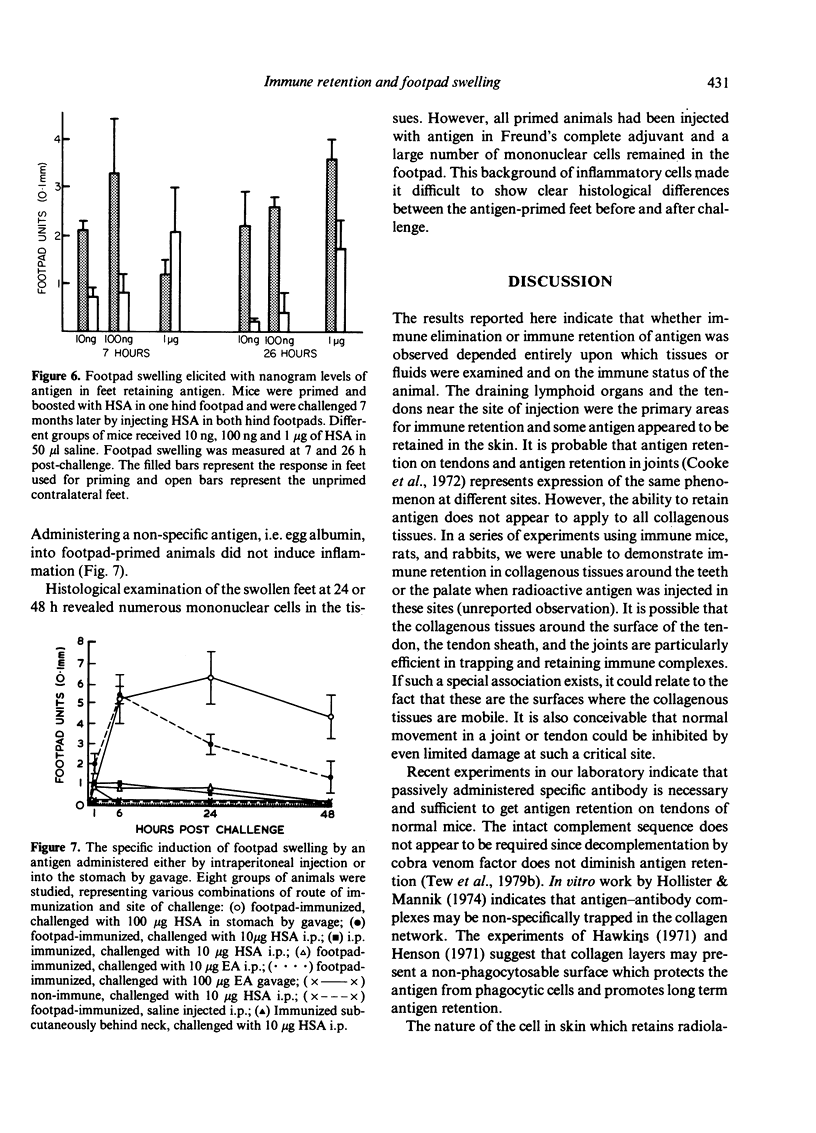
We determined whether (1) long term antigen retention is present in distal sites after footpad challenge of immune mice; (2) if antigen retained in the foot is selectively localized; and (3) if the foot is adversely affected by the retained antigen. Mice immune or non-immune to human serum albumin (HSA) were injected in the hind footpads with 125I-HSA. In immune mice rapid clearance of radiolabel occurred in the liver, lungs, kidney, blood and urine but radiolabel was retained in the hind feet, draining lymph nodes and spleen. Non-immune mice rapidly cleared radiolabel from these sites. Autoradiography revealed that most of the radiolabel in the feet was in flexor tendons and tendon sheaths. Electron microscope autoradiography indicated that antigen was associated with collagen at the tendon surface, but not with cells or cell processes. Radiolabel solubilized from the feet, lymph nodes and spleen could be specifically precipitated with rabbit anti-HSA. Histological examination of the tendon and surrounding tissues did not show that retained antigen was causing inflammation or chronic tissue damage. Nanogram levels of antigen could elicit swelling in the sensitized foot even if the antigen was injected at a remote site, but mice immunized by other routes or against other antigens did not show footpad swelling. Antigen retained on collagenous tissues may induce hypersensitivity and thus play a role in rheumatic diseases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke T. D., Hurd E. R., Ziff M., Jasin H. E. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. II. Preferential localization of antigen-antibody complexes to collagenous tissues. J Exp Med. 1972 Feb 1;135(2):323–338. doi: 10.1084/jem.135.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., MAURER P. H., WEIGLE W. O. Immunologic activity of pneumococcal polysaccharide fixed in the tissues of the mouse. J Immunol. 1955 Mar;74(3):188–191. [PubMed] [Google Scholar]
- Fox A., Glynn L. E. Is persisting antigen responsible for the chronicity of experimental allergic arthritis? Ann Rheum Dis. 1977 Feb;36(1):34–38. doi: 10.1136/ard.36.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf M. W., Uhr J. W. Regulation of antibody formation by serum antibody. I. Removal of specific antibody by means of immunoadsorption. J Exp Med. 1969 Nov 1;130(5):1175–1186. doi: 10.1084/jem.130.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E. J., Tew J. G., Stavitsky A. B. The differential localization of the in vitro spontaneous antibody and proliferative responses in lymphoid organs proximal and distal to the site of primary immunization. Cell Immunol. 1975 Aug;18(2):476–483. doi: 10.1016/0008-8749(75)90074-x. [DOI] [PubMed] [Google Scholar]
- Hawkins D. Biopolymer membrane: a model system for the study of the neutrophilic leukocyte response to immune complexes. J Immunol. 1971 Aug;107(2):344–352. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Hollister J. R., Mannik M. Antigen retention in joint tissues in antigen-induced synovitis. Clin Exp Immunol. 1974 Apr;16(4):615–627. [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E., Cooke T. D. The inflammatory role of immune complexes trapped in joint collagenous tissues. Clin Exp Immunol. 1978 Sep;33(3):416–424. [PMC free article] [PubMed] [Google Scholar]
- Rowden G., Lewis M. G., Sullivan A. K. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977 Jul 21;268(5617):247–248. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- SISKIND G. W., PATERSON P. Y., THOMAS L. INDUCTION OF UNRESPONSIVENESS AND IMMUNITY IN NEWBORN AND ADULT MICE WITH PNEUMOCOCCAL POLYSACCHARIDE. J Immunol. 1963 Jun;90:929–934. [PubMed] [Google Scholar]
- Stingl G., Wolff-Schreiner E. C., Pichler W. J., Gschnait F., Knapp W., Wolff K. Epidermal Langerhans cells bear Fc and C3 receptors. Nature. 1977 Jul 21;268(5617):245–246. doi: 10.1038/268245a0. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. E., Burgess A. W. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell Immunol. 1979 Jun;45(1):207–212. doi: 10.1016/0008-8749(79)90378-2. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. E., Miller G. A. Immune retention: immunological requirements for maintaining an easily degradable antigen in vivo. Aust J Exp Biol Med Sci. 1979 Aug;57(4):401–414. doi: 10.1038/icb.1979.40. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. The maintenance and regulation of serum antibody levels: evidence indicating a role for antigen retained in lymphoid follicles. J Immunol. 1978 Mar;120(3):1063–1069. [PubMed] [Google Scholar]
- Tew J. G., Self C. H., Harold W. W., Stavitsky A. B. The spontaneous induction of anamnestic antibody synthesis in lymph node cell cultures many months after primary immunization. J Immunol. 1973 Aug;111(2):416–423. [PubMed] [Google Scholar]
- Weigle W. O. Cyclical production of antibody as a regulatory mechanism in the immune response. Adv Immunol. 1975;21:87–111. doi: 10.1016/s0065-2776(08)60219-9. [DOI] [PubMed] [Google Scholar]