Abstract
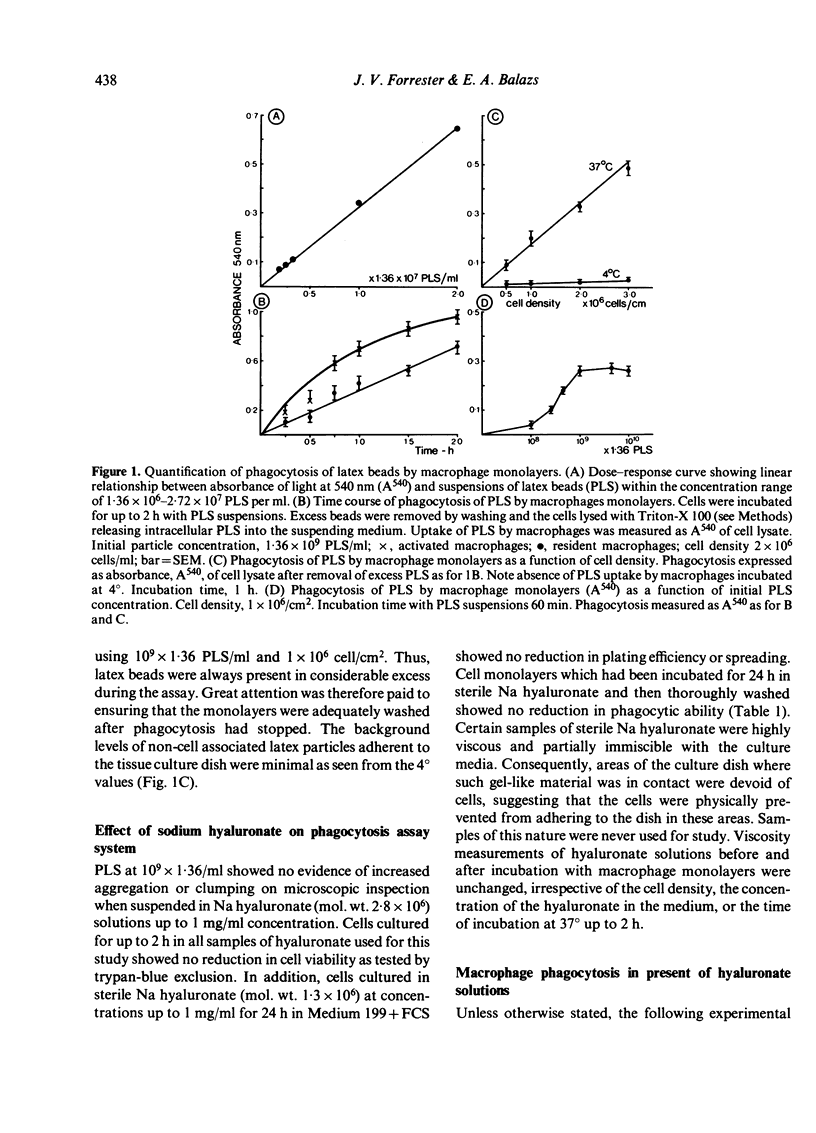
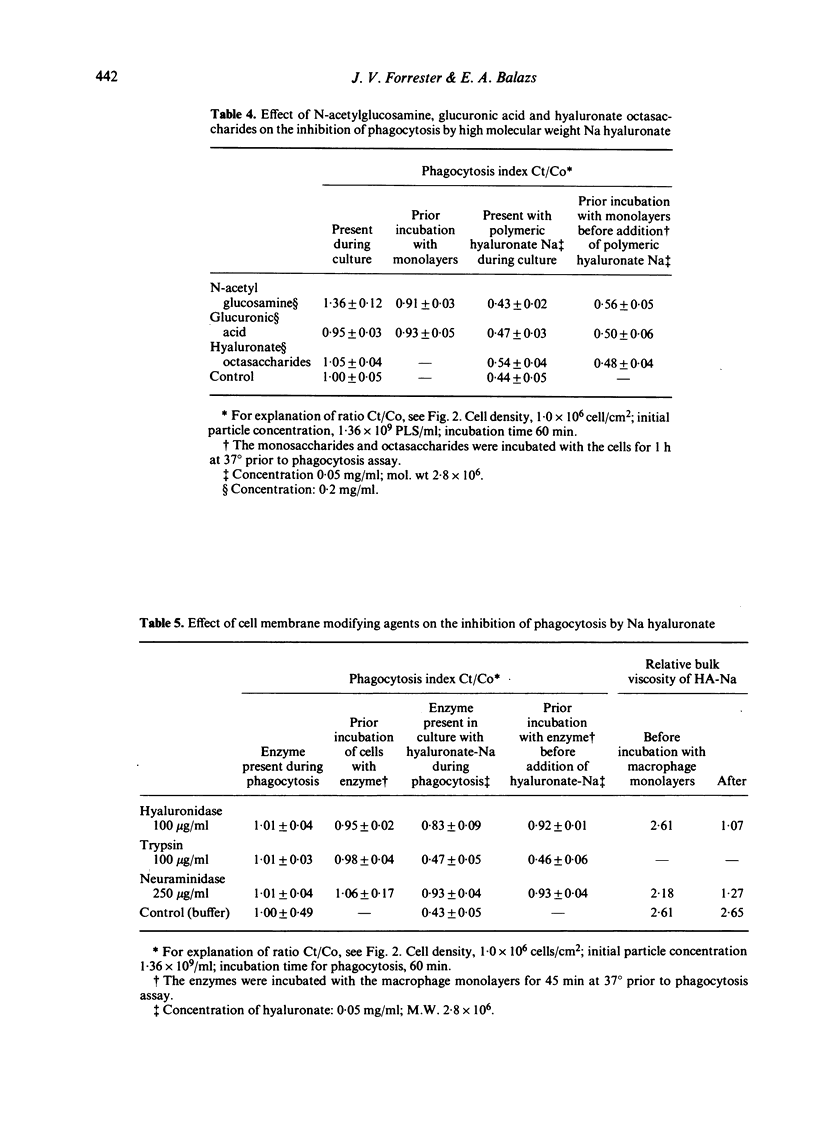
The effect of sodium hyaluronate on phagocytosis was studied using a sensitive polystyrene latex sphere assay in mouse peritoneal macrophage monolayers. Viscous solutions of high molecular weight hyaluronate (4.6 X 10(5)--2.8 X 10(6)) caused a dose-dependent inhibition of phagocytosis, but low molecular weight hyaluronate (9.0 X 10(4)) was not inhibitory at equivalent viscosity. The inhibitory effect of high molecular weight hyaluronate did not appear to be mediated by the polyanionic charge of the molecule since sulphated glycosaminoglycans with greater charge density (heparin and chondroitin sulphate) were ineffective. In addition, competitive inhibition studies indicated that a direct effect on possible cell surface membrane receptors was unlikely. Instead, physical factors such as steric hindrance by the continuous polymeric network, were considered of more importance. Alternatively, the hydrophilic polysaccharide may have inhibited phagocytosis by providing an unsuitable surface for adhesive contact between the latex beads and the cell surface.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. B., Wilkinson P. C. A visual analysis of chemotactic and chemokinetic locomotion of human neutrophil leucocytes. Use of a new chemotaxis assay with Candida albicans as gradient source. Exp Cell Res. 1978 Jan;111(1):191–203. doi: 10.1016/0014-4827(78)90249-5. [DOI] [PubMed] [Google Scholar]
- BALAZS E. A., LAURENT T. C., LAURENT U. B., DEROCHE M. H., BUNNEY D. M. Studies on the structure of the vitreous body. VIII. Comparative biochemistry. Arch Biochem Biophys. 1959 Apr;81(2):464–479. doi: 10.1016/0003-9861(59)90227-9. [DOI] [PubMed] [Google Scholar]
- Balazs E. A., Berntsen K. O., Karossa J., Swann D. A. An automated method for the determination of hexuronic acids. Anal Biochem. 1965 Sep;12(3):547–558. doi: 10.1016/0003-2697(65)90221-6. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B., Balazs E. A. Optical properties of hyaluronic acid. Ultraviolet circular dichroism and optical rotatory dispersion. J Mol Biol. 1973 Jun 25;78(1):135–141. doi: 10.1016/0022-2836(73)90433-6. [DOI] [PubMed] [Google Scholar]
- Clarris B. J., Fraser J. R. On the pericellular zone of some mammalian cells in vitro. Exp Cell Res. 1968 Jan;49(1):181–193. doi: 10.1016/0014-4827(68)90530-2. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Wang J. L. Ionic polysaccharides. 3. Dilute solution properties of hyaluronic acid fractions. Biopolymers. 1970;9(7):799–810. doi: 10.1002/bip.1970.360090706. [DOI] [PubMed] [Google Scholar]
- Cohn R. H., Banerjee S. D., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J Cell Biol. 1977 May;73(2):464–478. doi: 10.1083/jcb.73.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Balazs E. A. Effect of connective tissue intercellular matrix on lymphocyte stimulation. Exp Cell Res. 1971 May;66(1):113–123. doi: 10.1016/s0014-4827(71)80018-6. [DOI] [PubMed] [Google Scholar]
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A., Bazin S. Mucopolysaccharides, collagen, and nonfibrillar proteins in inflammation. Int Rev Connect Tissue Res. 1964;2:301–325. doi: 10.1016/b978-1-4831-6751-0.50013-6. [DOI] [PubMed] [Google Scholar]
- Filkins J. P., Di Luzio N. R. Effect of heparin and sulfated polysaccharides on in vitro hepatic phagocytosis. Proc Soc Exp Biol Med. 1966 Jun;122(2):548–551. doi: 10.3181/00379727-122-31187. [DOI] [PubMed] [Google Scholar]
- Fromageot H. P. Adsorption of human erythrocyte surface glycoprotein onto polystyrene latex spheres. J Lab Clin Med. 1977 Aug;90(2):324–329. [PubMed] [Google Scholar]
- Guss J. M., Hukins D. W., Smith P. J., Winter W. T., Arnott S. Hyaluronic acid: molecular conformations and interactions in two sodium salts. J Mol Biol. 1975 Jul 5;95(3):359–384. doi: 10.1016/0022-2836(75)90196-5. [DOI] [PubMed] [Google Scholar]
- Isles M., Foweraker A. R., Jennings B. R., Hardingham T., Muir H. Characterization of proteoglycan and the proteoglycan--hyaluronic acid complex by electric birefringence. Biochem J. 1978 Jul 1;173(1):237–243. doi: 10.1042/bj1730237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., RYAN M., PIETRUSZKIEWICZ A. Fractionation of hyaluronic acid. The polydispersity of hyaluronic acid from the bovine vitreous body. Biochim Biophys Acta. 1960 Aug 26;42:476–485. doi: 10.1016/0006-3002(60)90826-x. [DOI] [PubMed] [Google Scholar]
- Lackie J. M. The aggregation of rabbit polymorphonuclear leucocytes: effects of antimitotic agents, cyclic nucleotides and methyl xanthines. J Cell Sci. 1974 Oct;16(1):167–180. doi: 10.1242/jcs.16.1.167. [DOI] [PubMed] [Google Scholar]
- Markwald R. R., Fitzharris T. P., Bank H., Bernanke D. H. Structural analyses on the matrical organization of glycosaminoglycans in developing endocardial cushions. Dev Biol. 1978 Feb;62(2):292–316. doi: 10.1016/0012-1606(78)90218-x. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Stossel T. P., Vaughan M. Quantitative studies of phagocytosis by alveolar macrophages. Biochim Biophys Acta. 1973 May 28;304(3):864–870. doi: 10.1016/0304-4165(73)90233-x. [DOI] [PubMed] [Google Scholar]
- McBride W. H., Bard J. B. Hyaluronidase-sensitive halos around adherent cells. Their role in blocking lymphocyte-mediated cytolysis. J Exp Med. 1979 Feb 1;149(2):507–515. doi: 10.1084/jem.149.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer W. A., Steinberg M. S. Do rates of intercellular adhesion measure the cell affinities reflected in cell-sorting and tissue-spreading configurations? Dev Biol. 1976 Sep;52(2):246–262. doi: 10.1016/0012-1606(76)90244-x. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., STANIER J. E. The dimensions of the particle of hyaluronic acid complex in synovial fluid. Biochem J. 1951 Oct;49(5):585–590. doi: 10.1042/bj0490585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., STANIER J. E. The physiological function of hyaluronic acid in synovial fluid; viscous, elastic and lubricant properties. J Physiol. 1953 Feb 27;119(2-3):244–252. doi: 10.1113/jphysiol.1953.sp004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Tigwell M. J. Periodate oxidation and the shapes of glycosaminoglycuronans in solution. Biochem J. 1978 Jul 1;173(1):103–114. doi: 10.1042/bj1730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetlar M. R., Davitt W. F., Rosett R. L., Crass M. F., 3rd, Lautsch E. V., Kischer C. Glycosaminoglycan changes in healing myocardial infarction. Proc Soc Exp Biol Med. 1978 Jun;158(2):210–214. doi: 10.3181/00379727-158-40173. [DOI] [PubMed] [Google Scholar]
- Tagesson C., Magnusson K. E., Stendahl O. Physicochemical consequences of opsonization: perturbation of liposomal membranes by Salmonella typhimurium 395 MS opsonized with IgG antibodies. J Immunol. 1977 Aug;119(2):609–613. [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: modulation of enzyme production by anti-inflammatory steroids, mitotic inhibitors, and cyclic nucleotides. Cell. 1976 Jun;8(2):271–281. doi: 10.1016/0092-8674(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Westermark B., Lindahl U., Pontén J. Aggregation of feline lymphoma cells by hyaluronic acid. Int J Cancer. 1973 Jul 15;12(1):169–178. doi: 10.1002/ijc.2910120118. [DOI] [PubMed] [Google Scholar]
- Weisman R. A., Korn E. D. Phagocytosis of latex beads by Acanthamoeba. I. Biochemical properties. Biochemistry. 1967 Feb;6(2):485–497. doi: 10.1021/bi00854a017. [DOI] [PubMed] [Google Scholar]
- Winter W. T., Arnott S. Hyaluronic acid: the role of divalent cations in conformation and packing. J Mol Biol. 1977 Dec 15;117(3):761–784. doi: 10.1016/0022-2836(77)90068-7. [DOI] [PubMed] [Google Scholar]
- Winter W. T., Smith P. J., Arnott S. Hyaluronic acid: structure of a fully extended 3-fold helical sodium salt and comparison with the less extended 4-fold helical forms. J Mol Biol. 1975 Dec 5;99(2):219–235. doi: 10.1016/s0022-2836(75)80142-2. [DOI] [PubMed] [Google Scholar]
- van Oss C. J., Gillman C. F., Bronson P. M., Border J. R. Phagocytosis-inhibiting properties of human serum alpha-1 acid glycoprotein. Immunol Commun. 1974;3(4):321–328. doi: 10.3109/08820137409061112. [DOI] [PubMed] [Google Scholar]



