Abstract
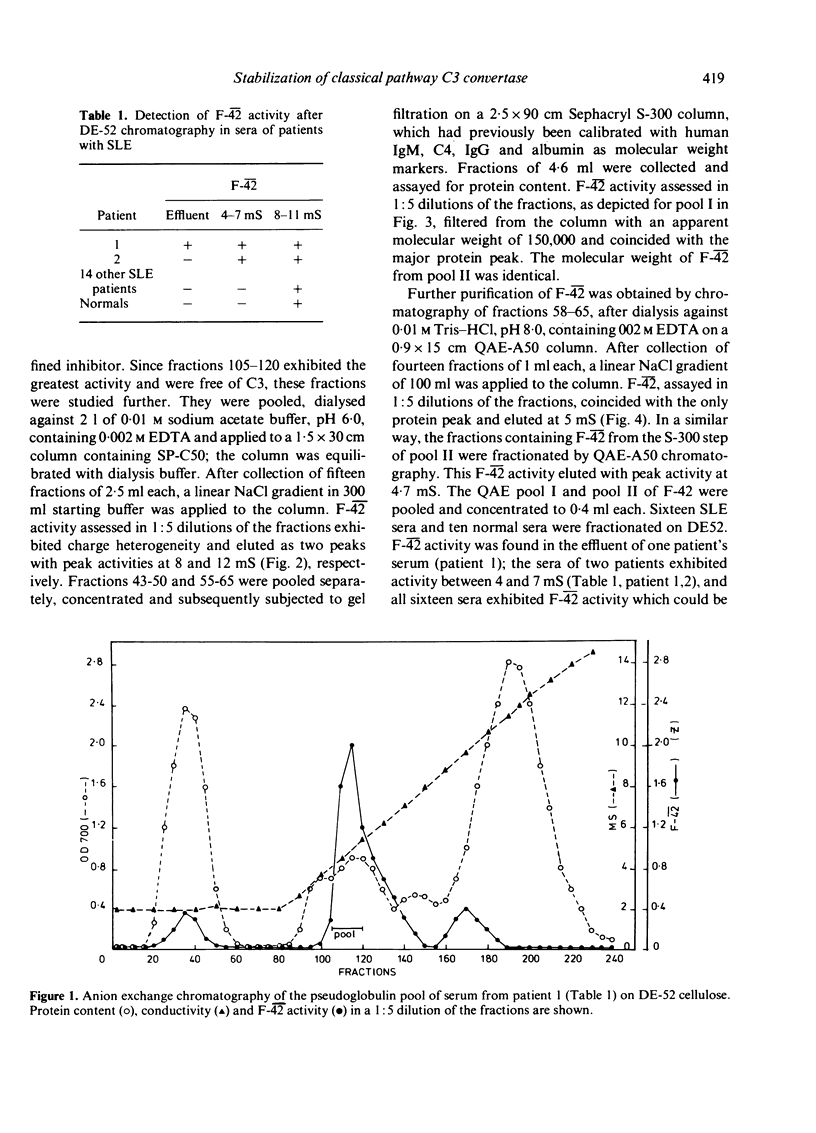
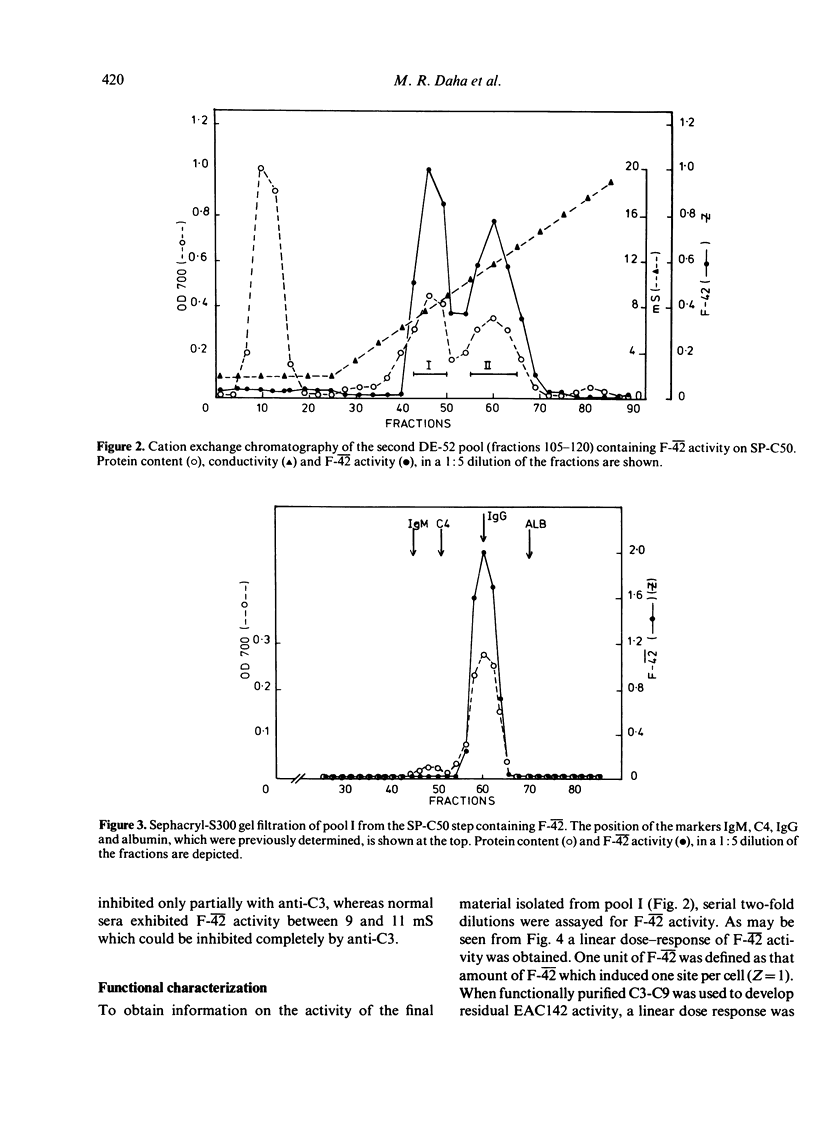
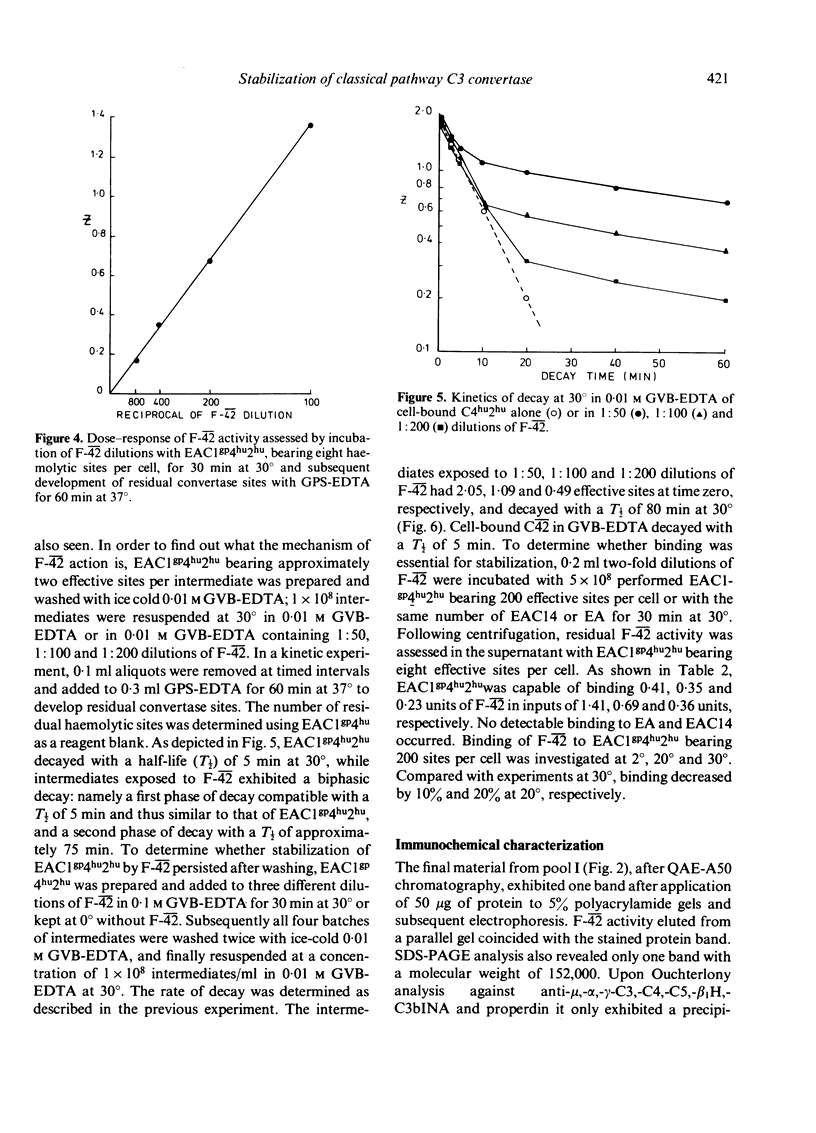
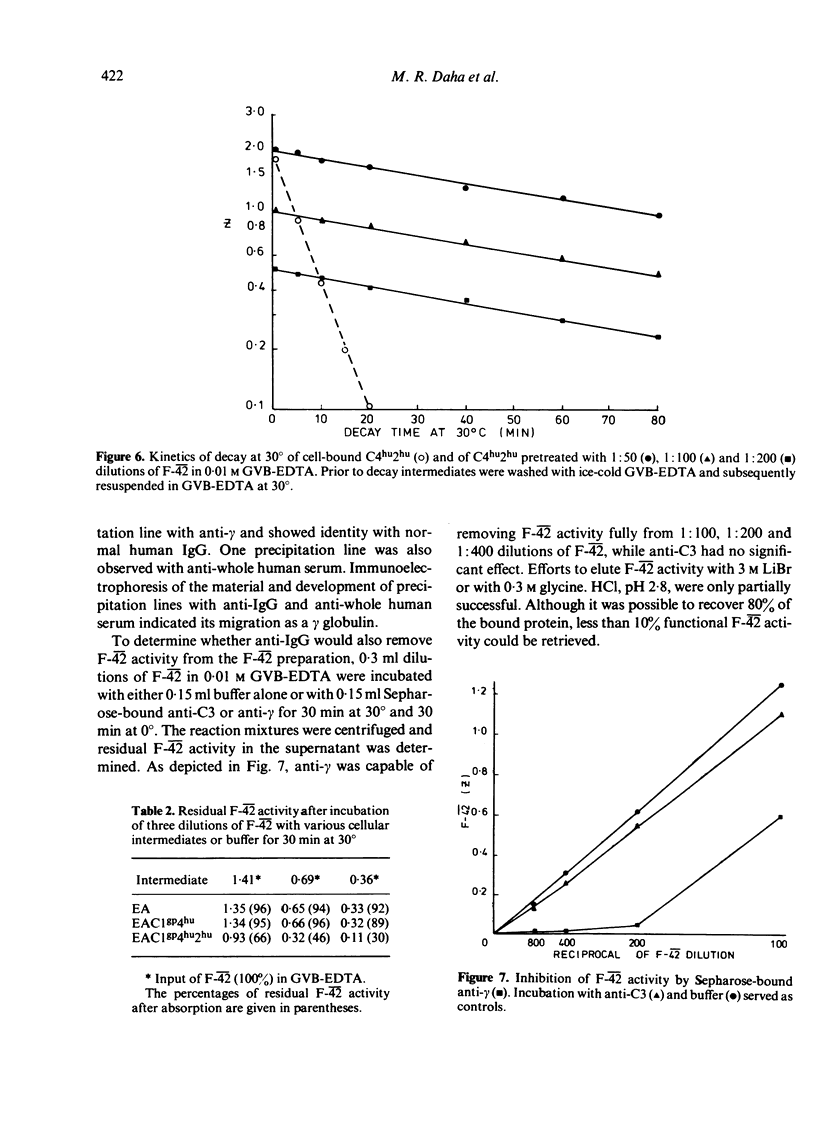
Sera from sixteen patients with SLE were investigated for the presence of a factor which would conserve convertase activity on preformed EAClgp 4hu2hu for 30 min at 30 degrees in EDTA. Although such a factor could not be detected readily in the sera, chromatography on DE-52 cellulose yielded fractions appearing as three peaks in one patient and as two peaks in a second patient. These peaks were capable of conserving C42 activity and were designated as F-42. Purification of F-42 from the second peak eluting between 4 and 7 mS on DE-52 was obtained by SP-C50, S-300 and QAE-A50 chromatography. F-42 exhibited charge heterogeneity upon SP-C50 chromatography. On polyacrylamide gel electrophoresis the final material migrated as one band, which coincided with the position of F-42 activity upon eluation from a parallel gel. F-42 had an apparent molecular weight of 150,000 and reacted with anti-IgG in Ouchterlony analysis. Sepharose-bound anti-IgG was capable of neutralizing F-42 activity. The purified material was shown to prolong the half-life (T 1/2) of performed cell-bound C42 in GVB-EDTA at 30 degrees from 5 to 80 min.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Daha M. R., Austen K. F., Fearon D. T. Heterogeneity, polypeptide chain composition and antigenic reactivity of C3 nephritic factor. J Immunol. 1978 Apr;120(4):1389–1394. [PubMed] [Google Scholar]
- Daha M. R., Austen K. F., Fearon D. T. The incorporation of C3 nephritic factor (C3NeF) into a stabilized C3 convertase, C3bBb(C3NeF), and its release after decay of convertase function. J Immunol. 1977 Sep;119(3):812–817. [PubMed] [Google Scholar]
- Daha M. R., Fearon D. T., Austen K. F. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976 Jan;116(1):1–7. [PubMed] [Google Scholar]
- Davis A. E., 3rd, Ziegler J. B., Gelfand E. W., Rosen F. S., Alper C. A. Heterogeneity of nephritic factor and its identification as an immunoglobulin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3980–3983. doi: 10.1073/pnas.74.9.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis E. J., Carpenter C. B., Schur P. H. Serum complement component levels in human glomerulonephritis. Ann Intern Med. 1971 Oct;75(4):555–560. doi: 10.7326/0003-4819-75-4-555. [DOI] [PubMed] [Google Scholar]
- McLean R. H., Nilson S. H. C3 nephritic factor stabilization of the classic C3 convertase: a role for C2 in C3 nephritic factor activity. Proc Soc Exp Biol Med. 1979 Jul;161(3):358–363. doi: 10.3181/00379727-161-40553. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Cleavage of C2 by C1s into the antigenically distinct fragments C2a and C2b: demonstration of binding of C2b to C4b. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2998–3001. doi: 10.1073/pnas.74.7.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- Schreiber R. D., Götze O., Müller-Eberhard H. J. Nephritic factor: its structure and function and its relationship to initiating factor of the alternative pathway. Scand J Immunol. 1976;5(6-7):705–713. doi: 10.1111/j.1365-3083.1976.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Scott D. M., Amos N., Sissons J. G., Lachmann P. J., Peters D. K. The immunogloblin nature of nephritic factor (NeF). Clin Exp Immunol. 1978 Apr;32(1):12–24. [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]


