Abstract
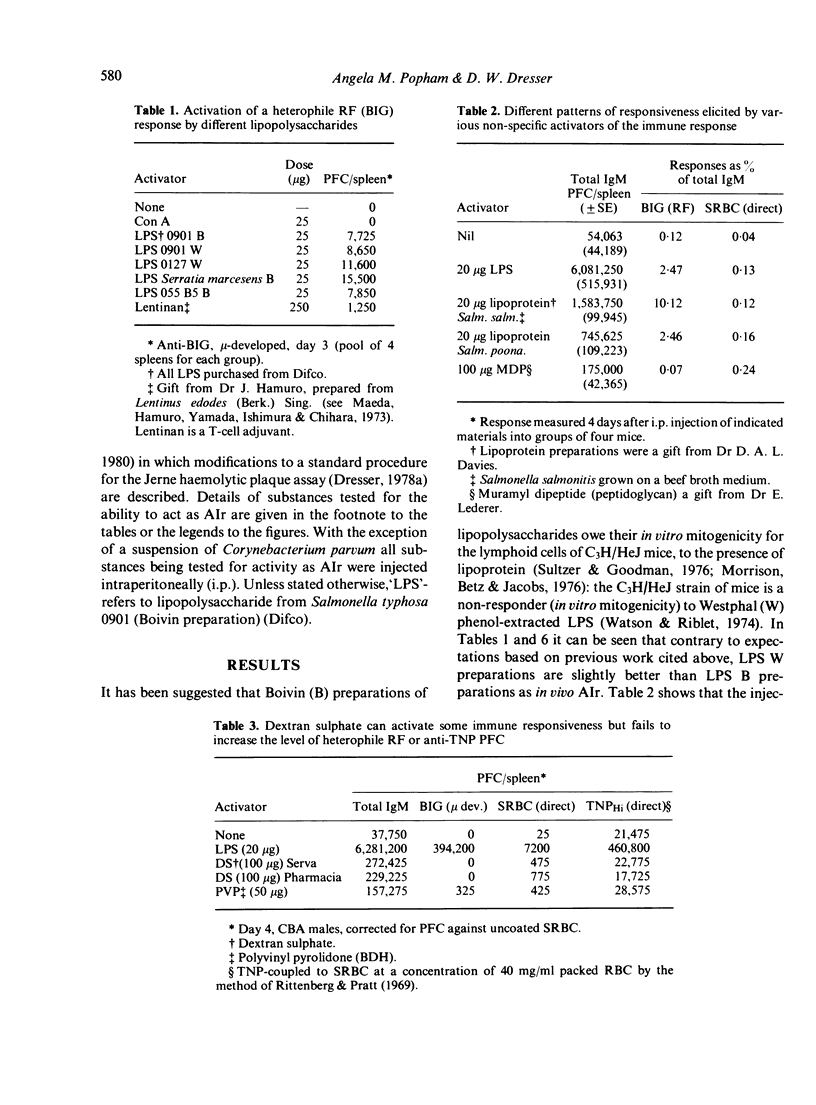
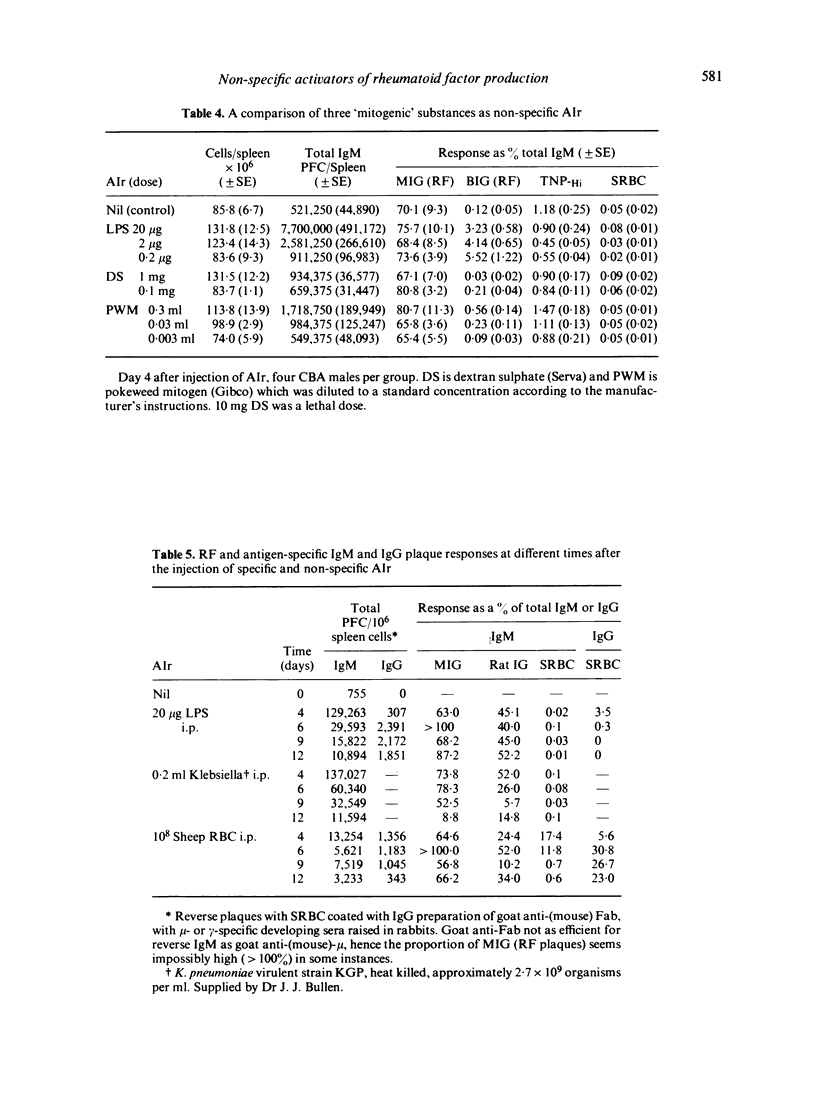
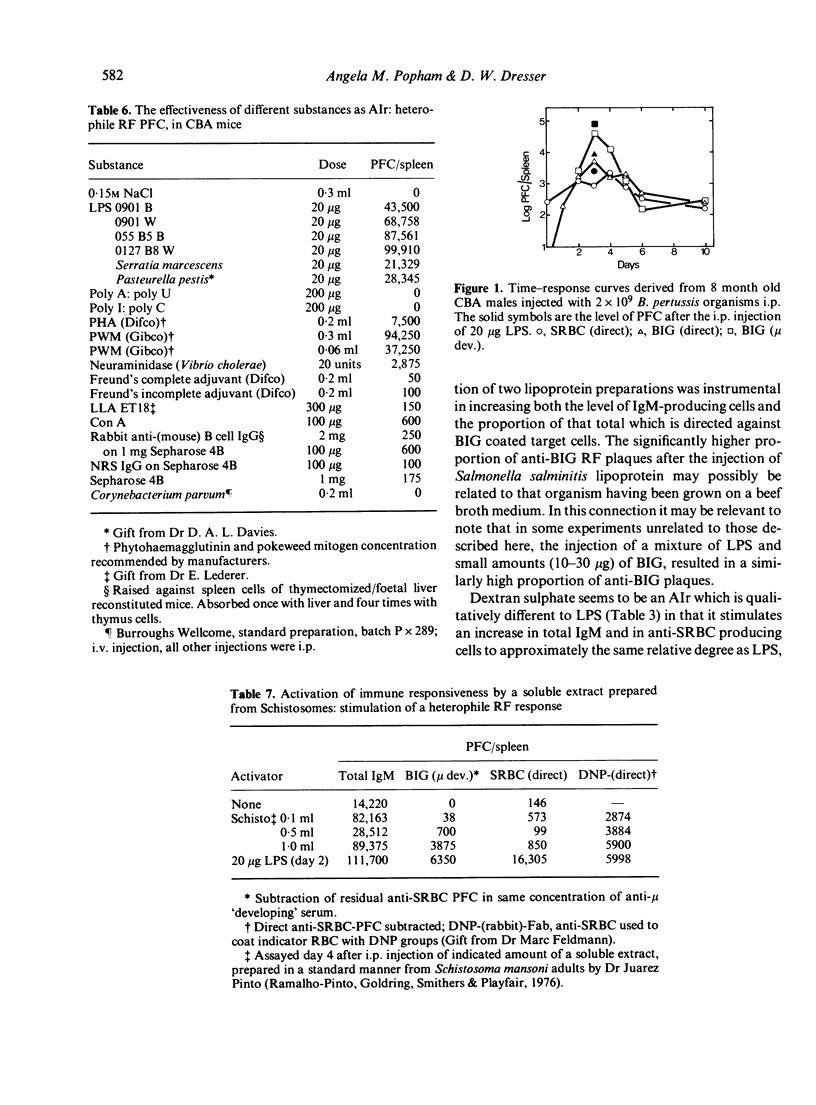
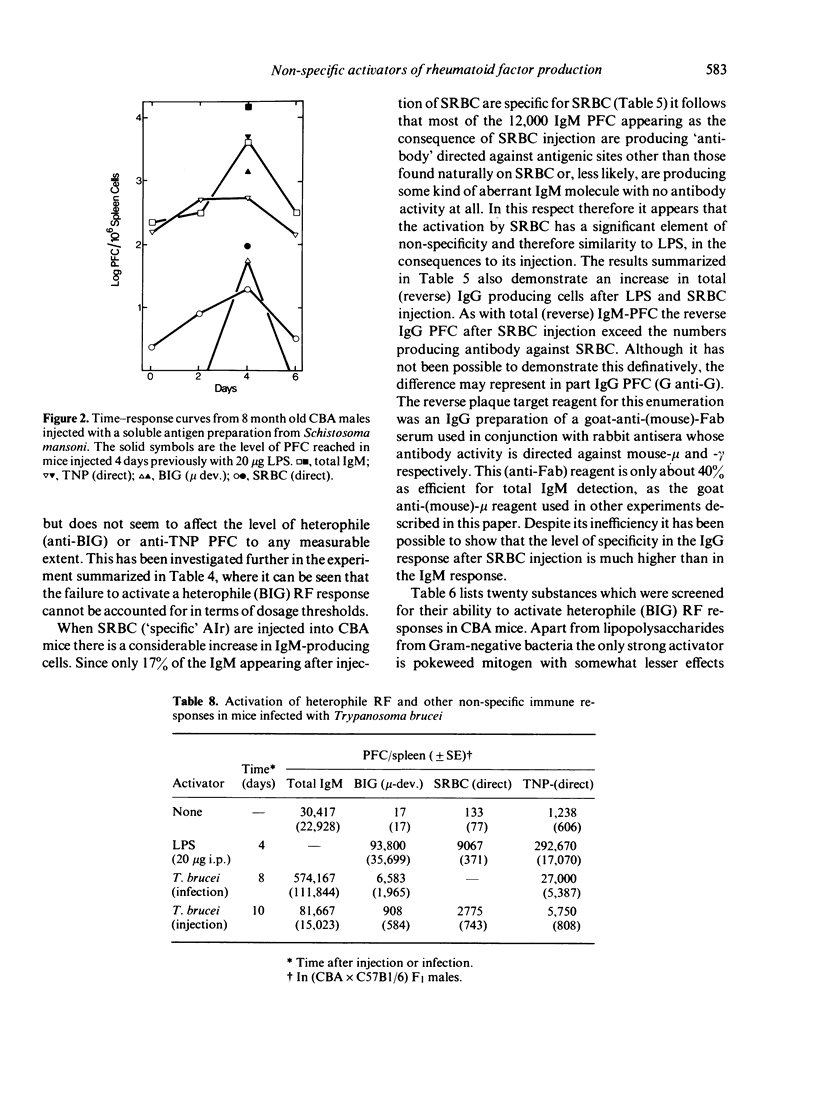
Although possibly the best, LPS is by no means the only non-specific activator of immune responsiveness (AIr). Plant lectins such as PHA and PWM have some ability to stimulate both total IgM production (reverse IgM PFC) and more particularly heterophile and homophile IgM rheumatoid factor (RF) responses, together with IgM 'antibody' responses to target antigens such as xenogeneic red blood cells or haptens such as TNP. Some parasitic infections and/or parasite antigens were shown to be powerful non-specific AIr, while the injection of dextran sulphate was shown to lead to a great increase in spleen cellularity and total IgM (reverse) PFC but failed to stimulate a heterophile (BIG) RF PFC response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. Most IgM-producing cells in the mouse secrete auto-antibodies (rheumatoid factor). Nature. 1978 Aug 3;274(5670):480–483. doi: 10.1038/274480a0. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. Induction of an IgM anti-(bovine)-IgG response in mice by bacterial lipopolysaccharide. Nature. 1976 Dec 9;264(5586):552–554. doi: 10.1038/264552a0. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. Rheumatoid factors in mice: plaque assay for homophile and heterophile rheumatoid factors. Immunology. 1980 Nov;41(3):569–577. [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Parish C. R. Polyclonal B-cell activation during rodent malarial infections. Clin Exp Immunol. 1978 Apr;32(1):41–45. [PMC free article] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Izui S., Kobayakawa T., Zryd M. J., Louis J., Lambert P. H. Mechanism for induction of anti-DNA antibodies by bacterial lipopolysaccharides in mice; II. Correlation between anti-DNA induction and polyclonal antibody formation by various polyclonal B lymphocyte activators. J Immunol. 1977 Dec;119(6):2157–2162. [PubMed] [Google Scholar]
- Izui S., Zaldivar N. M., Scher I., Lambert P. H. Mechanism for induction of anti-DNA antibodies by bacterial lipopolysaccharides in mice. I. Anti-DNA induction by LPS without significant release of DNA in circulating blood. J Immunol. 1977 Dec;119(6):2151–2156. [PubMed] [Google Scholar]
- Keeler K. D., Phillips J. M., Dresser D. W. IgM rheumatoid factor as a source of non-specificity in murine anti-allotype sera. Immunology. 1979 May;37(1):263–271. [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa T., Louis J., Izui S., Lambert P. H. Autoimmune response to DNA, red blood cells, and thymocyte antigens in association with polyclonal antibody synthesis during experimental African trypanosomiasis. J Immunol. 1979 Jan;122(1):296–301. [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. S., Sultzer B. M., Bullock W. W. Purified protein derivative of tuberculin induces immunoglobulin production in normal mouse spleen cells. J Exp Med. 1973 Jan 1;137(1):127–139. doi: 10.1084/jem.137.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Goldring O. L., Smithers S. R., Playfair H. L. T-cell helper response to antigens of Schistosoma mansoni in CBA mice. Clin Exp Immunol. 1976 Nov;26(2):327–333. [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y. J. Autoimmune and polyclonal B cell responses during murine malaria. Nature. 1978 Jul 13;274(5667):170–172. doi: 10.1038/274170a0. [DOI] [PubMed] [Google Scholar]
- Steele E. J., Cunningham A. J. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978 Aug 3;274(5670):483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


