Abstract
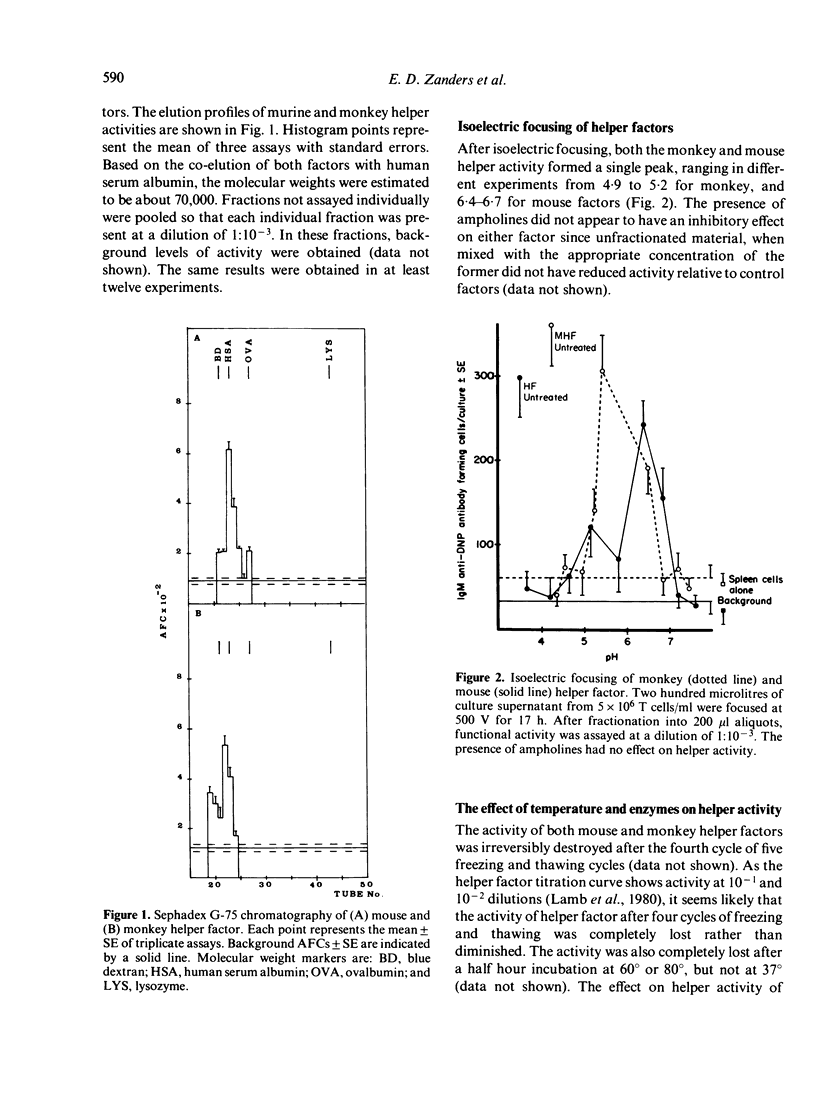
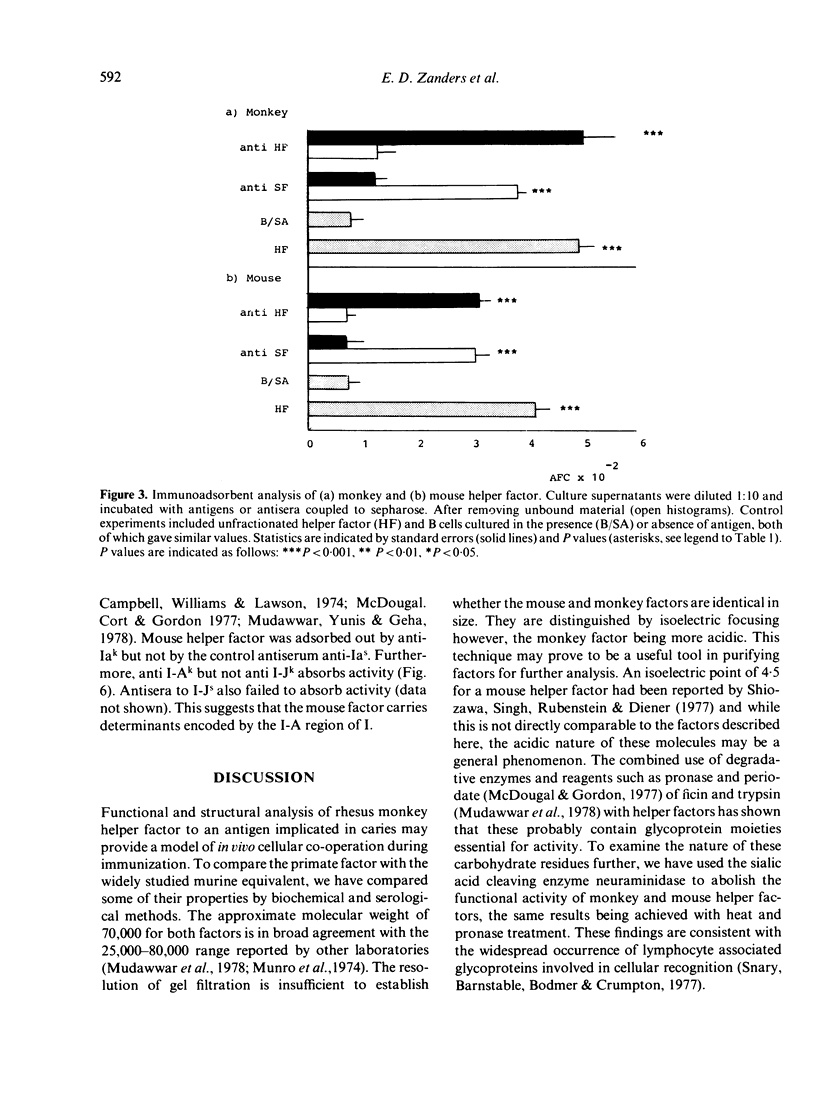
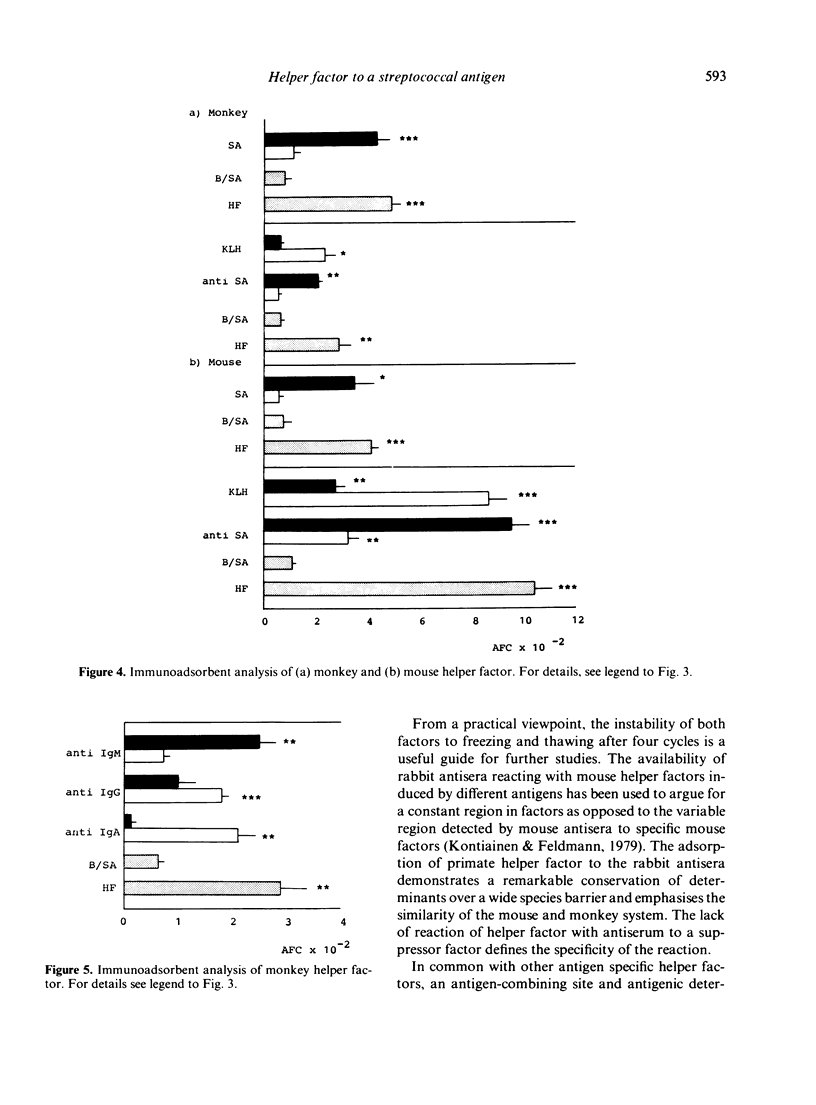
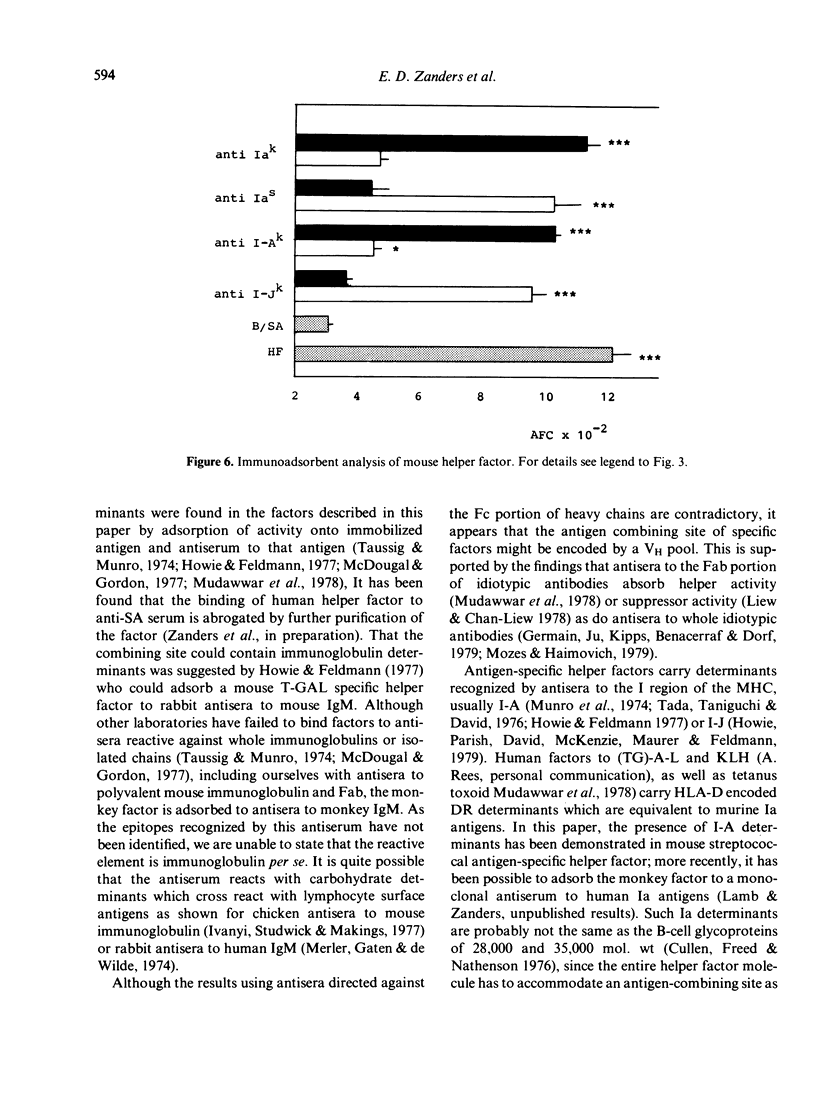
Helper factors specifically stimulating cooperative antibody responses by normal mouse spleen cells to a dinitrophenylated protein antigen from Streptococcus mutans (DNP-SA) were produced in vitro from monkey peripheral blood leucocytes and mouse spleen cells. The factors were partially characterized by gel filtration on Sephadex G-75, isoelectric focusing, treatment with heat and degradative enzymes and binding to specific immunoadsorbents. Gel filtration of both the monkey and mouse factors showed coelution with human serum albumin, suggesting a molecular weight of approximately 70,000. The isoelectric points fell within the range of 4.9-5.2 for monkey and 6.4-6.7 for the mouse helper factors. The glycoprotein nature of both factors was suggested by their lability to heat and sensitivity to pronase and neuraminidase. The factors carried a small fragment of the stimulating antigen and showed specific binding to SA but not to keyhole limpet haemocyanin (KLH). Monkey factor bound to rabbit antisera directed against the Fc portion of monkey IgM, but not to the IgG or IgA isotypes. The mouse factor contained determinants coded for by the I-Ak but not I-Jk subregion of the MHC. Both factors were absorbed by an antiserum to helper factor raised in rabbits against a KLH-specific mouse helper factor as immunogen. A corresponding antiserum to suppressor factor failed to adsorb either factor. This emphasizes the specific identities of helper and suppressor factors and suggests an evolutionary relationship between those derived from monkey and mouse leucocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. V. Specific collaboration via complexes of antigen and thymus-derived cell immunoglobulin. J Exp Med. 1972 Oct 1;136(4):737–760. doi: 10.1084/jem.136.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Ju S. T., Kipps T. J., Benacerraf B., Dorf M. E. Shared idiotypic determinants on antibodies and T-cell-derived suppressor factor specific for the random terpolymer L-glutamic acid60-L-alanine30-L-tyrosine10. J Exp Med. 1979 Mar 1;149(3):613–622. doi: 10.1084/jem.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Thèze J., Kapp J. A., Benacerraf B. Antigen-specific T-cell-mediated suppression. I. Induction of L-glutamic acid60-L-alanine30-L-tyrosine10 specific suppressor T cells in vitro requires both antigen-specific T-cell-suppressor factor and antigen. J Exp Med. 1978 Jan 1;147(1):123–136. doi: 10.1084/jem.147.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie S., Parish C. R., David C. S., McKenzie I. F., Maurer P. H., Feldmann M. Serological analysis of antigen-specific helper factors specific for poly-L(Tyr, Glu)-poly-DLAla--poly-LLys [(T, G)-A--L] and L Glu60-LAla30-LTyr10 (GAT). Eur J Immunol. 1979 Jul;9(7):501–506. doi: 10.1002/eji.1830090703. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Strudwick L., Makings C. A heterophile carbohydrate moiety common to mammalian IgM and erythrocytes detected by chicken IgM antibody. Eur J Immunol. 1977 Apr;7(4):204–209. doi: 10.1002/eji.1830070403. [DOI] [PubMed] [Google Scholar]
- Kantor F., Feldmann M. Induction of human antigen-specific and non-specific helper factors in vitro. Clin Exp Immunol. 1979 Apr;36(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- Kontianen S., Feldmann M. Induction of specific helper cells in vitro. Nat New Biol. 1973 Oct 31;245(148):285–286. doi: 10.1038/newbio245285a0. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Lehner T., Challacombe S. J., Caldwell J. Immunological and bacteriological basis for vaccination against dental caries in rhesus monkeys. Nature. 1975 Apr 10;254(5500):517–520. doi: 10.1038/254517a0. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Chan-Liew W. L. Regulation of delayed-type hypersensitivity. II. Specific suppressor factor for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):168–171. doi: 10.1002/eji.1830080305. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Cort S. P., Gordon D. S. Generation of T helper cells in vitro. III. Helper cell culture-derived factors are related to alloantigens coded for the I region of the H-2 major histocompatibility complex. J Immunol. 1977 Dec;119(6):1933–1937. [PubMed] [Google Scholar]
- McDougal J. S., Gordon D. S. Generation of T-helper cells in vitro. II. Analysis of supernates derived from T-helper cell cultures. J Exp Med. 1977 Mar 1;145(3):693–708. doi: 10.1084/jem.145.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merler E., Gatien J., DeWilde G. Significance of immunofluorescent staining of lymphocytes with antisera to IgM immunoglobulins. Nature. 1974 Oct 18;251(5476):652–654. doi: 10.1038/251652a0. [DOI] [PubMed] [Google Scholar]
- Mudawwar F. B., Yunis E. J., Geha R. S. Antigen-specific helper factor in man. J Exp Med. 1978 Oct 1;148(4):1032–1043. doi: 10.1084/jem.148.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro A. J., Taussig M. J., Campbell R., Williams H., Lawson Y. Antigen-specific T-cell factor in cell cooperation: physical properties and mapping in the left-hand (K) half of H-2. J Exp Med. 1974 Dec 1;140(6):1579–1587. doi: 10.1084/jem.140.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa C., Singh B., Rubinstein S., Diener E. Molecular control of B cell triggering by antigen-specific T cell-derived helper factor. J Immunol. 1977 Jun;118(6):2199–2205. [PubMed] [Google Scholar]
- Snary D., Barnstable C., Bodmer W. F., Goodfellow P., Crumpton M. J. Human Ia antigens--purification and molecular structure. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):379–386. doi: 10.1101/sqb.1977.041.01.045. [DOI] [PubMed] [Google Scholar]
- Sorg C., Bloom B. R. Products of activated lymphocytes. I. The use of radiolabeling techniques in the characterization and partial purification of the migration inhibition factor of the guinea pig. J Exp Med. 1973 Jan 1;137(1):148–170. doi: 10.1084/jem.137.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbman M. A., Smith D. J. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody and experimental dental caries in rats. Infect Immun. 1974 Jun;9(6):1079–1091. doi: 10.1128/iai.9.6.1079-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J. T cell factor which can replace T cells in vivo. Nature. 1974 Mar 15;248(445):234–236. doi: 10.1038/248234a0. [DOI] [PubMed] [Google Scholar]


