Abstract
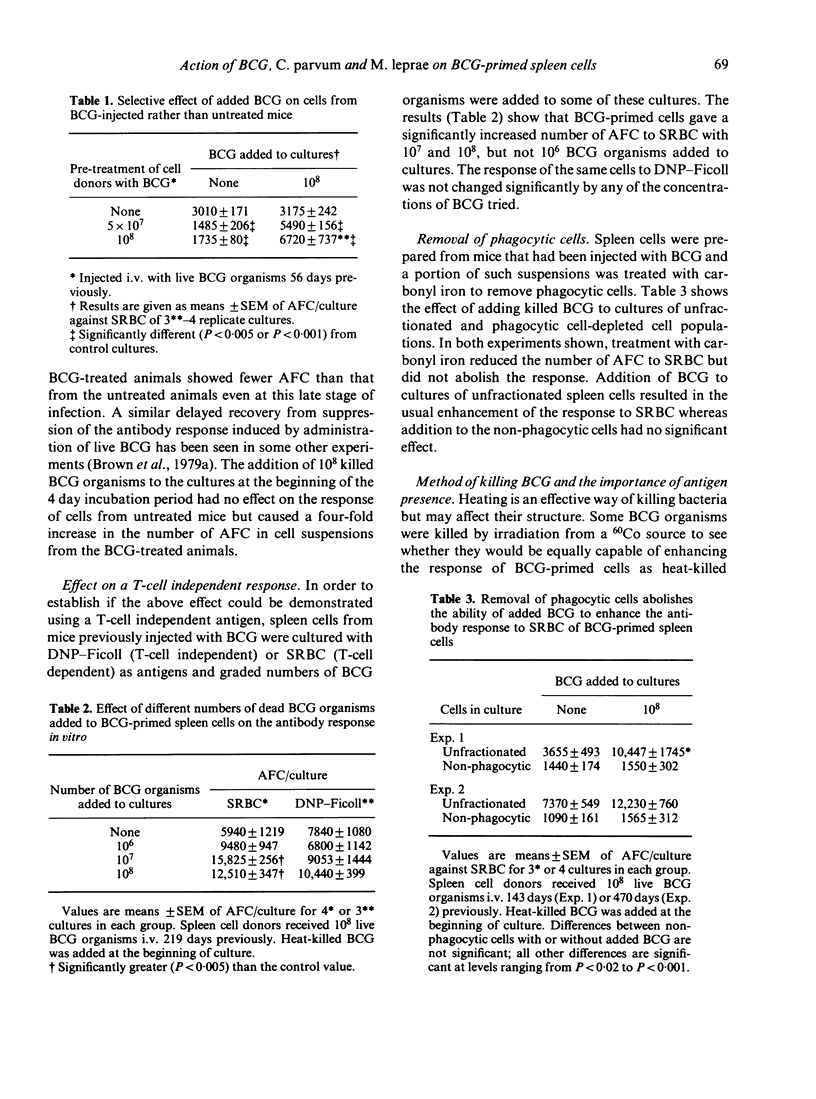
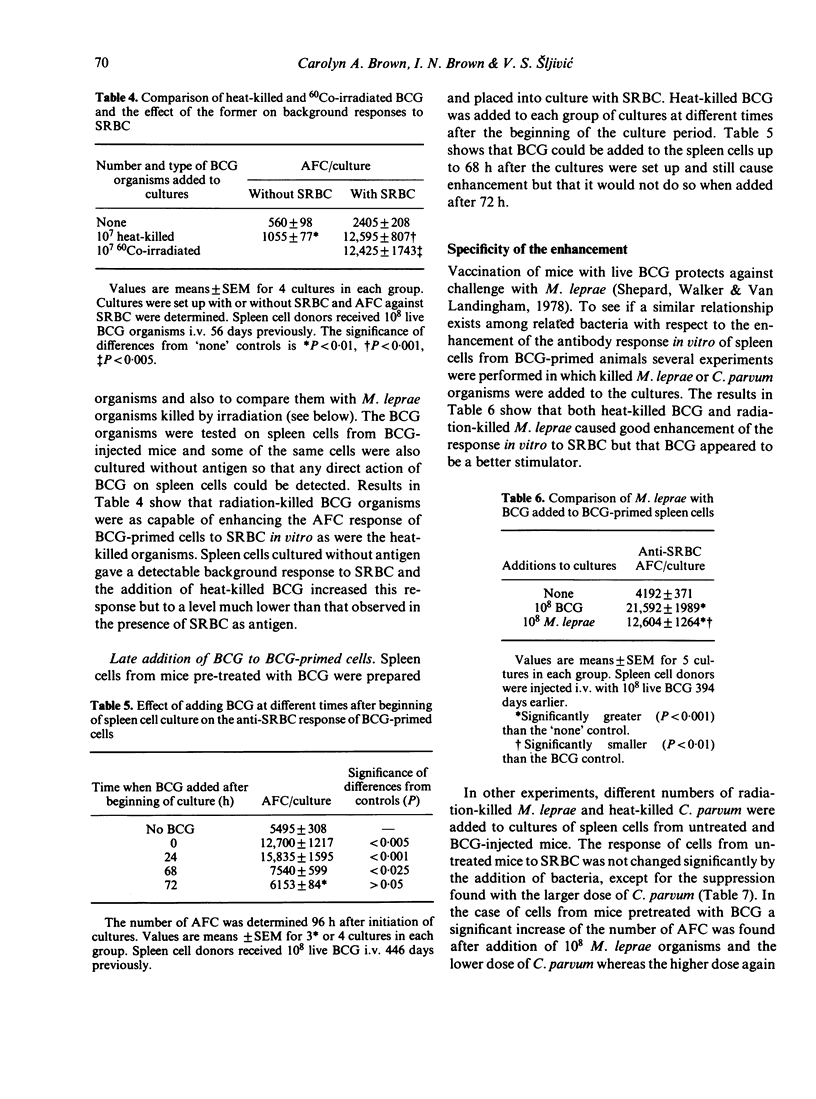
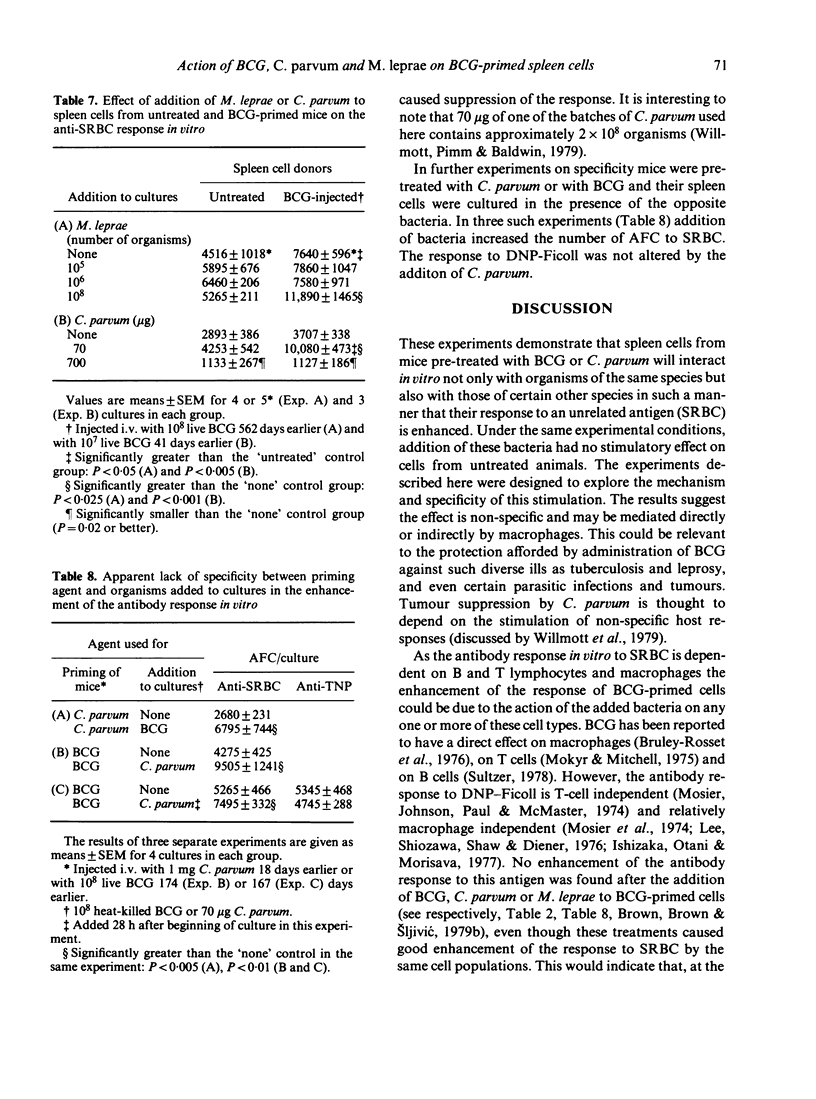
A few weeks after mice were injected i.v. with 10(8) live Mycobacterium bovis, BCG, the antibody response of their spleen cells to SRBC in vitro was comparable with the response of cells from untreated mice. Addition of BCG organisms to the culture vessels resulted in enhanced antibody-forming cell (AFC) responses by the primed cells but not by the cells from the untreated mice. No evidence was found for a direct stimulation of B cells and cell depletion experiments suggested macrophages were directly involved. BCG added to the cultures up to 68 h after they were set up, but not later, still caused enhancement. No enhancement was found when DNP-Ficoll was used as antigen. The ability to stimulate the anti-SRBC response was not restricted to the organism used for priming. Enhancement was also found if C. parvum or M. leprae were added to BCG-primed cells and if BCG was added to C. parvum-primed cells. The relevance of the results to the search for a leprosy vaccine is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Ajisaka M., Yamamura Y. Polysaccharides of Mycobacterium bovis Ushi 10, Mycobacterium smegmatis, Mycobacterium phlei, and Atypical Mycobacterium P1. Infect Immun. 1970 Sep;2(3):347–349. doi: 10.1128/iai.2.3.347-349.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Pimm M. V. BCG in tumor immunotherapy. Adv Cancer Res. 1978;28:91–147. doi: 10.1016/s0065-230x(08)60647-8. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Brown I. N., Sljivić V. S. Active suppression masks an underlying enhancement of antibody production in vitro by spleen cells from BCG-infected mice. Immunology. 1980 Jul;40(3):303–309. [PMC free article] [PubMed] [Google Scholar]
- Brown C. A., Brown I. N., Sljivić V. S. Phagosome/lysosome fusion: a possible prerequisite for the enhancement of antibody responses in vitro by BCG, Mycobacterium leprae and Corynebacterium parvum. Parasite Immunol. 1979 Winter;1(4):309–316. doi: 10.1111/j.1365-3024.1979.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Brown I. N., Sljivić V. S. Suppressed or enhanced antibody responses in vitro after BCG treatment of mice: importance of BCG viability. Immunology. 1979 Nov;38(3):481–488. [PMC free article] [PubMed] [Google Scholar]
- Bruley-Rosset M., Florentin I., Khalil A. M., Mathé G. Nonspecific macrophage activation by systemic adjuvants. Evaluation by lysosomal enzyme and in vitro tumoricidal activities. Int Arch Allergy Appl Immunol. 1976;51(5):594–607. doi: 10.1159/000231638. [DOI] [PubMed] [Google Scholar]
- Buys C. H., Elferink M. G., Bouma J. M., Gruber M., Nieuwenhuis P. Proteolysis of formaldehyde-treated albumin in Kupffer cells and its inhibition by suramin. J Reticuloendothel Soc. 1973 Aug;14(2):209–223. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T. The Clayton memorial lecture, 1978: "Is immunoprophylaxis in leprosy feasible?". Lepr Rev. 1978 Dec;49(4):305–317. doi: 10.5935/0305-7518.19780042. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977 Jul 30;2(6082):293–295. doi: 10.1136/bmj.2.6082.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K., Hämmerling U., Simon M., Oettgen H. F. Macrophage requirements of CR- and CR+ B lymphocytes for antibody production in vitro. J Immunol. 1976 May;116(5):1447–1451. [PubMed] [Google Scholar]
- Ishizaka S., Otani S., Morisawa S. Effects of carrageenan on immune responses. Studies on the macrophage dependency of various antigens after treatment with carrageenan. J Immunol. 1977 Apr;118(4):1213–1218. [PubMed] [Google Scholar]
- Jennings J. J., Rittenberg M. B. Evidence for separate subpopulations of B cells responding to T-independent and T-dependent immunogens. J Immunol. 1976 Nov;117(5 PT2):1749–1752. [PubMed] [Google Scholar]
- Lee K. C., Shiozawa C., Shaw A., Diener E. Requirement for accessory cells in the antibody response to T cell-independent antigens in vitro. Eur J Immunol. 1976 Jan;6(1):63–68. doi: 10.1002/eji.1830060114. [DOI] [PubMed] [Google Scholar]
- Mokyr M. B., Mitchell M. S. Activation of lymphoid cells by BCG in vitro. Cell Immunol. 1975 Feb;15(2):264–273. doi: 10.1016/0008-8749(75)90005-2. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M., Paul W. E., McMaster P. R. Cellular requirements for the primary in vitro antibody response to DNP-ficoll. J Exp Med. 1974 May 1;139(5):1354–1360. doi: 10.1084/jem.139.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L. Suramin effects on macrophage phagolysosome formation and antimicrobial activity. Infect Immun. 1978 May;20(2):503–511. doi: 10.1128/iai.20.2.503-511.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research in leprosy. A report of a committee set up by the medical research council to study future prospects. Clin Exp Immunol. 1979 Apr;36(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M. Immunity to Mycobacterium leprae infections induced in mice by BCG vaccination at different times before or after challenge. Infect Immun. 1978 Feb;19(2):391–394. doi: 10.1128/iai.19.2.391-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]


