Abstract
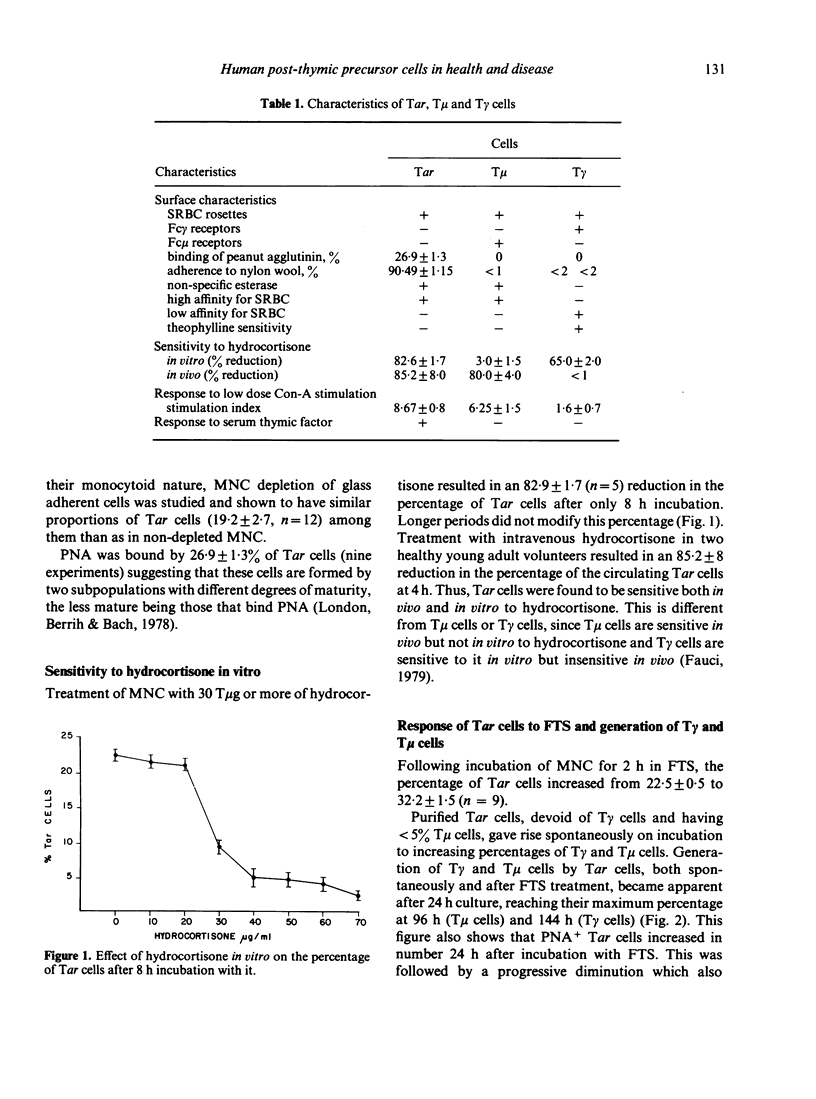
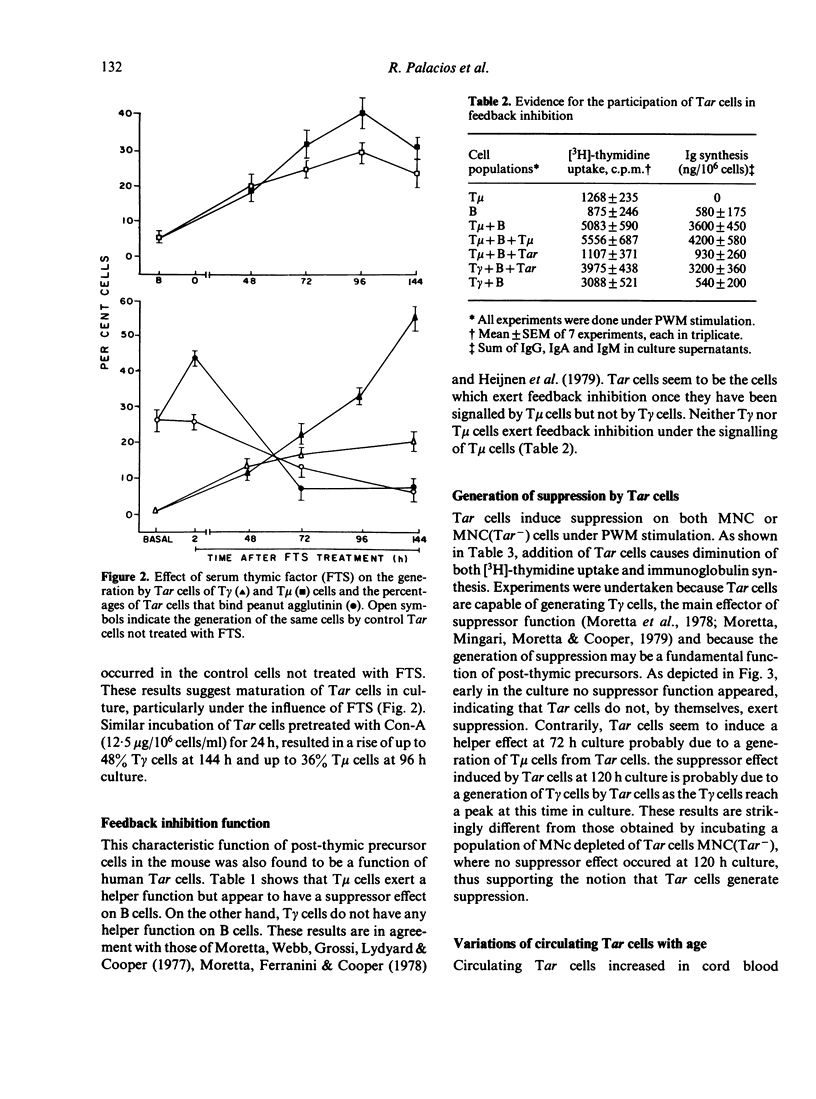
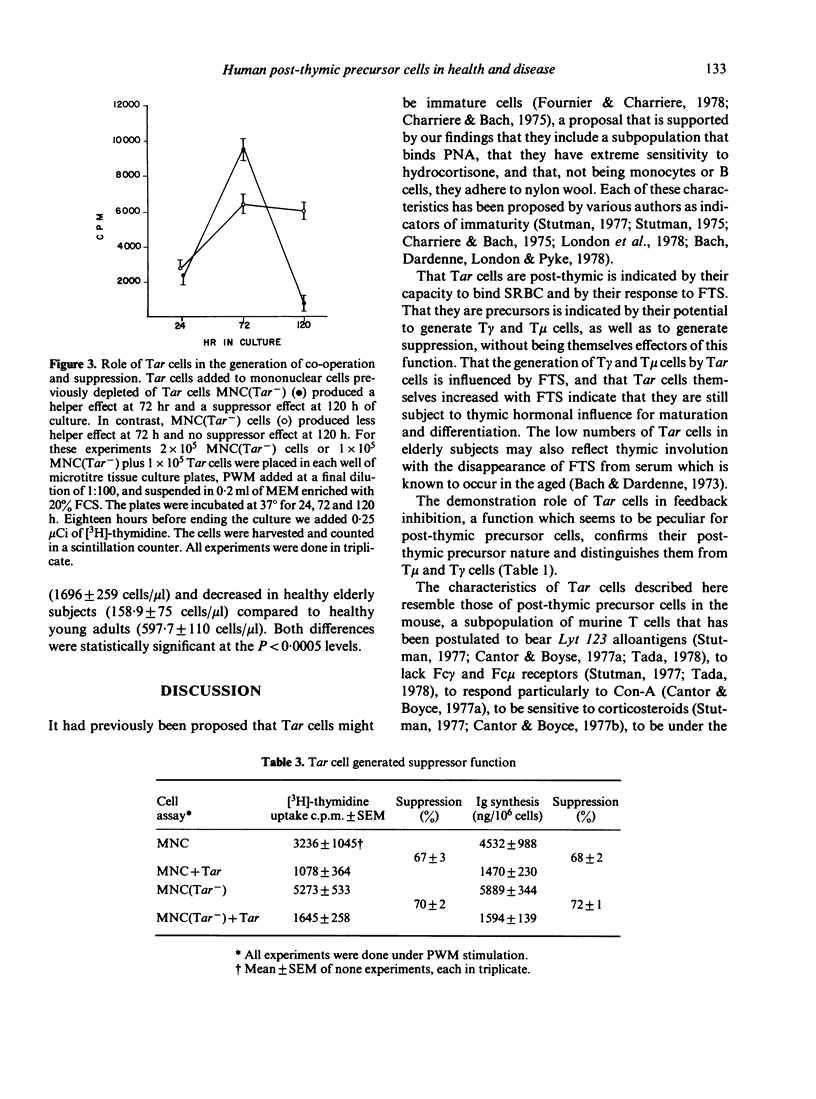
Human autologous-rosette-forming T cells (Tar cells) have many of the characteristics of post-thymic precursor cells. Thus, they bind to sheep erythrocytes but have neither receptors for the Fc portion of IgG nor for that of IgM. They include a subpopulation that binds peanut agglutinin which suggests that they are immature and, as opposed to T cells with either receptors for the FC portion of IgM (T mu) or of IgG (T gamma), Tar cells adhere to nylon wool, another possible indicator of immaturity, as is their extreme sensitivity to hydrocortisone both in vitro and in vivo. There are more Tar cells in cord blood than in the peripheral blood of young adults and there are more Tar cells in the peripheral blood of young adults than in the peripheral blood of elderly subjects. By co-culturing T mu and B cells, or T mu, or Tar and B cells in the presence of pokeweek mitogen (PWM) we were able to determine that these cells cause feedback inhibition, a function considered characteristic of post-thymic precursors. In co-cultures in which we placed mononuclear cells (MNC) or MNC plus Tar cells, or MNC depleted of Tar cells or MNC depleted of Tar cells plus Tar cells stimulated with PWM, we determined that Tar cells play a role in the generation of suppression thereby confirming that human Tar cells are precursor cells. We also found that Tar cells proliferated and generated T gamma and T mu cells both spontaneously and in greater numbers, under the effect of serum thymic factor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon-Segovia D., Ruiz-Arguelles A., Llorente L. Antibody penetration into living cells. II. Anti-ribonucleoprotein IgG penetrates into Tgamma lymphocytes causing their deletion and the abrogation of suppressor function. J Immunol. 1979 May;122(5):1855–1862. [PubMed] [Google Scholar]
- Alarcón-Segovia D., Fishbein E. Determination by laser nephelometry of immunoglobulins produced by mononuclear cells in culture. Rev Invest Clin. 1980 Jul-Sep;32(3):295–297. [PubMed] [Google Scholar]
- Bach J. F., Dardenne M. Studies on thymus products. II. Demonstration and characterization of a circulating thymic hormone. Immunology. 1973 Sep;25(3):353–366. [PMC free article] [PubMed] [Google Scholar]
- Bach M. A., Charreire J. Role of a circulating thymic factor in self-recognition and self-tolerance. Ann N Y Acad Sci. 1979;332:55–63. doi: 10.1111/j.1749-6632.1979.tb47097.x. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. Regulation of the immune response by T-cell subclasses. Contemp Top Immunobiol. 1977;7:47–67. doi: 10.1007/978-1-4684-3054-7_2. [DOI] [PubMed] [Google Scholar]
- Cantor H., McVay-Boudreau L., Hugenberger J., Naidorf K., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. II. Physiologic role of feedback inhibition in vivo: absence in NZB mice. J Exp Med. 1978 Apr 1;147(4):1116–1125. doi: 10.1084/jem.147.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charreire J., Bach J. F. Binding of autologous erythrocytes to immature T-cells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3201–3205. doi: 10.1073/pnas.72.8.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of the immunosuppressive and anti-inflammatory effects of glucocorticosteroids. J Immunopharmacol. 1978 1979;1(1):1–25. doi: 10.3109/08923977809027327. [DOI] [PubMed] [Google Scholar]
- Fournier C., Charreire J. Activation of a human T cell subpopulation bearing receptors for autologous erythrocytes by concanavalin A. J Immunol. 1978 Aug;121(2):771–776. [PubMed] [Google Scholar]
- Gluckman J. C., Montambault P. Spontaneous autorosette-forming cells in man. A marker for a subset population of T lymphocytes? Clin Exp Immunol. 1975 Nov;22(2):302–310. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Thurman G. B., Low T. L., Rossio J. L., Trivers G. E. Hormonal influences on the reticuloendothelial system: current status of the role of thymosin in the regulation and modulation of immunity. J Reticuloendothel Soc. 1978 Apr;23(4):253–266. [PubMed] [Google Scholar]
- Heijnen C. J., Uytdehaag F., Pot C. H., Ballieux R. E. Feedback inhibition by human primed T-helper cells. Nature. 1979 Aug 16;280(5723):589–591. doi: 10.1038/280589a0. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Limatibul S., Shore A., Dosch H. M., Gelfand E. W. Theophylline modulation of E-rosette formation: an indicator of T-cell maturation. Clin Exp Immunol. 1978 Sep;33(3):503–513. [PMC free article] [PubMed] [Google Scholar]
- London J., Berrih S., Bach J. F. Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol. 1978 Aug;121(2):438–443. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Cooper M. D. Characterization of human T-cell subpopulations as defined by specific receptors for immunoglobulins. Contemp Top Immunobiol. 1978;8:19–53. doi: 10.1007/978-1-4684-0922-2_2. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Moretta A., Cooper M. D. Human T lymphocyte subpopulations: studies of the mechanism by which T cells bearing Fc receptors for IgG suppress T-dependent B cell differentiation induced by pokeweed mitogen. J Immunol. 1979 Mar;122(3):984–990. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A., Bagwell C. B., Hudson J. L., Irvin G. L., 3rd Differences in flow cytometry and 3H-thymidine analyses of perturbed human lymphocytes. J Histochem Cytochem. 1979 Jan;27(1):486–490. doi: 10.1177/27.1.220326. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. II. Acquisition of mitogen responsiveness and of theta antigen during the ontogeny of thymocytes and "T" lymphocytes. Cell Immunol. 1972 Aug;4(4):367–380. doi: 10.1016/0008-8749(72)90039-1. [DOI] [PubMed] [Google Scholar]
- Stutman O. Humoral thymic factors influencing postthymic cells. Ann N Y Acad Sci. 1975 Feb 28;249:89–105. doi: 10.1111/j.1749-6632.1975.tb29060.x. [DOI] [PubMed] [Google Scholar]
- Stutman O. Two main features of T-cell development: thymus traffic and postthymic maturation. Contemp Top Immunobiol. 1977;7:1–46. doi: 10.1007/978-1-4684-3054-7_1. [DOI] [PubMed] [Google Scholar]
- West W. H., Payne S. M., Weese J. L., Herberman R. B. Human T lymphocyte subpopulations: correlation between E-rosette-forming affinity and expression of the Fc receptor. J Immunol. 1977 Aug;119(2):548–554. [PubMed] [Google Scholar]


