Abstract
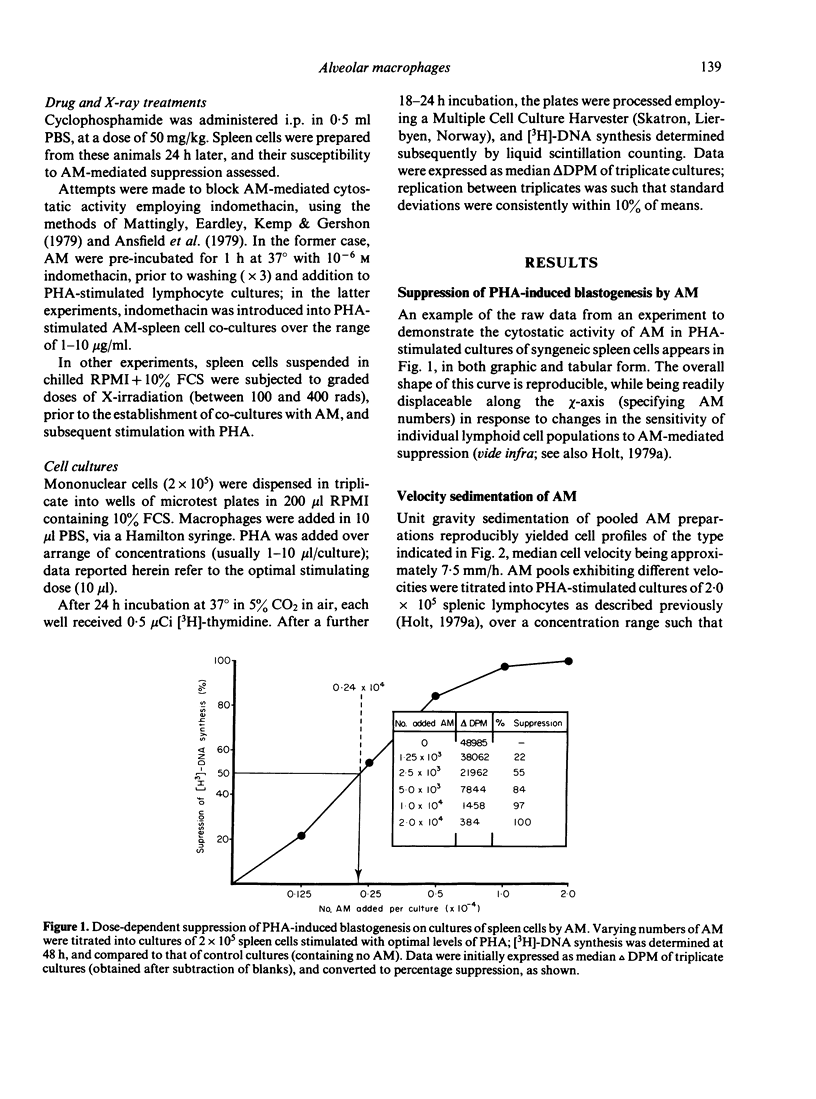
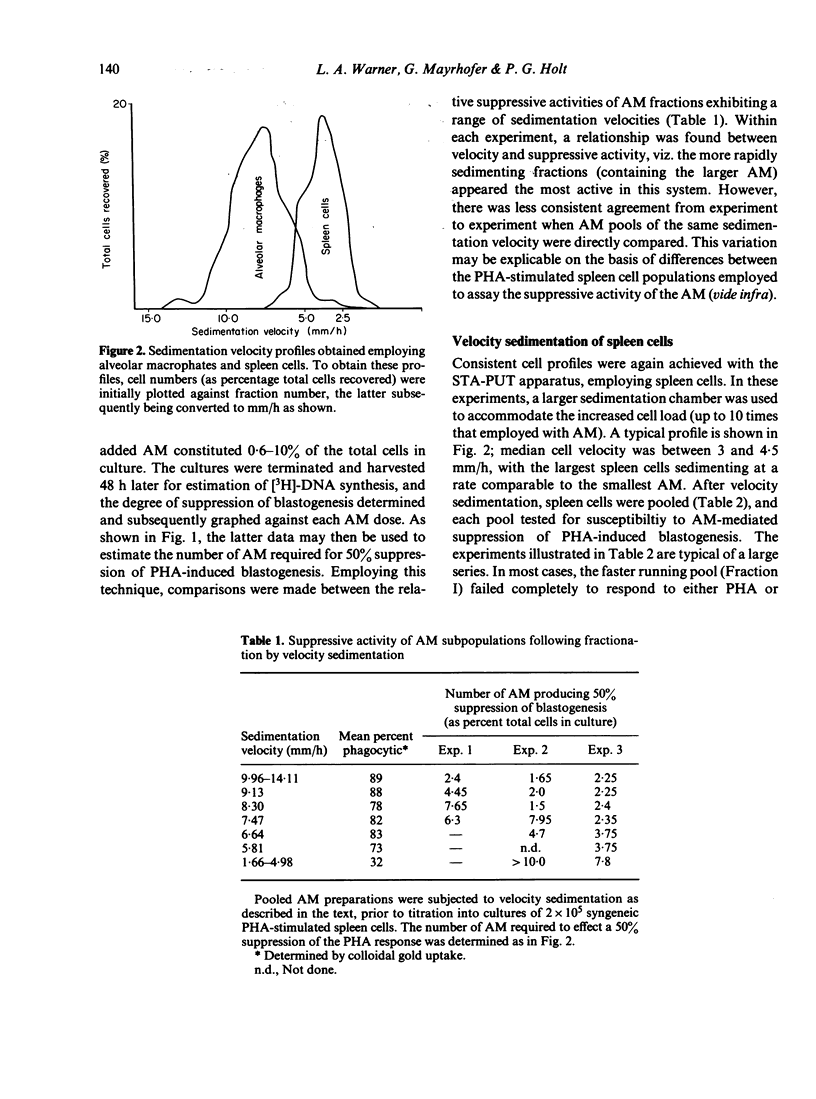
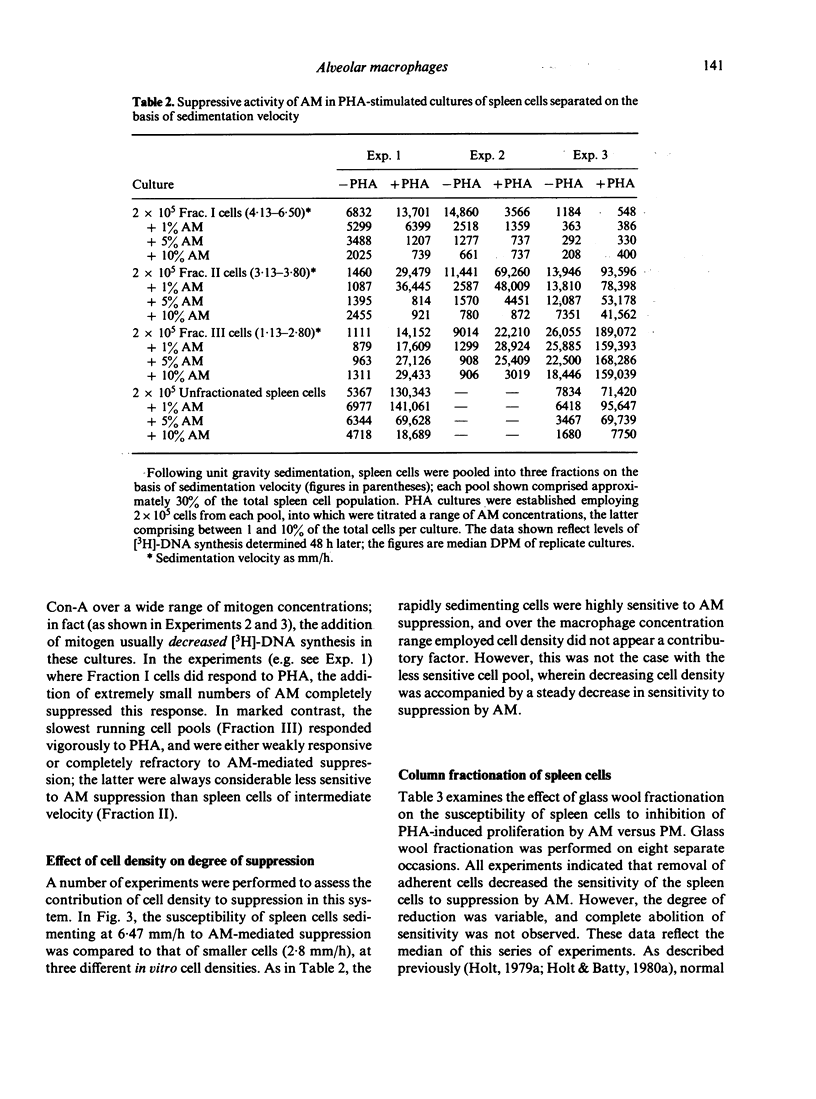
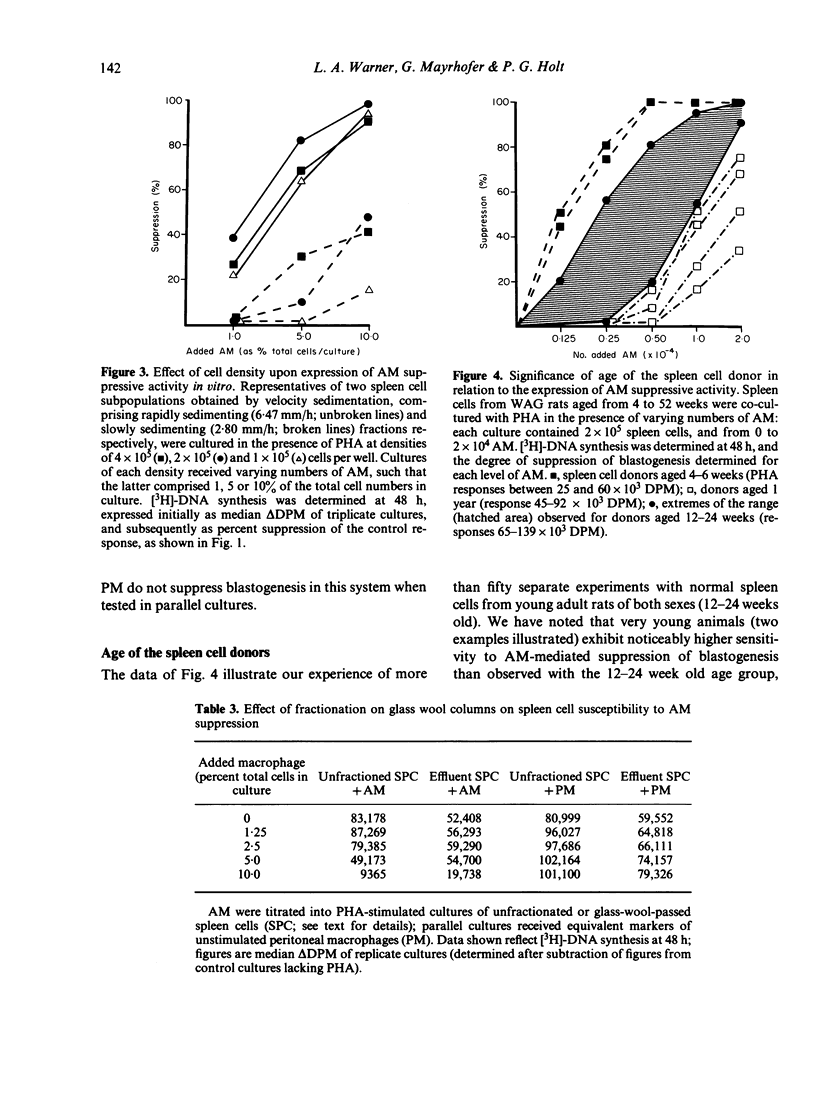
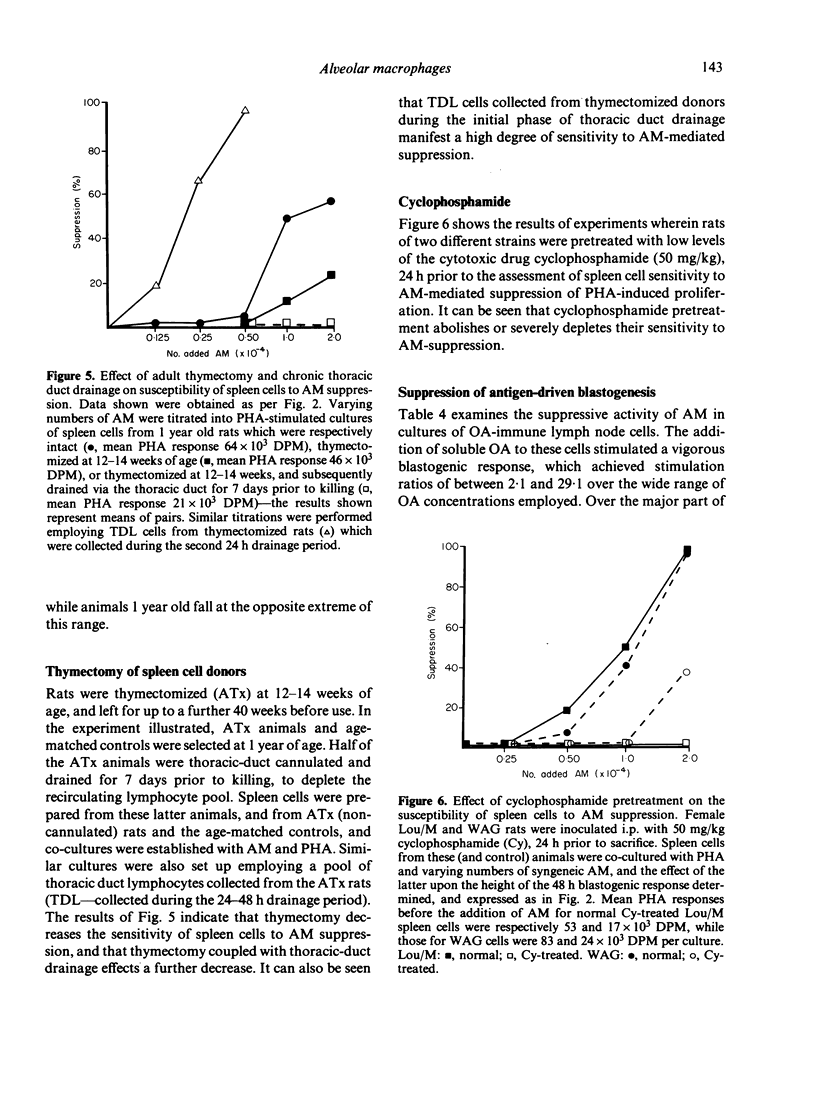
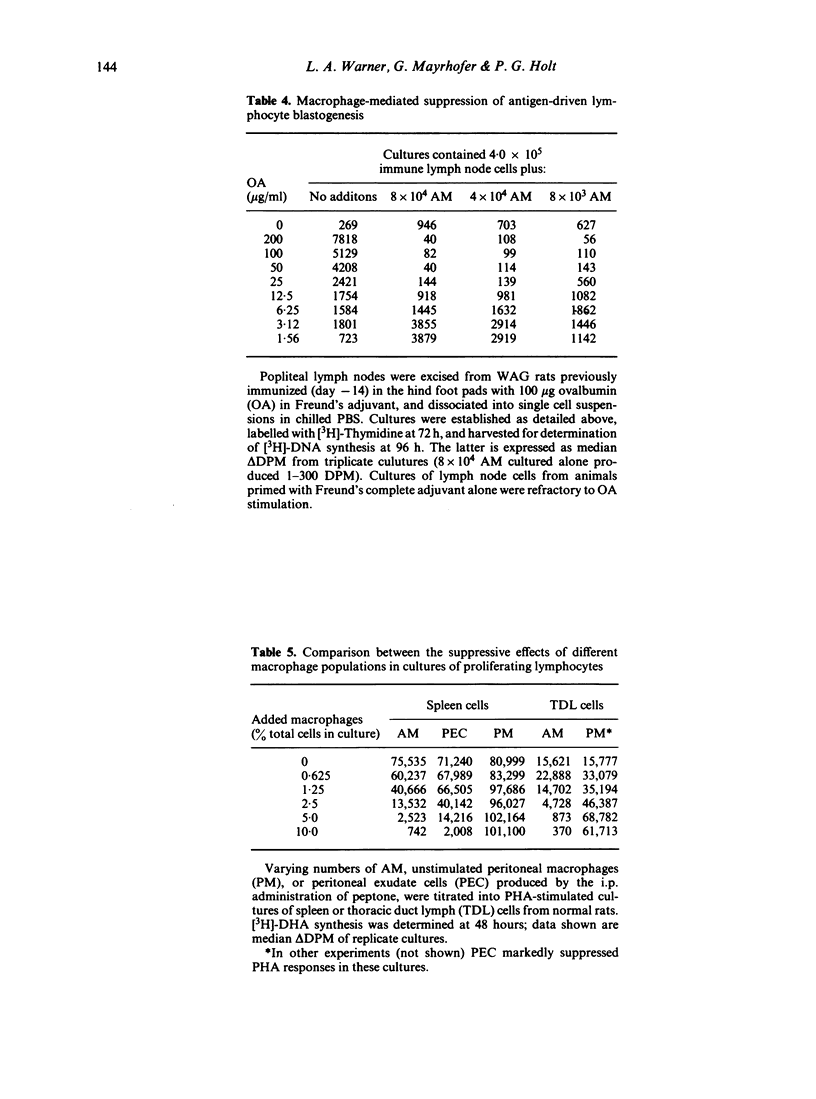
Alveolar macrophages (AM) from normal rats suppressed antigen- or mitogen-stimulated blastogenic responses in cultures of splenic or lymph node lymphocytes, high levels of suppression often being observed when added AM comprised as few 0.6% of the total cells in culture. The efficiency of AM-mediated suppression of spleen cell blastogenesis declined with the age of the spleen cell donors, was severely curtailed by pretreatment of donors with low levels of cyclophosphamide, and was depleted by adult thymectomy coupled with thoracic duct drainage. The suppressive activity of AM was most obvious at high cell density, was unaffected by the presence of indomethacin in the cultures, or by prior X-irradiation of the spleen cells. Fractionation of spleen cells by velocity sedimentation yielded cell populations of greatly varying sensitivities to AM-mediated suppression, from small splenocytes (sedimentation velocity 1.1-2.8 mm/h) which were almost totally refractory to AM-suppression when assayed in isolation from the remainder of the spleen cell population, to larger cells (sedimentation velocity greater than 3.,5 mm/h) exhibiting high levels of sensitivity. Fractionation of spleen cells by glass wool adherence indicated decreased sensitivity to AM-suppression in the effluent population. Examination of the suppressive activity of individual subpopulations of AM separated by velocity sedimentation indicated that the larger macrophages were the most active in vitro. Suppressive activity of this nature was not seen with unstimulated peritoneal macrophages, but was observed when 'activated' peritoneal exudate cells were tested. These data are discussed in terms of a two-cell model for suppression of blastogenesis, the ultimate effector cell being a macrophage, the activity of which is controlled by a long-lived, recirculating lymphocyte, which we have provisionally designated as a T lymphocyte.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansfield M. J., Kaltreider H. B., Caldwell J. L., Herskowitz F. N. Hyporesponsiveness of canine bronchoalveolar lymphocytes to mitogens: inhibition of lymphocyte proliferation by alveolar macrophages. J Immunol. 1979 Feb;122(2):542–548. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol. 1974 Jul;113(1):127–139. [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. II. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology. 1979 Jun;37(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. III. Studies on the mechanisms of inhibition of T-cell proliferation. Immunology. 1979 Jun;37(2):437–445. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. IV. Interspecies differences in activity in proliferating lymphocyte cultures. Cell Immunol. 1980 Mar 1;50(1):210–215. doi: 10.1016/0008-8749(80)90020-9. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Batty J. E. Alveolar macrophages. V. Comparative studies on the antigen presentation activity of guinea-pig and rat alveolar macrophages. Immunology. 1980 Oct;41(2):361–366. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Inhibitory activity of unstimulated alveolar macrophages on T-lymphocyte blastogenic response. Am Rev Respir Dis. 1978 Oct;118(4):791–793. doi: 10.1164/arrd.1978.118.4.791. [DOI] [PubMed] [Google Scholar]
- Hopper K. E., Wood P. R., Nelson D. S. Macrophage heterogeneity. Vox Sang. 1979;36(5):257–274. doi: 10.1111/j.1423-0410.1979.tb04434.x. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Eardley D. D., Kemp J. D., Gershon R. K. Induction of suppressor cells in rat spleen: influence of microbial stimulation. J Immunol. 1979 Mar;122(3):787–790. [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Pennline K. J., Conrad R. E., Gerber H. R., Herscowitz H. B. Suppressive effect of alveolar macrophages on the in vitro immune response of rabbit lymphocytes. J Reticuloendothel Soc. 1979 May;25(5):495–512. [PubMed] [Google Scholar]
- Raff H. V., Hinrichs D. J. Suppressor cell infleunce in selected strains of inbred rats. II. Macrophage-associated suppression of cell-mediated immune responsiveness. Cell Immunol. 1977 Mar 1;29(1):109–117. doi: 10.1016/0008-8749(77)90279-9. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Hinrichs D. J. Suppressor cell influence in selected strains of inbred rats. III. Evidence for nonspecific suppression by a lymphocyte-macrophage cooperation. Cell Immunol. 1977 Mar 1;29(1):118–128. doi: 10.1016/0008-8749(77)90280-5. [DOI] [PubMed] [Google Scholar]
- Waksman B. H. Tolerance, the thymus, and suppressor T cells. Clin Exp Immunol. 1977 Jun;28(3):363–374. [PMC free article] [PubMed] [Google Scholar]


