Abstract
Many behavioral responses require the coordination of sensory inputs with motor outputs. Aging is associated with progressive declines in both motor function and muscle structure. However, the consequences of age-related motor deficits upon behavior have not been clearly defined. Here, we examined the effects of aging on behavior in the nematode, Caenorhabditis elegans. As animals aged, mild locomotory deficits appeared that were sufficient to impair behavioral responses to sensory cues. In contrast, sensory ability appeared well-maintained during aging. Age-related behavioral declines were delayed in animals with mutations in the daf-2/insulin-like pathway governing longevity. A decline in muscle tissue integrity was correlated with the onset of age-related behavioral deficits, although significant muscle deterioration did not. Treatment with a muscarinic agonist significantly improved locomotory behavior in aged animals, indicating that improved neuromuscular signaling may be one strategy for reducing the severity of age-related behavioral impairments.
Introduction
Aging is a process of gradual functional decline leading to death. Age-related functional declines correlate with progressive cellular deterioration, although the pathways leading to these declines are poorly understood. Furthermore, it is not known whether specific cellular processes are particularly sensitive to the effects of age, or if all cellular processes suffer equally from age-related decline. A better understanding of how aging affects cellular function is necessary to answer these questions. Such studies may also reveal basic mechanisms behind the increased prevalence of certain diseases with age. For example, dopaminergic neurons of the substantia nigra are the major targets of Parkinson's disease, although how the biological function of these cells may be related to disease is not understood. Defining the ways that aging can affect cellular function may lead to new strategies for treating age-related diseases.
One approach for investigating the relationship between age and cellular function is to examine the problem in a genetically tractable organism with a relatively short lifespan, such as the nematode, Caenorhabditis elegans, which has been widely used for genetic studies of longevity. Genetic screens have identified several genes that determine C. elegans lifespan, including genes comprising the C. elegans daf-2/insulin-like signaling pathway (1, 2). While the search for genes that determine lifespan has been fruitful in C. elegans, less is known about how cellular function changes with age in this organism. Body movement declines gradually with increasing age in C. elegans (3-8). Studies have shown that the rate of age-related decline in body movement is a good predictor of lifespan (7, 8). This correlation suggests that locomotory decline and senescence may share common components in this species. Analysis of cell structure in aged animals showed that muscles, but not neurons, deteriorate significantly in older animals (6). Longitudinal studies showed that tissue deterioration correlates well with both locomotory behavior and lifespan (6, 9)
One area that has not been investigated is the impact of age upon behavioral responses that require the integration of sensory and motor function. C. elegans nematodes display characteristic responses to the presence of chemical attractants and repellents in their environment (10-12). It is not known whether sensory ability declines with age in C. elegans. In addition, the impact of age-related locomotory decline upon responses to sensory cues has not been investigated, although an early study reported that aging was associated with reduced movement towards bacterial food (13). In order to address these questions, we examined performance of several characteristic C. elegans behaviors during aging between young adulthood and the age of 50% mortality. During this period, mild locomotory deficits appeared which progressed in severity and compromised responses to sensory cues. In contrast, sensory function itself was not apparently affected by aging. Responses to sensory cues were protected from age-related decline in long-lived daf-2/insulin pathway mutants. Interestingly, early age-related locomotory deficits occurred without evidence of significant muscle deterioration, although there was evidence of reduced muscle integrity. These studies reveal that aging in C. elegans manifests early as mild locomotory impairments that impede normal behavioral responses. In addition, they provide several novel and useful approaches for monitoring physiological aging in C. elegans.
Methods
Strains
Animals were maintained at 15°C and propagated on nematode growth medium (NGM) plates with Escherichia coli strain OP50 as a food source according to standard protocols (14). Strains used in this study were: N2 (Bristol), wildtype; BA17, fem-1(hc17); DR1572, daf-2(e1368); CB444, unc-52(e444) and CB190, unc-54(e190).
Age-synchronized populations
Aged populations of wildtype and daf-2(e1368) animals were obtained from synchronized egg lays and the larvae were raised to young adulthood at 15°C with ample bacterial food. For ease of manipulation, some experiments were carried out with fem-1(hc17), an adult sterile strain with the same lifespan characteristics as wildtype. For fem-1(hc17), larval development occurred at 25°C, the nonpermissive temperature for adult fertility. For all strains, the day following the fourth and final larval molt was designated day 0 of adulthood and animals were transferred to 25°C on fresh media at densities of 30-50 animals/6-cm plate. We did not expose the aging cohorts to 5-fluorodeoxyuracil (FUDR), which is sometimes used in lifespan studies to suppress progeny production. In control experiments, FUDR had a deleterious effect on motility, also noted by others (3). For wildtype and daf-2(e1368) strains, adult animals were gently transferred to fresh media every day while fertile to prevent overcrowding by progeny and approximately every 2-3 days thereafter. Animals damaged during transfers, as reflected by lack of movement within a few minutes of transfer, were excluded from further analysis. Animals were scored as dead when they failed to move in response to gentle prodding with a platinum wire.
Behavioral assays
Mechanosensation and aversive odor. To confirm that all animals were capable of locomotion, only animals that could move away after a touch with a platinum wire (harsh touch) and could thrash in a drop of buffer were used for behavioral assays. Responses to gentle touch and aversive odor were assayed as described (15, 16). In brief, response to gentle touch was assayed by gently stroking the body with an eyebrow hair up to three times and scoring the animal's response as movement away from the stimulus. Different neurons mediate responses to harsh and gentle touch, so it is reasonable to assay these responses independently (15). Response to aversive odor was tested by exposing forward-moving animals to an octanol-saturated hair. The times required for animals to (a) stop forward movement and (b) start backing away from the octanol were both measured. Spontaneous stopping time in the absence of octanol was 9 ± 5.4 seconds for day 2 adults and 10.5 ± 4.7 seconds for day 8 adults; time to stop in response to octanol was significantly different (p<0.01, t-test. Animals that did not back away <60 seconds after stopping were scored as non-reversers.
Chemotaxis and locomotion. Chemotaxis and spontaneous locomotion were assayed at 25°C, essentially as described (10, 12, 17-19). Animals were washed briefly in a drop of M9 buffer and 20-30 same-aged animals were placed onto the center of a prewarmed 9-cm dish containing 10 mL of solid assay agar (agar (1.6%), CaCl2 (1mM), MgSO4 (1mM), NH4Cl (4μM), phosphate buffer (25mM)) with a 1 μL drop of chemical odorant at one edge. After all animals were transferred onto the agar, a second drop of odorant was added to the same spot as the first along with one drop of sodium azide (10%). Assay plates were gently placed into a 25°C incubator for one hour. Chemotaxis was quantified as net displacement away from the attractant, in the general direction of the odorant, as opposed to net displacement from the attractant, because diffusion of the odorant during the assay period would lead to uncertainty in the precision position of the odorant. Similar results were observed with several attractive (isoamyl alcohol (1%), Cl- (1M NH4Cl), diacetyl (1%), benzaldehyde (1%)) and noxious (octanol, (10%)) odorants. In separate experiments, we examined the effect of a control spot of sodium azide at the opposite edge of the plate from the odorant, but did not observe a significant difference in outcomes from the presence or absence of the control spot in experiments with aging populations (12, 17). For locomotion analyses performed in the presence of arecoline, 0.25 mM arecoline was added directly to the molten assay medium before pouring. All experiments were performed with at least three independent populations for wildtype and at least two populations for daf-2(e1368) to minimize population effects. Therefore, error bars represent the variation observed between independent populations.
Displacement measurements. To determine net distance traveled in chemotaxis and locomotion assays, each animal's position after 1 hour was marked on the bottom of the plate. The marked plate was digitally imaged and each animal's net displacement from the origin (plate center) was measured using Adobe Photoshop (Adobe Inc., San Jose, CA) or Openlab (Improvision Inc., Lexington, MA) software.
Muscle analyses
Phalloidin staining. For phalloidin staining of actin filaments, fem-1(hc17) adults were transferred onto a lysine-spread slide, freeze-cracked and fixed in 100% methanol (-20°C) for 4 minutes. Animals were stained with 1-4 μL of 0.1 mM AlexaFluor 488 phalloidin (Molecular Probes, Eugene, OR) and viewed with an Endow GFP filter set (Chroma Technology Corp., Rockingham, VT) on a Nikon E800 microscope. Digital images were collected with a Spot/RT CCD camera (Diagnostic Instruments, Sterling Heights, MI). Similar results were obtained with an alternate protocol in which same-aged animals were washed from an agar plate in M9 or PBS buffer and fixed for 10 minutes in acetone (-20°C) before staining with FITC-conjugated phalloidin (100nM). Specimens were mounted on an agarose pad with n-propyl gallate to reduce photobleaching. Similar results were obtained with fem-1(hc17) and N2 wildtype animals.
Quantitation of muscle damage. Several images were collected of body-wall muscles in each phalloidin-stained animal. These images were examined for signs of sarcomere damage, such as irregular staining or deformed sarcomeres. Images in which muscles had broken into pieces during the staining process were discarded and did not appear to be more prevalent at older ages. If the majority of an animal's muscles showed signs of damage, the animal's muscles were scored as damaged. If the majority of muscles from an animal did not show significant damage, the animal was scored as intact. The fraction of animals containing damaged muscles was determined for each of several independent staining experiments. The average occurrence of animals with damaged muscles was then calculated for all the trials at each age of adult life.
Electron microscopy. For electron microscopy, fem-1(hc17) adults were cut transversely in fixative (formaldehyde (2%), gluteraldehyde (2%), CaCl2 (3 mM) in 0.1 M cacodylate buffer, pH 7.2) and incubated for 1 hour at room temperature. Specimens were postfixed in 2% OsO4 in 0.1 M cacodylate buffer (pH 7.2) with 3 mM CaCl2 for 1 hour and stained in 2% uranyl acetate for 30 minutes at room temperature. Cut specimens were aligned and embedded in low melting temperature agarose (2.5%, US Biological). The agarose blocks were dehydrated in an ethanol series (5 minutes each in 50%, 70%, 90% and 100% ethanol) followed by propylene oxide before embedding in Epon. Transverse sections (70 nm) were stained with aqueous 2% uranyl acetate followed by 0.03% lead citrate and examined on a Phillips BioTwin CM120 transmission electron microscope or a Zeiss Electron Microscope EM10A. In this study, animals were not segregated by behavioral ability prior to muscle examination, although dead or severely incapacitated animals were removed from the population.
Body-length shrinkage assays: For body contraction assays, fem-1(hc17) animals were first photographed on NGM agar plates, transferred to media supplemented with 0, 50, 100 or 500 μM levamisole (Sigma) and photographed again after 20 minutes. The length of each animal before and after levamisole treatment was determined using software tracing and measuring tools (Openlab 3.1.5, Improvision Inc.). Similar results were obtained with N2 wildtype animals.
Statistical analyses
Statistical analyses were performed using student's t-test in a Microsoft Excel spreadsheet to compare results between multiple independent populations.
Results
Sensory ability remains intact during C. elegans aging
In this work, all ages refer to the age of adult life and do not include developmental time in order to minimize lifespan differences due to developmental phenotypes. The first day of C. elegans adult lifespan (adult day 0) occurs after the 4th and final larval molt, when animals became reproductive adults. The majority of eggs were laid on days 1-3 of adult lifespan, and reproduction ceased by adult day 5 (Fig. 1A). Under our laboratory conditions, the mean adult lifespan of wildtype and fem-1(hc17) animals at 25°C was 13 ± 2.7 days and maximum adult lifespan was 18 days (n=131 animals).
Figure 1.
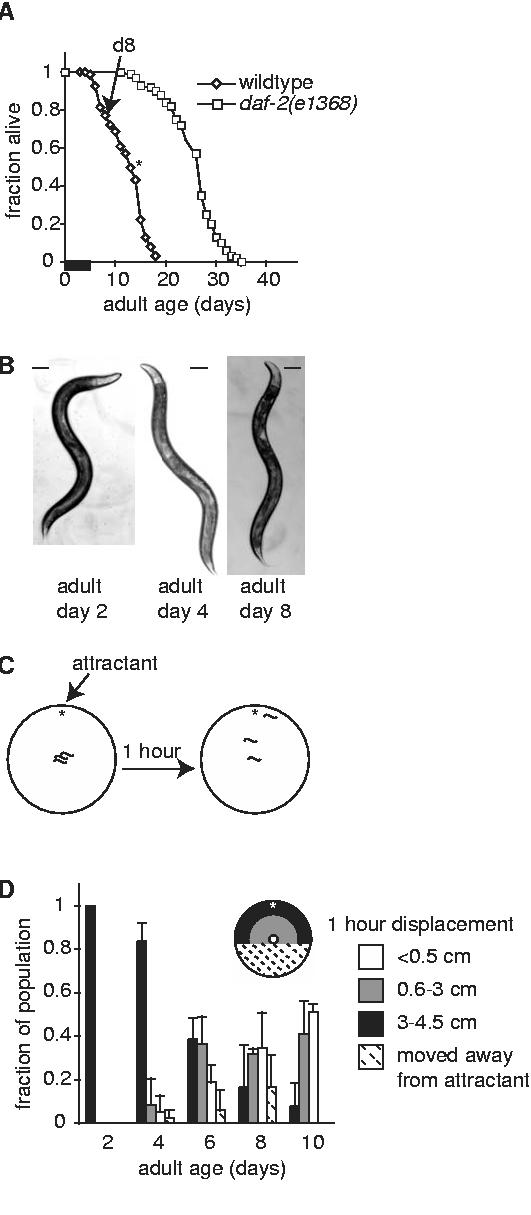
Aging in C. elegans is associated with decreased chemotaxis behavior. (A) Adult lifespan curves for wildtype (diamonds, n=131 animals) and daf-2(e1368) (squares, n=100 animals) beginning with day 0 of adulthood. Arrow indicates day 8 of adulthood. Asterisk indicates 50% survival in wildtype populations. Similar results were obtained with fem-1(hc17). A thickened bar on the x-axis indicates the period of fertility in wildtype animals (days 1-5). Reproductive lifespan of daf-2(e1368) is slightly longer (31). (B) Physical appearance of fem-1(hc17) day 2, 4 and 8 adults representative of those used in this study; scale bar represents 100 μm. Appearance of wildtype animals was similar. Animals incapable of normal sinusoidal movement were not used for behavioral analyses, which slightly reduced the apparent rate of behavioral decline as compared with the population as a whole. (C) One hour chemotaxis assays were performed by placing animals in the center of an agar plate with an attractive odorant near one edge (star). All chemotaxis experiments were repeated at least twice with independent populations. (D) Chemotaxis behavior declined in wildtype animals between adult days 2 and 10. Chemotaxis behavior was quantified as net displacement away from the original position (origin, center of a 9-cm plate) and is presented as color-coded bars according to the region of the plate where the animal was located. Uncolored bars: fraction of animals moving < 0.5 cm/1 hour; grey bars: animals moving between 0.6-2.9 cm/1 hour; darkened bars: animals moving > 3 cm. Hatched bars represent animals that moved away from the attractant. At least 20 animals were tested/trial; data represents the average of (n) trials, each with an independent population; adult day 2, 1 trial; day 4, 2 trials; day 6, 7 trials; day 8, 3 trials; day 10, 2 trials. Error bars represent variation between trials with independent populations.
Several studies have documented progressive declines in locomotory behavior during C. elegans aging (3, 5-8). We were concerned that severe locomotory deficits would complicate the analysis of sensory ability during aging. In preliminary experiments, we found that many animals younger than adult day 10 were capable of moving in normal sinusoidal fashion (Fig. 1B). In populations older than 10 days, the numbers of animals moving normally was substantially decreased (not shown). We therefore limited our analysis to animals between days 2 and 10 of adulthood that could move normally.
We examined whether adult age altered the performance of characteristic behaviors in response to several stimuli. The ability of young and old animals to detect gentle touch was measured by movement in response to gentle mechanical stimulation (15). In this assay, any animal failing to produce detectable movement in response to the stimulus within 5 seconds was scored as non-responsive. The majority of adult day 2 and day 8 animals exhibited appropriate responses to the touch stimulus (Table 1). We noted that the magnitude of response, as judged by distance moved in response to the touch, was reduced in older animals (not shown).
Table 1.
Sensory responses of wildtype animals during aging
| Young adult (days 2-3) | Aged adult (day 8) | |
|---|---|---|
| Gentle touch † | 92 ± 2.5 | 96 ± 1.5 |
| Aversive odor | ||
| Time to stop (sec.) § | ||
| 100 % octanol | 2.4 ± 0.7 | 4.1 ± 0.6** |
| 1% octanol | 3.0 ± 1.5 | 5.3 ± 3.3** |
| Time to back away (sec.) ¶ | ||
| 100% octanol | 2.2 ± 2.1 | 15 ± 11** |
| 1% octanol | 4.7 ± 3.8* | 14 ± 14 |
All values are ± sd
p<0.01 vs young.
Fraction of animals responding to gentle touch with a hair as described in methods; young, n=157 animals; aged, n=169 animals.
Mean time to stop after introduction of octanol near the nose; n≥15 trials, 5 animals.
Mean time between stop and backing away from octanol; n=15 trials, 5 animals
p<0.05 vs young, undiluted. Time to back up is only shown for animals that backed away within 60 seconds; young animals backed away in 100% of trials; aged animals backed away in 93% (100% octanol) and 47% (10% octanol) of trails.
The second sensory behavior we examined was response to an aversive odor, octanol, that held on a hair directly in front of the animal (16). When presented with this stimulus, young adult animals stopped forward movement within 2-3 seconds and then backed away from the odor in the subsequent 2-5 seconds (Table 1). By day 8 of adulthood, animals took almost twice as long to stop forward movement in response to octanol. However, aging was not associated with an obvious decline in sensitivity to octanol, as day 8 animals were able to stop forward movement when presented with 100-fold diluted octanol. Older animals exhibited defects in coordinating movement away from the aversive stimulus. The interval between stopping and backing away was significantly longer for adult day 8 animals and nearly half of day 8 animals failed to back away within the cut-off time (60 seconds) when presented with 100-fold diluted octanol. From these experiments, we concluded that sensory ability, as measured by responses to gentle touch and aversive odor, was similar between young and aged animals, although aged animals exhibited defects in performing some responses to those stimuli.
To further investigate the effects of age on responses to sensory stimuli, we examined chemotaxis behavior between adult days 2 and 10 (Fig. 1C). When placed onto an agar plate containing a small drop of the attractive odorant, such as diacetyl (2,3-butanedione), all young adult animals moved rapidly to the attractant, consistent with earlier reports (10, 12) (Fig. 1D). Increased age was associated with a progressive decline in this response. Adult day 8 and 10 animals exhibited significant declines in movement toward diacetyl (Fig 1D). Nearly half of day 8 and 10 animals did not initiate any significant movement toward diacetyl and instead remained within 0.5 cm of the original position after one hour (Fig. 1D, white bars). We observed that these animals sometimes moved alternately forward and backward, but did not make appreciable progress toward the attractant. Other day 8 and 10 animals were able to respond to diacetyl, consistent with our finding that sensory ability remained intact with age, although these animals did not travel as far as young adult animals during the assay period (Fig. 1D, grey bars). The presence of a control spot of sodium azide at the opposite edge of the agar surface did not affect the outcome of these experiments, presumably because animals were able to detect and respond to the presence of the attractant or did not move at all.
In adult day 8 animals, chemotaxis was not significantly improved by altering diacetyl concentration. Animals that had moved slightly toward the attractant in one hour assays usually continued progress toward the attractant when assay times were increased to 2 or 3 hours. Animals that had not left the origin in the first hour did not do so after longer incubation times (not shown). Chemotaxis defects were not odorant-specific; similar results were observed with several attractive (1% isoamyl alcohol, Cl- (1M NH4Cl), 1% diacetyl, 1% benzaldehyde) and noxious (10% octanol) odorants (not shown). It should be noted that, by confining behavioral analysis to animals capable of normal movement, these results likely indicate a slightly slower rate of behavioral decline than would be observed in the population as a whole.
Early age-associated behavioral declines correlate with movement defects
The finding that age had a negative impact on chemotaxis behavior, despite apparently normal sensory abilities in older animals, suggested that chemotaxis defects in day 8 animals were linked to locomotory deficits. To investigate this possibility, we examined movement at days 2, 4 and 8 of adulthood. Body movement in C. elegans consists of three basic movements. Forward sinusoidal movement is interspersed with reversals, which are spontaneous periods of backwards movement, and omega turns, in which animals change direction (19). Although many adult day 8 animals were capable of normal forward sinusoidal movement, other aspects of movement behavior were altered in these animals. First, adult day 8 animals moved slower; the number of body waves performed in a 20-second interval declined by half between days 2 and 8 of adulthood (Fig. 2A). Day 8 animals also exhibited different movement patterns. When monitored over a 3-minute assay period, day 2 animals moved forward almost continuously (Fig. 2B). The duration of forward movement was shorter in day 8 animals, which also did not move for a larger fraction of the assay period. In young animals, forward movement was interspersed by a small number of reversals and omega turns (Fig. 2C). In contrast, day 8 animals performed a greater number of reversals during the testing period than younger animals (Fig. 2C). The frequency of omega turns was similar between ages.
Figure 2.
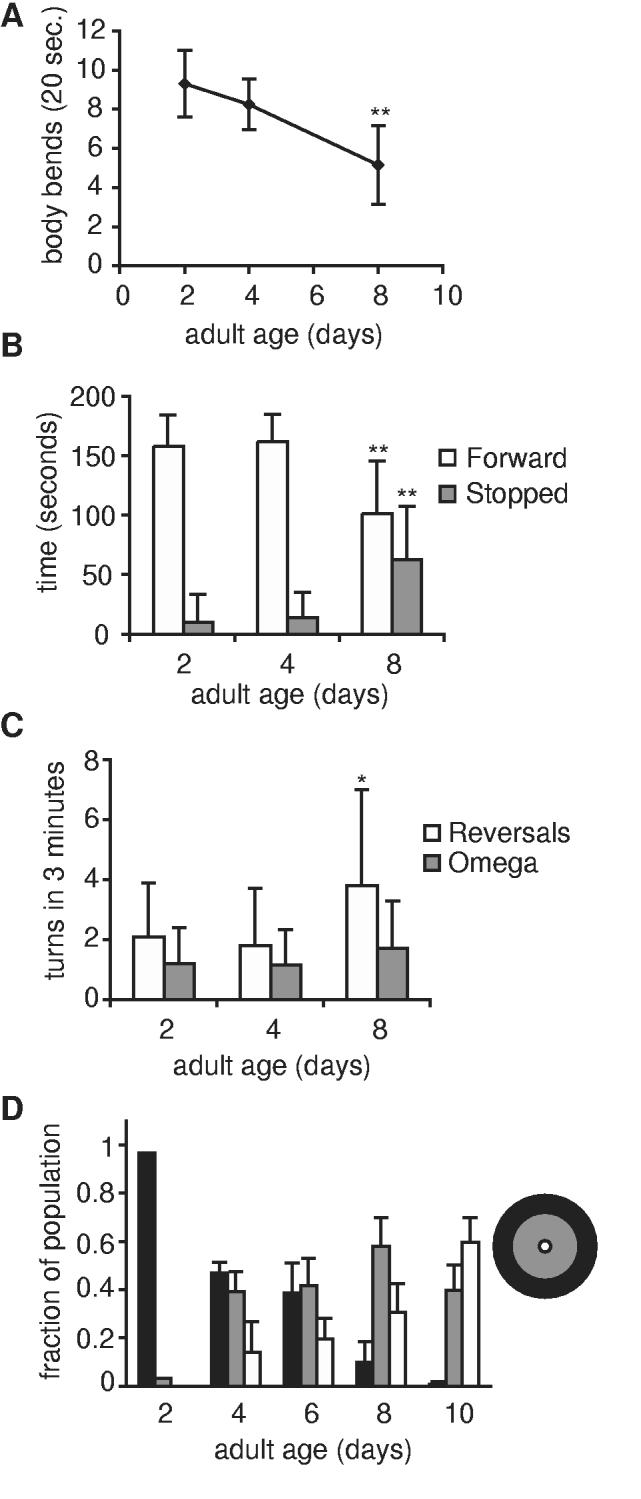
Locomotory deficits are evident early during C. elegans aging. (A) Rate of movement declined between days 2 and 8 of adulthood in fem-1(hc17) animals. Locomotion rate was measured as number of body bends performed in 20 seconds on NGM agar in the absence of bacterial food. Only animals that moved for the full 20-seconds were included; day 2, n=10 animals; day 4 and day 8, n=20 animals; ** p<0.01, t-test. (B) Time spent moving forward declined between days 2 and 8 of adulthood. fem-1(hc17) animals were observed for 3 minutes on NGM agar without bacterial food and time spent moving forward and stopped were recorded; day 2, n=25 animals; day 4, n=20 animals; day 8, n=20 animals. (C) Some day 8 fem-1(hc17) animals performed more reversals, but not omega turns. Frequency of reversals and omega turns, recorded during a 3-minute interval, on NGM agar without bacterial food; day 2, n=25 animals; day 4, n=20 animals; day 8, n=20 animals; *p<0.05, t-test. (D) Spontaneous locomotion declined with age. Spontaneous locomotion in any direction was scored and displayed as for chemotaxis, but without regard for directionality. At least 20 wildtype animals were scored per trial with independent populations; day 2, 1 trial; day 4, 2 trials; day 6, 6 trials; day 8, 6 trials; day 10, 3 trials. Error bars represent variation between populations.
The changes in movement were correlated with defects in spontaneous locomotion, or movement in the absence of odorant cues. Spontaneous movement was assayed similarly to chemotaxis behavior, except odorant cues were omitted (18, 19). In the absence of food or other sensory cues, day 2 adults dispersed from their original position at the center of the plate and, after one hour, most were found near the outer edges of the plate, between 3 and 4.5 cm from the origin (Fig. 2D). With increased age, net displacement from the origin decreased. As early as day 4, the number of animals found at least 3 cm from the origin had declined by half (Fig. 2D, black bars). This decline in spontaneous locomotion progressed as age increased and paralleled the decline in chemotaxis behavior. Analysis of tracks from individual animals in dispersal assays corroborated these observations. During the one hour assay period, day 2 adults moved away from the original position and were often found at the outer edge of the plate. In contrast, adult day 8 animals were more likely to remain in the middle of the plate, and rarely reached the plate edge (data not shown). We noted that day 4 animals exhibited greater displacement in chemotaxis assays than in locomotion assays, suggesting that day 4 animals could overcome mild locomotory deficits in the presence of sensory cues (Fig. 1D vs 2D).
Behavioral responses are protected from age-associated declines in long-lived daf-2 mutants
Longevity in C. elegans is regulated by an insulin-like signaling pathway that includes the gene, daf-2, encoding a protein most closely related to vertebrate insulin and IGF-I receptors (1, 20). Regulation of lifespan by insulin-like pathways appears to be evolutionarily conserved (21). Mutations in daf-2 result in a 2- to 3-fold increase in adult lifespan (see Fig. 1A). Previous studies showed that mutations in daf-2 and age-1, which also functions in the daf-2 pathway to regulate lifespan, can protect animals from movement declines during aging (5, 8, 22, 23). Locomotory declines were also delayed in long-lived recombinant inbred lines of C. elegans (7).
To determine if daf-2 mutations delayed age-related declines in chemotaxis and locomotion, we examined these behaviors in long-lived daf-2(e1368) animals. Young adult daf-2(e1368) animals exhibited normal chemotaxis responses, and this behavior was better maintained through adult day 10 than in wildtype animals (Fig. 3A vs. Fig. 1D). Similar results were obtained with a more severe daf-2(e1370) allele (not shown). By day 20, chemotaxis by daf-2(e1368) animals had significantly declined. Locomotory behavior in daf-2(e1368) animals was relatively normal at adult day 2 and declined over lifespan more slowly than in wildtype animals (Fig. 3B). Together these results show that long-lived daf-2(e1368) animals were subject to similar age-related behavioral declines as wild-type animals, but the progression of these declines was slowed.
Figure 3.
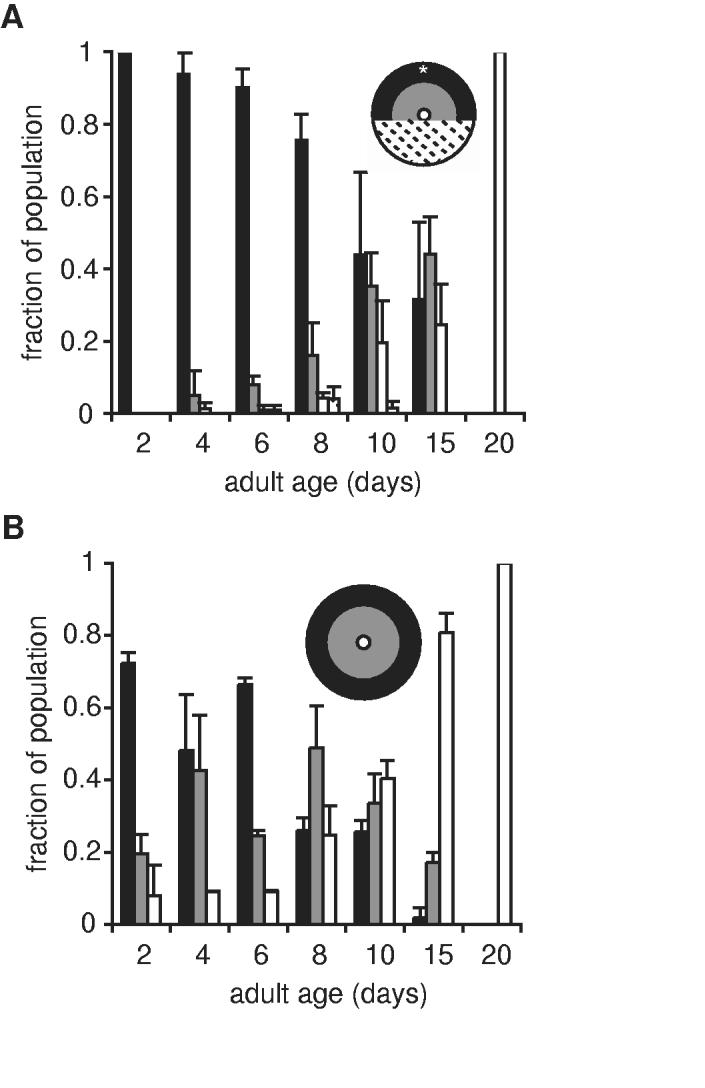
Chemotaxis and locomotory behavior was protected from age-related declines in long-lived daf-2(e1368) animals. (A) Chemotaxis behavior and (B) spontaneous locomotion in daf-2(e1368) animals were assayed at the nonpermissive temperature, 25°C; n=2 trials using independent populations, with at least 20 animals per trial. Error bars represent variation between cohorts. Refer to Fig. 1D and 2D for comparison to wildtype animals.
Behavioral declines precede extensive muscle deterioration
Aging in C. elegans is accompanied by sarcopenia, the progressive deterioration of muscle tissue (6). Thus, muscle deterioration may be the basis for locomotory declines between adult days 2 and 8. To investigate this possibility, we examined muscle structure during this period of adulthood. Movement in C. elegans is mediated by striated body wall muscles oriented in four quadrants longitudinally along the anterior/posterior body axis. Each muscle cell contains approximately eight sarcomeres which are composed of alternating bundles of thick filaments, containing myosin, and thin filaments, containing actin (24).
We first examined the overall structure of muscle tissue by staining animals with phalloidin to visualize the actin-containing thin filaments of the body wall muscles. Sarcomeres in most day 2 adults appeared straight and evenly stained, as expected for intact muscle cells in young adult animals (Fig. 4A). Phalloidin-stained muscles at adult days 4 and 6 were similar to day 2, with a slight increase in the fraction of animals with patched or wrinkled sarcomeres (Fig. 4B,C). In contrast, the majority of day 8 adults contained muscles that appeared patched or wrinkled when stained with phalloidin indicating that muscles were altered or damaged, either in vivo or as a consequence of the staining process (Fig. 4D). We determined the fraction of animals at each age with muscle damage in this assay (see Methods). This analysis showed that day 8 adults were significantly more likely to contain damaged muscles, as visualized by phalloidin, than day 2 adults (p<0.001, t-test) (Table 2). Day 4 and 6 adults exhibited a trend towards increased damage, but this difference was not statistically significant in most cases.
Figure 4.
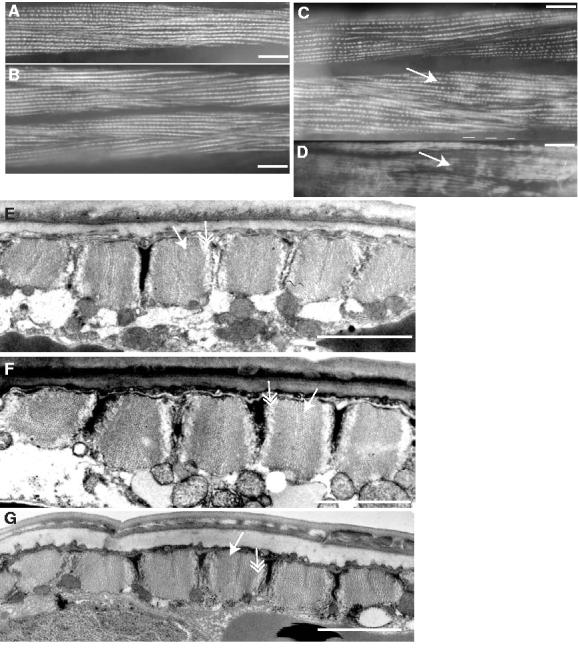
Muscle structure in fem-1(hc17) animals between days 2 and 8 of adulthood. (A-D) Muscle structural integrity was examined by phalloidin staining of actin in body wall muscle sarcomeres or, (E-G) by electron microscopy; (A, E) adult day 2; (B, F) day 4; (C) day 6; (D, G) day 8; (A-D), scale bar represents 20 μm; (E-G), scale bar represents 2 μm. Patchiness in phalloidin staining pattern is apparent in (C) day 6, and (D) day 8, muscles (white arrows). Similar results were obtained with wildtype animals. (E-G) By electron microscopy, characteristic structure of alternating myosin-containing thick filaments (white arrows), with actin-containing thin filaments (double-headed arrow) is evident at days 2, 4 and 8. For EM studies, 2-4 sections from 8-10 animals of each age were examined.
Table 2.
Condition of muscle sarcomeres after phalloidin staining over lifespan.
| Adult age | Muscle appearance |
sd | trials (n) | animals (total) | p vs d1,2 (t-test) | p vs d8 (t-test) | |
|---|---|---|---|---|---|---|---|
| Intact (%)† | Damaged § (%) | ||||||
| days 1, 2 | 85% | 15% | 18.3% | 7 | 101 | <0.001 | |
| day 4 | 66% | 34% | 25.7% | 6 | 74 | 0.25 | 0.013 |
| day 6 | 50% | 50% | 26.6% | 3 | 59 | 0.14 | 0.13 |
| day 8 | 12% | 88% | 1.8% | 3 | 66 | <0.001 | |
Mean of all trials.
Phalloidin-stained sarcomeres appeared patched or wrinkled.
We also examined adult day 2, 4 and 8 muscles at higher resolution by electron microscopy. All day 2 and 4 adults displayed normal sarcomere organization and cellular composition (Fig. 4E,F). In the majority of day 8 adults examined, muscles did not show evidence of significant deterioration and appeared to retain normal sarcomere organization (Fig. 4G). Both thin and thick filaments were present and appeared normal. One day 8 animal, of eight animals examined, contained disordered sarcomeres, possibly due to sarcopenia (not shown). Muscle cytoplasmic volume appeared to be reduced in three day 8 animals, although cytoplasmic volume appeared normal in the remaining five specimens (not shown). The relatively normal appearance of day 8 muscles by EM, together with the fact that day 8 animals performed normal sinusoidal movement, indicates that day 8 muscles were not extensively deteriorated, but were perhaps more sensitive than younger muscles to mechanical stresses, including those associated with phalloidin staining.
Adult day 8 muscles can contract normally in response to nicotinic acetylcholine receptor agonistss
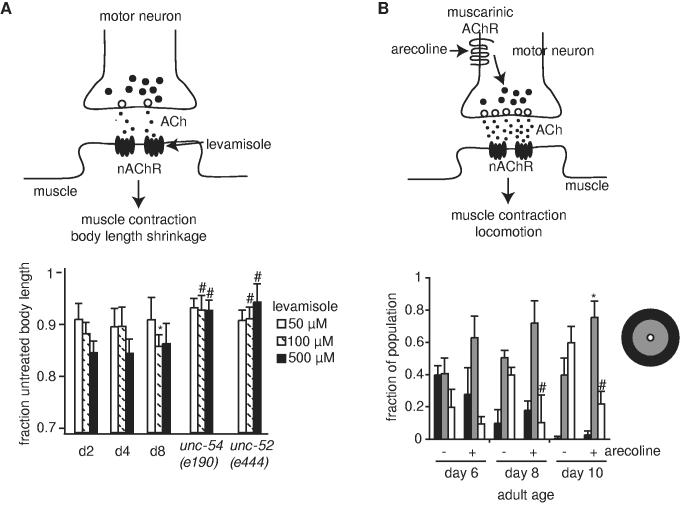
We next investigated whether specific aspects of muscle function were altered in adult day 8 animals that might be correlated with reduced movement at this age. One marker for muscle function is the ability to contract in the presence of cholinergic agonists. Acetylcholine is the major excitatory neurotransmitter in C. elegans (25). Acetylcholine released by motor neurons triggers muscle contraction via nicotinic acetylcholine receptors in the body wall muscle. Pharmacologic activation of muscle nicotinic receptors by the nicotinic agonist, levamisole, caused muscle contraction, thus shortening the animal's body length (Fig. 5A, upper panel) (26, 27). We reasoned that the extent of body length shrinkage may reflect the relative ability of muscles to contract after levamisole treatment, and this might be useful for assaying muscle function at adult days 2, 4 and 8. When examined over a 10-fold range of levamisole concentration, adult animals of all three ages exhibited similar reductions in body length at most concentrations tested (Fig. 5A, lower panel).
Figure 5.
Muscle contraction was similar between days 2 and 8 of adulthood and locomotory behavior in old animals was improved by increasing neuromuscular signaling. (A) Body length shrinkage after treatment with levamisole is a measure of muscle function. (Upper panel) Acetylcholine (ACh, small circles in synapse), released from vesicles in pre-synaptic motor neurons, activates nicotinic acetylcholine receptors on the post-synaptic cells (nAChR, black oval shapes in muscle membrane). Levamisole stimulates nAChRs directly, leading to muscle contraction and body shrinkage. (Lower panel) Body length shrinkage in fem-1(hc17), at days 2, 4, and 8 of adulthood, or locomotion-defective mutants, unc-52(e444) and unc-54(e190), as young adults. The unc-52(e444) and unc-54(e190) mutations both significantly impair mobility and result in abnormal sarcomere structure, and both strains exhibited defects in body shrinkage with levamisole. White bars, 50 μM levamisole; hatched bars, 100 μM levamisole; black bars, 500 μM levamisole; *p<0.05 vs day 2 fem-1(hc17); # p<0.001 vs day 2 and day 8 fem-1(hc17). (B) Arecoline treatment improved locomotory behavior in aged animals. (Upper panel) Arecoline is an agonist of muscarinic acetylcholine receptors. Arecoline treatment stimulates acetylcholine release by motor neurons. In control experiments, arecoline treatment increased paralysis of wildtype day 8 animals by aldicarb, consistent with the expectation that arecoline treatment stimulated acetylcholine release by motor neurons (not shown). (Lower panel) One hour spontaneous locomotion assays were conducted in the presence (+) or absence (-) of 0.25mM arecoline, using separate wildtype populations for treatment and control, and net displacement scored, as for Fig. 2D. 22 to 66 animals were tested in each trial; average of 3 trials is shown; *p<0.05; # p<0.01 (t-test).
As controls, we examined body shrinkage in two mutant strains with non-functional body-wall muscles, unc-54(e190) and unc-52(e444). unc-54(e190) animals contain a null mutation in the major body wall muscle myosin heavy chain, rendering animals limp and unable to move (28). unc-52(e190) animals exhibit progressive paralysis due to a mutation in perlecan (29). As compared to wildtype animals, both mutant strains displayed significant defects in body length shrinkage after treatment with 100μM or 500μM levamisole. Thus, impairment of levamisole-induced body contraction is a reasonable marker for impaired muscle function.
Stimulating acetylcholine release from motor neurons with muscarinic agonists improved movement at days 8 and 10 of adulthood
Since muscles did not appear to undergo significant structural or functional deterioration between days 2 and 8 of adulthood, we hypothesized that locomotory deficits in older animals may result from deficits in neuromuscular coordination, possibly due to decreased muscle integrity. In this case, stimulating motor neuron acetylcholine release might alleviate the locomotory deficits of day 8 adults. Muscarinic receptors are one pathway for modulating motor neuron acetylcholine release in C. elegans. Arecoline is a muscarinic agonist that can increase acetylcholine release by C. elegans motor neurons (Fig. 5B, upper panel) (30). Spontaneous locomotion at days 8 and 10 of adulthood was improved in assays performed in the presence of arecoline (Fig. 5B, lower panel). In particular, arecoline treatment of day 10 adults was associated with a significant increase in the fraction of animals that initiated movement away from the original position (Fig. 5B, grey bars).
Discussion
In this study, we characterized mild age-related locomotory deficits that appeared early during adult aging and correlated with declines in responses to attractive odorants. Other studies have also documented age-associated locomotory declines in C. elegans and the rate of locomotory decline appears to be predictive of lifespan (4-8). Several pieces of evidence support a link between the deficits in locomotory and chemotaxis behavior in day 8 adults. First, both chemotaxis and spontaneous locomotion were similarly reduced between days 4 and 8 of adulthood. Second, day 8 adults exhibited altered locomotory patterns that could interfere with chemotaxis. Specifically, day 8 adults did not sustain forward movement for as long as young animals. Third, delayed locomotory decline in daf-2(e1368) animals was correlated with delayed decline in chemotaxis behavior. Finally, sensory abilities appeared to be similar between day 2 and day 8 adults, as measured by responses to touch and an aversive odor placed in front of the animal. Together, these findings suggest that an early effect of aging in C. elegans is a decline in coordinated movement that ultimately compromises behavioral responses.
Several factors could contribute to the loss of movement between days 2 and 8 of adulthood. Locomotory decline may be a consequence of age-related muscle deterioration, or sarcopenia, which is a feature of aging in C. elegans and other organisms (6). Alternatively, age-related declines in muscle function between days 2 and 8 of adulthood may precede significant structural deterioration. We investigated these possibilities by examining several aspects of muscle structure and function between days 2 and 8 of adulthood.
Our examination of muscle tissue between days 2 and 8 revealed signs of general decline, as evidenced by increased sarcomere damage when day 8 muscles were stained with phalloidin. Significant structural deterioration was absent from the majority of day 8 muscles when examined by EM, although the relatively small size of the EM study precludes elimination of the possibility of structural deterioration at day 8. Nevertheless, these findings suggest that day 8 muscles suffered from frailty, or a general decline in cellular integrity, but had not progressed to the state of severe deterioration characteristic of later ages (6). It is possible that day 8 muscles were more sensitive that day 2 muscles to stress from the manipulations for phalloidin staining. It should be noted that similar structural alterations were observed using two different protocols, suggesting that damage in phalloidin-stained day 8 muscles was not the result of a specific protocol, but may reflect general weakness of the tissue.
The day 8 animals examined in this study did not exhibit obvious defects in the ability to perform sinusoidal movement. However, closer inspection revealed that day 8 animals moved more slowly than day 2 and 4 adults, consistent with earlier studies (4, 7). Slower movement alone may not necessarily impair chemotaxis behavior, as slow-moving unc-52(e444) animals are reported to chemotax normally, although slowly, to attractants (10). In addition to slower movement rates, day 8 animals did not maintain forward movement for long intervals. In these experiments, day 8 animals did move in reverse normally. Interestingly, in the aversive odor assay, day 8 animals took significantly longer to start backing away from the odor than day 2 animals. One possible explanation for this incongruity is that day 8 animals may also suffer from defects in coordinating changes of direction.
We further examined whether a significant decline in muscle function could be detected between days 2 and 8 of adulthood by evaluating muscle contraction by the nicotinic agonist, levamisole. Body length shrinkage by levamisole has been a useful assay for probing post-synaptic components at C. elegans neuromuscular junctions (26, 27, 30). Our control experiments with unc-52(e444) and unc-54(e190) animals indicated that muscle function can also be measured by relative changes of body length in the presence of levamisole. The finding that levamisole treatment caused similar reductions of body length at days 2 and 8 is consistent with the hypothesis that day 8 muscles have not suffered from significantly structural deterioration or functional decline. Rather, locomotory impairments at day 8 were better correlated with a decline in cellular integrity, possibly a sign of muscle frailty, evident from phalloidin staining of muscle sarcomeres.
Locomotory declines associated with C. elegans aging were partially remedied by treatment with the muscarinic agonist, arecoline. In C. elegans, arecoline treatment has been shown to stimulate motor neuron acetylcholine release (30). The finding that arecoline treatment improved locomotory behavior during aging indicates that body movement in aged animals is not limited by muscle function. If age-related functional declines in muscle were the sole limiting factor for locomotory ability, then we would have expected arecoline to have no effect on locomotory behavior in aged animals. Rather, arecoline stimulated locomotion in adult day 8 and 10 animals. One potential explanation for this observation is that aged muscles may function less efficiently than young muscles in the presence of physiological levels of neurotransmitter. Therefore, we hypothesize that stimulation of neuromuscular signaling by arecoline attained a threshold necessary for a more youthful pattern of behavior. Alternatively, aging may negatively impact neuromuscular signaling. Further investigations are necessary to distinguish these scenarios.
In this study, we have described several changes associated with early stages of aging in C. elegans, before animals take on the deteriorated appearance characteristic of older ages. This time period may be analogous to the period between young adulthood and middle age in mammals, coinciding with the cessation of reproduction and the onset of general decline. An understanding of the cellular changes that occur early during aging is important, as these events may be the basis for subsequent deterioration of the organism as aging progresses. This study showed that the early period of C. elegans aging was characterized by a decline in behavioral responses correlated with the onset of locomotory deficits. After day 4 of adulthood, animals were unable to overcome these locomotory deficits, despite the presence of motivational cues, such as attractive odorants. Thus, these locomotory and behavioral alterations are the earliest signs of age-related declines that culminate with the complete loss of mobility at older ages.
Acknowledgements
We thank the Caenorhabditis Genetics Center for providing strains used in this work. We are also grateful to Lauren Zeitels, Mark Wilson and other colleagues at the NIA and in the Baltimore/Washington C. elegans community for helpful discussions.
References
- 1.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 2.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 3.Bolanowski M, Russell R, Jacobson L. Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech. Ageing and Develop. 1981;15:279–95. doi: 10.1016/0047-6374(81)90136-6. [DOI] [PubMed] [Google Scholar]
- 4.Croll N, Smith J, Zuckerman B. The aging process of the nematode Caenorhabditis elegans in bacterial and axenic culture. Exp Aging Res. 1977;3:175–199. doi: 10.1080/03610737708257101. [DOI] [PubMed] [Google Scholar]
- 5.Duhon SA, Johnson TE. Movement as an index of vitality: comparing wild type and the age-1 mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 1995;50:B254–B261. doi: 10.1093/gerona/50a.5.b254. [DOI] [PubMed] [Google Scholar]
- 6.Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TE. Aging can be genetically dissected into component processes using long-lived lines of Caenorhabditis elegans. Proc. Natl. Acad. Sci. 1987;84:3777–3781. doi: 10.1073/pnas.84.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl. Acad. Sci. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garigan D, Hsu A-L, Graser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward S. Chemotaxis by the nematode C. elegans: Identification of attractants and analysis of the response by use of mutants. Proc. Natl. Acad. Sci. 1973;70:817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dusenbery D. Analysis of chemotaxis in the nematode Caenorhabditis elegans by countercurrent separation. J Exp Biol. 1974;188:41–48. doi: 10.1002/jez.1401880105. [DOI] [PubMed] [Google Scholar]
- 12.Troemel E, Kimmel B, Bargmann C. Reprogramming Chemotaxis Responses: Sensory Neurons Define Olfactory Preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 13.Hosono R. Age dependent changes in the behavior of Caenorhabditis elegans on attraction to Escherichia coli. Exp Geront. 1978;13:31–36. doi: 10.1016/0531-5565(78)90027-x. [DOI] [PubMed] [Google Scholar]
- 14.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of C. elegans. Developmental Biology. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 16.Troemel E, Chou J, Dwyer N, Colbert H, Bargmann C. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 17.Bargmann C, Hartwieg E, Horvitz H. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 18.Pierce-Shimomura J, Morse T, Lockery S. The fundamental role of pirouettes in C. elegans chemotaxis. J Neurosci. 1999;19:9557–9569. doi: 10.1523/JNEUROSCI.19-21-09557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croll N. Components and patterns in the behavior of the nematode C. elegans. J Zoology. 1975;176:159–176. [Google Scholar]
- 20.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 21.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 22.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 23.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moerman DG, Fire A. Muscle: Structure, function, and development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor Laboratory Press; Plainview, NY: 1997. pp. 417–470. [PubMed] [Google Scholar]
- 25.Rand J, Nonet M. Synaptic transmission. In: Riddle D, Blumenthal T, Meyer B, Priess J, editors. C. elegans II. Cold Spring Harbor Press; Plainview, NY: 1997. pp. 611–643. [PubMed] [Google Scholar]
- 26.Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired by not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 27.Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson C, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejsovec A, Anderson P. Myosin heavy-chain mutations that disrupt Caenorhabditis elegans thick filament assembly. Genes Dev. 1988;2:1307–1317. doi: 10.1101/gad.2.10.1307. [DOI] [PubMed] [Google Scholar]
- 29.Rogalski TM, Williams B, Mullen GP, Moerman DG. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev. 1993;139:159–169. doi: 10.1101/gad.7.8.1471. [DOI] [PubMed] [Google Scholar]
- 30.Lackner M, Nurrish S, Kaplan J. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 31.Gems D, Sutton AJ, Sundermeyer ML, et al. Two pleiotropic classes of daf-2 mutations affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]