Abstract
Background
Accumulating evidence indicates that cAMP-dependent protein kinase A (PKA) is involved in the neurobiological responses to ethanol. Previous reports indicate that mice lacking the RIIβ subunit of PKA (RIIβ−/−) voluntarily consume more ethanol than wild-type controls (RIIβ+/+) using two-bottle testing procedures. While such procedures primarily measure consummatory behavior, operant self-administration procedures allow analysis of consummatory as well as appetitive or “ethanol-seeking” behavior (i.e., lever pressing is required to gain access to the ethanol solution). Therefore, we determined if the high ethanol consumption characteristic of RIIβ−/− mice would be complimented by increased appetitive ethanol-seeking behavior in an operant paradigm.
Methods
RIIβ−/− (n=8) and RIIβ+/+ (n=8) mice were initially sucrose-faded until they were lever responding for non-sweetened ethanol (10, 14, and 18%). Following the self-administration testing, RIIβ+/+ and RIIβ−/− mice were given access to 2 bottles, one containing water and the other ethanol to replicate the voluntary ethanol drinking data previously from our laboratory. Finally, immediately after voluntary consumption all mice were again tested for self-administration of 10% ethanol. Alterations in the reinforcement schedule were also explored as RIIβ+/+ and RIIβ−/− mice were tested for self-administration of 10% ethanol at FR-3 and FR-5 schedules.
Results
The RIIβ−/− mice displayed lower operant responding for ethanol and food reinforcement compared to RIIβ+/+ controls. However, this effect was driven by a significant increase in lever responses made by female RIIβ+/+ mice. When the excessive lever responses of the female RIIβ+/+ mice are accounted for, the RIIβ −/− mice show ethanol lever responses comparable to controls. Following operant self-administration testing, RIIβ−/− mice of both sexes consumed more ethanol solution compared to RIIβ+/+ mice during two-bottle testing.
Conclusions
Increased ingestion of ethanol by RIIβ−/− mice is likely the result of altered PKA activity within neuronal pathways that control ethanol consummatory behaviors. On the other hand, the RIIβ subunit of PKA appears to not play a critical role in neuronal pathways that regulate appetitive behaviors directed at obtaining ethanol. Finally, increased operant self-administration of food and ethanol by female wild-type mice was absent in female RIIβ−/− mice, suggesting that normal PKA signaling may be part of a general, and sex-dependent, mechanism involved with reinforcement-seeking behavior.
Keywords: ethanol, protein kinase A, C57BL/6J mice, consummatory, appetitive
INTRODUCTION
Activation of G-protein-coupled receptors is a common mechanism by which a variety of neurotransmitters, neuromodulators, and hormones transduce their signal into neurons. The activation of G protein-coupled receptors can inhibit or enhance adenosine monophosphate (cAMP) levels through adenylyl cyclase activity, which in turn directly alters c-AMP dependent protein kinase A (PKA) activity. PKA is a holoenzyme comprised of a regulatory (R) subunit homodimer and two catalytic (C) subunits. Mice possess four R genes (RIα, RIβ, RIIα, RIIβ) and two C genes (Cα and Cβ) which are expressed in tissue-specific patterns (McKnight, 1991).
The PKA system has been implicated in a variety of behaviors including learning and memory (Connolly et al., 1996; Goodwin et al., 1997; Kandel and Schwartz, 1982; Skoulakis et al., 1993; Villacres et al., 1998; Wong et al., 1999), drug tolerance and dependence (Andretic et al., 1999; Moore et al., 1998; Self and Nestler, 1995; Yoshimoto et al., 1992), and sensitization in nociception (Taiwo and Levine, 1991). In addition, evidence is accumulating for a role for PKA in modulating the acute and chronic cellular responses to ethanol. In vitro experiments have found that ethanol treatment (200 mM) of NG108-15 cells translocates the Cα subunit of PKA from the Golgi area to the nucleus (Dohrman et al., 1996), and long-term ethanol exposure (12-hr) translocates both the Cα and RIIβ subunits to the nucleus (Dohrman et al., 2002). Additionally, chronic ethanol administration significantly increases cAMP and PKA levels in the nucleus accumbens of rats (Ortiz et al., 1995). These observations suggest that PKA signaling modulates neurobiological responses associated with ethanol exposure.
Recent pharmacological and genetic studies have provided additional evidence for the importance of the cAMP/PKA system in regulating the neurobiological responses to ethanol. Pharmacological approaches demonstrate that PKA inhibition (using micro-infusions of the PKA inhibitor Rp-cAMPS) in the central and basolateral nucleus of the amygdala reduce CREB phosphorylation, with PKA reductions specifically in the central nucleus of the amygdala associated with increased ethanol consumption in rats (Pandey et al., 2003). Deletion of the regulatory type II PKA subunit (PKA-RII) gene in Drosophila melanogaster results in reduced sensitivity to the sedative properties of ethanol (Park et al., 2000). Similarly, inhibition of PKA activity in specific brain regions of Drosophila reduces ethanol sensitivity (Rodan et al., 2002). Consistent with Drosophila data, we have found that mice lacking production of the RIIβ subunit of PKA (RIIβ−/−) are less sensitive to the sedative effects of ethanol and voluntarily consume higher amounts of 6%, 10%, and 20% ethanol (v/v) when compared to wild-type (RIIβ+/+) control mice (Thiele et al., 2000). More recently, we have replicated and extended these findings by showing that basal anxiety-like behavior in RIIβ−/− mice does not correlate with high ethanol drinking (Fee et al., 2004). Taken together, these pharmacological and genetic studies suggest that blunted PKA activity is associated with reduced ethanol sensitivity and increased ethanol consumption. On the other hand, there is also evidence that reduced PKA activity increases ethanol sensitivity and inhibits ethanol drinking. For example, enhanced sensitivity to the motor impairment effects of ethanol is seen in genetically modified Drosophila that lack a neuropeptide that activates the cAMP pathway (Moore et al., 1998), and genetically modified mice that have reduced cAMP/PKA activity consume less ethanol but are more sensitive to ethanol-induced sedation compared to controls (Wand et al., 2001). Regardless of these inconsistencies, these data provide compelling evidence that PKA signaling modulates neurobiological responses to ethanol and ethanol consumption.
Ingestive behavior (i.e., feeding and drinking) is complex and may be divided into at least two components. Appetitive behaviors are those used to locate and acquire stimuli (e.g., food and water) in the environment while consummatory behaviors are those used to directly consume the stimuli once they have been obtained (Samson & Hodge, 1995). Previous experiments evaluating ethanol drinking in RIIβ−/− mice have primarily measured consummatory behavior, that is, the mice engaged in simple consumption of the ethanol solution from a sipper tube that extended into the cage. Operant procedures allow for the analysis of consummatory behavior as well as appetitive or “seeking” responses (i.e., lever pressing is required to gain access to the ethanol solution). The distinction between appetitive and consummatory behavior has a useful clinical application. Some human alcoholics report a subjective “craving” component toward alcohol (Jellinek, 1955) which may ultimately drive intentional behaviors involved in obtaining access to alcohol (i.e., the appetitive component). Additionally, alcoholism is thought to entail loss of control over ethanol drinking (Marlatt and George, 1984) once consumption has been initiated (i.e., the consummatory component). Furthermore, drugs acting on dopamine or glutamate receptors have been found to uniquely influence consummatory or appetitive behaviors associated with ethanol ingestion (Czachowski et al., 2001a; Czachowski et al., 2001b; Czachowski et al., 2002). Because different neuronal pathways appear to modulate appetitive versus consummatory behaviors during ethanol self-administration, we determined if the high ethanol drinking (consummatory behavior) characteristic of mice lacking the RIIβ subunit of PKA was associated with increased operant self-administration of ethanol. Such results would suggest that the RIIβ subunit of PKA modulates appetitive, in addition to consummatory, behaviors associated with ethanol ingestion.
The present experiment was divided into three phases. Phase 1: Operant responding for ethanol in RIIβ+/+ and RIIβ−/− mice. Phase 2: Homecage ethanol consumption in RIIβ+/+ and RIIβ−/− mice. Phase 3: Operant responding for ethanol in RIIβ+/+ and RIIβ−/− mice following homecage ethanol access. We predicted that RIIβ−/− mice would lever press significantly more for ethanol compared RIIβ+/+ mice. Further, we expected to replicate and extend the high ethanol homecage drinking in RIIβ−/− mice by examining homecage ethanol drinking immediately following operant self-administration training. Because differences in voluntary ethanol consumption exist between male and female C57BL/6J mice (Middaugh and Kelley, 1999a; Middaugh et al., 1999b), both sexes of RIIβ+/+ and RIIβ−/− mice were used. Further, this allowed us to examine the possibility that deletion of the RIIβ subunit of PKA interacts with sex on measures of ethanol consumption and operant self-administration.
MATERIALS AND METHODS
Animals
Disruption of the RIIβ gene by homologous recombination in embryonic stem cells from 129 SvJ mice has been reported elsewhere (Brandon et al., 1998). Chimeras were bred with C57BL/6J mice to obtain heterozygotes (50% 129 SvJ x 50% C57BL/6J). Heterozygote mice were then backcrossed with C57BL/6J mice for eight generations to yield RIIβ+/− mice on an approximately 100% C57BL/6J genetic background. Finally, non-littermate RIIβ+/− mice were bred to yield RIIβ−/−and RIIβ+/+ F2 littermate mice that were used in this experiment. Mouse genotyping was performed using polymerase chain reaction procedures as published elsewhere (Thiele et al., 2000). Mice (RIIβ+/+, n=8; RIIβ−/−, n=8) were single-housed in standard polypropylene cages with standard rodent chow (Teklad, Madison, WI) and water continuously available. Mice were approximately 3 months of age and weighed between 17–25 g at the start of the experiment. Equal numbers of male and female mice from each genotype were used. The colony room was kept on a 12:12 light-dark cycle (lights on at 06:00 hr) with an ambient room temperature of 21 ºC. All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council, 1985) and the Institutional Animal Care and Use Committee (IACUC) at the University of North Carolina at Chapel Hill.
Operant Self-Administration Apparatus
The self-administration was conducted with eight modular mouse operant chambers (Med Associates, St. Albans, VT) with dimensions of 21.6 x 17.8 x 12.7 cm and a stainless steel grid floor. All chambers were housed in a sound-attenuating shell with a ventilation fan. A liquid receptacle with a nose-poke sensor was located in the center of the right wall with a stainless steel response lever to the right of the receptacle. Liquid solutions (sucrose and ethanol) were infused using 10 ml plastic syringes which were mounted on a programmable pump (PHM-100, 3.33 rpm). The pump delivered 0.01 ml of solution per activation. A food pellet receptacle was located in the center of the left wall with a stainless steel response lever to the right of the receptacle. Responses on the food lever delivered a 20 mg food pellet (Research Diets Inc., New Brunswick, NJ). A yellow stimulus light was illuminated directly above each response lever when a bar press was performed. Finally, a 150 ml plastic water bottle fitted with a stainless steel sipper tube was located to the left of the food pellet receptacle. A house light inside the operant chambers was turned on for the first and last 2 hours of the 16 hr operant session to correspond to the light phase in the mouse colony room.
Data recorded during each operant session included: sucrose/ethanol responses, sucrose/ethanol reinforcers, food responses, food reinforcers, ethanol (g/kg) and water (ml) consumption. The operant chambers were interfaced to an IBM computer and all data were automatically recorded using Med Associates software (MED-PC for Windows®, Version IV).
Phase 1: Operant Responding for Ethanol in RIIβ+/+ and RIIβ−/− mice
Mice were given a 1 hr habituation session where they were placed inside the operant chambers to acclimate to the test environment. Response levers were not active during the initial habituation session. The next day, mice were placed in the chambers for a 16 hr overnight training session (16:00-08:00 hr) with 10% sucrose (w/v). The sucrose and food response levers were set on a FR-1 schedule of reinforcement. Once stable sucrose administration was achieved, mice were trained to administer ethanol using a modified sucrose substitution procedure (Samson, 1986; Schroeder et al., 2003). All sucrose solutions were prepared as weight/volume and all ethanol solutions were volume/volume. Ethanol (2, 4, 8, and 10%) was added to 10% sucrose with a total of 4 days at each increasing concentration. Sucrose (10, 5, and 2%) was then faded out of the solution with 4 days at each decreasing concentration. After the fading procedure, mice were allowed to respond for 10% (3 days), 14% (3 days), and 18% (6 days) ethanol (v/v). Mice were tested for 6 days at 18% ethanol to test whether extended training was needed to observe genotype differences in ethanol responding. Mouse body weights were recorded immediately prior to the overnight training sessions to calculate ethanol consumed in grams per kilogram bodyweight.
Phase 2: Homecage Ethanol Consumption in RIIβ+/+ and RIIβ−/− mice
Following the final operant self-administration session with 18% ethanol (v/v), RIIβ+/+ and RIIβ−/− mice from Phase 1 were tested for voluntary ethanol consumption in a homecage two-bottle choice procedure. All mice were given 2 bottles on their homecage, one containing tap water and the other ethanol with food available ad libitum. An empty cage was used for the placement of dummy bottles (one ethanol and one water) and fluid lost from each of these bottles was subtracted off the consumption totals as a control for fluid spillage. The concentrations of ethanol (v/v) were gradually increased every 4 days. The ethanol concentrations increased in the following manner: 3%, 5%, 8%, 10%, 13%, 15%, 18%, and 20% and is a typical ethanol-ramping procedure used in our laboratory. The positions of the bottles were alternated every 2 days to control for position preferences. Body weights (g) were recorded on days when the ethanol concentrations were changed to minimize handling-induced stress. To account for differences in mouse body size, the amount of ethanol consumed was calculated in grams per kilogram body weight. To assess ethanol preference for each genotype, an ethanol preference ratio for each ethanol concentration was calculated by dividing the total ethanol intake by the total fluid consumed (water plus ethanol intake). The amount of water consumed (ml/kg body weight) during the ethanol phases was also recorded for all mice.
Phase 3: 10% Ethanol Operant Responding in RIIβ+/+ and RIIβ−/− mice following Homecage Ethanol Drinking: FR-3 and FR-5 Schedule Testing
After the two-bottle choice procedure, RIIβ+/+ and RIIβ−/− mice used in Phases 1 and 2 were again tested for operant responding to 10% ethanol (v/v) for 2 sessions (16 hr) using an FR-1 schedule of reinforcement. All mice were further tested for 10% ethanol responding by employing FR-3 and FR-5 response schedules (2 sessions per schedule). Testing FR-3 and FR-5 ethanol responding was done to determine if enhanced ethanol lever pressing in RIIβ−/− mice could be revealed by increasing the lever response requirement. The food levers were maintained on an FR-1 reinforcement schedule and water bottles were placed inside the chambers during all test sessions.
Statistical Analysis
SPSS (Version 12, Chicago, IL) was used to analyze the data from this experiment. All data in this report are presented as means ± SEM. The data were subjected to analysis of variance (ANOVA) with repeated measures. Genotype served as a between group factor. Because male and female mice were used in this experiment, sex was also included as a between group factor in all analyses. Session served as a repeated within subject factor. For all figures, data was collapsed across sessions for ethanol responding, food responding, and water consumption as the session factor did not significantly interact with genotype. Also, the time factor did not interact significantly with any of the factors tested and data are collapsed across the 16 hour test sessions. For the operant experiments, the mean number of lever responses was used as data for the analyses and mean g/kg/day or ml/kg/day data was used in the analyses of ethanol and water homecage drinking, respectively. Planned comparisons were evaluated using t-tests (Winer et al., 1991). The level of statistical significance for all analyses was set at p<0.05. As a standardized measure of effect size (i.e., the magnitude of the experimental treatment effect independent of sample size), partial eta squared (ηp2) is provided in the results section for each significant outcome (Kirk, 1982; Pierce et al., 2004). A large treatment effect is indicative of an effect size 0.15 (Cohen, 1977). The effect size value can thus be used to evaluate the strength of the association between the experimental manipulation (deletion of the RIIβ subunit) and behavioral changes in voluntary drinking and operant self-administration.
RESULTS
10% Sucrose and Sucrose/Ethanol Solutions
For brevity, the data for 10% sucrose and the various sucrose/ethanol fading solutions is not presented. The general pattern of results for the sucrose/ethanol fading solutions was similar to that found for pure 10% sucrose, that is, male and female RIIβ−/− mice generally did not show differences in the sucrose/ethanol solutions and food responding compared to male RIIβ +/+ controls. However, excessive lever responding for 10% sucrose and various sucrose/ethanol mixtures by female RIIβ +/+ mice were consistently observed above all other groups.
Phase 1: 10% Ethanol Lever Responding
Self-administration of 10% ethanol (v/v) in RIIβ +/+ and RIIβ−/− mice are presented in Fig. 1a. RIIβ−/− mice made significantly fewer lever presses for 10% ethanol compared to RIIβ +/+ controls (F1,12 = 12.23, p=0.004, ηp2= 0.51). In addition, sex (F1,12 = 13.89, p=0.003, ηp2= 0.54) and the genotype x sex interaction (F1,12 = 20.15, p=0.01, ηp2= 0.63) were statistically significant. Planned comparisons confirmed that female RIIβ +/+ mice responded more for 10% ethanol than all other mice (male RIIβ +/+ mice and male and female RIIβ−/− mice).
Figure 1.

Operant self-administration (FR-1) of 10% ethanol (v/v), food, and water in RIIβ +/+ and RIIβ−/− mice (a–c), for all figures male mice appear as (▪) and female mice (□). Operant self-administration of 14% ethanol (v/v), food, and water in RIIβ +/+ and RIIβ−/− mice (D–F). Operant self-administration of 18% ethanol (v/v), food, and water in RIIβ +/+ and RIIβ−/− mice (g–i). All data are mean ± SEM. * p<0.05, female RIIβ +/+ mice relative to all other groups.
Similarly, analysis of the food response data (Fig. 1b) indicated that RIIβ−/− mice had significantly fewer lever responses for food compared to RIIβ +/+ controls (F1,12 = 27.17, p<0.05, ηp2= 0.69), an effect explained by female RIIβ+/+ mice responding more for food than all other mice (F1,12 = 28.18, p<.05, ηp2= 0.70). No other factors examined for food responding achieved statistical significance.
Calculations of the average amount of 10% ethanol consumed during the 16 hr operant sessions by RIIβ +/+ and RIIβ−/− mice are shown in Table 1. RIIβ−/− mice drank significantly less ethanol (g/kg) than RIIβ +/+ mice (F1,12 = 11.18, p=0.006, ηp2= 0.48). The significant genotype effect must be qualified as a significant genotype x sex interaction revealed that female RIIβ +/+ mice consumed more ethanol than male RIIβ +/+ mice and male and female RIIβ−/− mice (F1,12 = 22.34, p<.05, ηp2= 0.65). There were no significant genotype differences in water consumption during 10% ethanol testing (Fig 1c).
Table 1.
Mean Ethanol Consumption (g/kg) in Male and Female RIIβ +/+ and RIIβ−/− mice During Ethanol Self-Administration Testing (16 Hr).
| Sex | Genotype | 1 0% EtOH | 1 4% EtOH | 1 8% EtOH | + 1 0% EtOH |
|---|---|---|---|---|---|
| Male | RIIβ +/+ | 5.7 ± 0.4 | 6.3 ± 0.9 | 9.7 ± 2.0 | 1 2.2 ± 1.5 |
| Male | RIIβ−/− | 8.5 ± 0.6 | 1 0.5 ± 1.1 | 1 3.7 ± 1.4 | 1 0.9 ± 1.8 |
| Female | RIIβ +/+ | *24.3 ± 2.3 | *29.2 ± 3.4 | *32.2 ± 2.9 | 28.6 ± 4.4 |
| Female | RIIβ −/− | 8.0 ± 1.3 | 8.5 ± 1.4 | 1 5.2 ± 1.6 | 1 5.0 ± 4.5 |
Significantly different (p<0.05) from male RIIβ +/+ mice and male and female RIIβ−/− mice.
Self-administration testing following homecage voluntary ethanol testing.
14% Ethanol Lever Responding
Lever responses for 14% ethanol (v/v) performed by RIIβ +/+ and RIIβ−/− mice are shown in Fig 1d. Consistent with the 10% ethanol responding data, RIIβ−/− mice also made significantly fewer lever presses for 14% ethanol compared to RIIβ +/+ controls (F1,12 = 10.08, p=0.008, ηp2 = 0.46). Sex (F1,12 = 11.08, p=0.006) and the genotype x sex interaction (F1,12 = 20.97, p=0.01, ηp2= 0.64) were significant and planned comparisons indicated that female RIIβ+/+ mice responded more for 14% ethanol than male RIIβ +/+ mice and male and female RIIβ−/− mice.
During 14% ethanol testing, RIIβ−/− mice performed significantly fewer lever presses for food (Fig. 1e) compared to RIIβ +/+ controls (F1,12 = 9.11, p=0.011, ηp2= 0.43). Further, the sex (F1,12 = 13.25, p=0.003, ηp2= 0.53) and genotype x sex interaction (F1,12 = 8.03, p=0.015, ηp2 = 0.40) achieved statistical significance with planned contrasts showing that female RIIβ +/+ mice responded more for food than male RIIβ +/+ mice and male and female RIIβ−/− mice. No significant genotype differences were detected for water consumption during 14% ethanol testing (Fig 1f).
RIIβ−/− mice drank significantly less 14% ethanol on average over the 16 hr operant test session (F1,12 = 7.60, p=0.017, ηp2= 0.39) (see Table 1). Again, significantly lower ethanol consumption in the knockout mice is explained by an excessive amount of ethanol lever responding in female RIIβ +/+ mice above male RIIβ +/+ mice and male and female RIIβ−/− mice (F1,12 = 17.31, p=0.001, ηp2= 0.59).
18% Ethanol Lever Responding
Lever responses made by RIIβ +/+ and RIIβ−/− mice for 18% ethanol (v/v) are presented in Fig. 1g. A significant reduction in lever pressing for 18% ethanol was found in RIIβ−/− mice when compared to RIIβ +/+ controls (F1,12 = 6.70, p=0.024, ηp2= 0.36). Additional analyses revealed a significant main effect of sex (F1,12 = 17.54, p=0.001, ηp2= 0.59) and genotype x sex interaction (F1,12 = 13.92, p=0.003, ηp2= 0.54). Further comparisons supported that female RIIβ+/+ mice performed significantly more lever presses for 18% ethanol compared to male RIIβ+/+ mice and male and female RIIβ−/− mice.
Lever responding of RIIβ +/+ and RIIβ−/− mice for food pellets during 18% ethanol testing is displayed in Fig. 1h. RIIβ−/− mice responded less for food compared to RIIβ +/+ mice (F1,12 = 8.00, p=0.015, ηp2= 0.40) and female RIIβ +/+ mice made significantly more lever responses compared to male RIIβ +/+ mice and male and female RIIβ−/− mice (F1,12 = 6.10, p=0.029, ηp2= 0.34). Analysis of the water consumption data showed that there were no significant differences between RIIβ +/+ and RIIβ−/− mice during 18% ethanol testing (Fig. 1i).
A significant genotype x sex interaction was detected (see Table 1) for the consumption of 18% ethanol (g/kg) as female RIIβ +/+ mice drank significantly more ethanol than male RIIβ +/+ mice and male and female RIIβ−/− mice (F1,12 = 8.32, p=0.014, ηp2= 0.41).
Phase 2: Homecage Ethanol Consumption
Homecage ethanol intake in male and female RIIβ +/+ and RIIβ−/− mice is displayed in Fig. 2a. RIIβ−/− mice drank significantly more ethanol compared to RIIβ +/+ controls (F1,12 = 12.58, p=0.004, ηp2= 0.51). This effect is in contrast to the data observed in the ethanol self-administration paradigm, where the RIIβ−/− mice consistently did not respond more for 10%, 14%, and 18% ethanol over controls. Planned t-tests showed that RIIβ−/− mice drank significantly more 3%, 5%, 15%, 18%, and 20% ethanol (v/v) in comparison to RIIβ +/+ controls. Further, RIIβ−/− mice displayed a significant ethanol preference over RIIβ +/+ mice (F1,12 = 5.98, p=0.031, ηp2= 0.33) at 3%, 5%, 15%, 18%, and 20% ethanol (see Fig. 2b). Importantly, unlike operant self-administration of ethanol, sex by genotype interaction was not statistically significant in the analysis of data obtained from voluntary ethanol drinking.
Figure 2.

Homecage two-bottle voluntary ethanol consumption (a), ethanol preference (b), and water consumption (c) in male and female RIIβ +/+ and RIIβ−/− mice. Male RIIβ +/+ mice appear as (•) and female RIIβ +/+ mice (▪). Male RIIβ−/− mice appear as (○) and female RIIβ−/− mice (□). All data are mean ± SEM. * p<0.05, overall significant genotype effect (RIIβ−/− mice drank more ethanol compared to RIIβ +/+ mice).
Homecage Water Consumption
Voluntary homecage water drinking across all phases of ethanol testing are shown in Fig. 2c. During the experiment, water consumption significantly increased for both genotypes (F7,84 = 47.59, p<0.05, ηp2= 0.80). Planned comparisons revealed that RIIβ+/+ mice consumed more water than RIIβ−/− mice during the 5% and 15% ethanol test phases. All other factors were not statistically significant.
Phase 3: 10% Ethanol Operant Responding Following Homecage Ethanol Drinking: FR-3 and FR-5 Schedule Testing
Data from 10% ethanol responding (FR-1 schedule) and calculated ethanol intake (g/kg) in RIIβ +/+ and RIIβ−/− mice following homecage ethanol drinking are presented in Fig. 3a and Table 1, respectively. Analysis of the data showed a non-significant genotype effect (F1,12 = 4.62, p=0.053, ηp2= 0.28). Although not statistically significant, the general trend in the data was that RIIβ−/− mice responded less for 10% ethanol after homecage ethanol drinking compared to controls (Mean lever responding: RIIβ−/− = 433.7, RIIβ +/+ = 767.8). No statistically significant effects were found for food and water consumption data during 10% ethanol responding.
Figure 3.
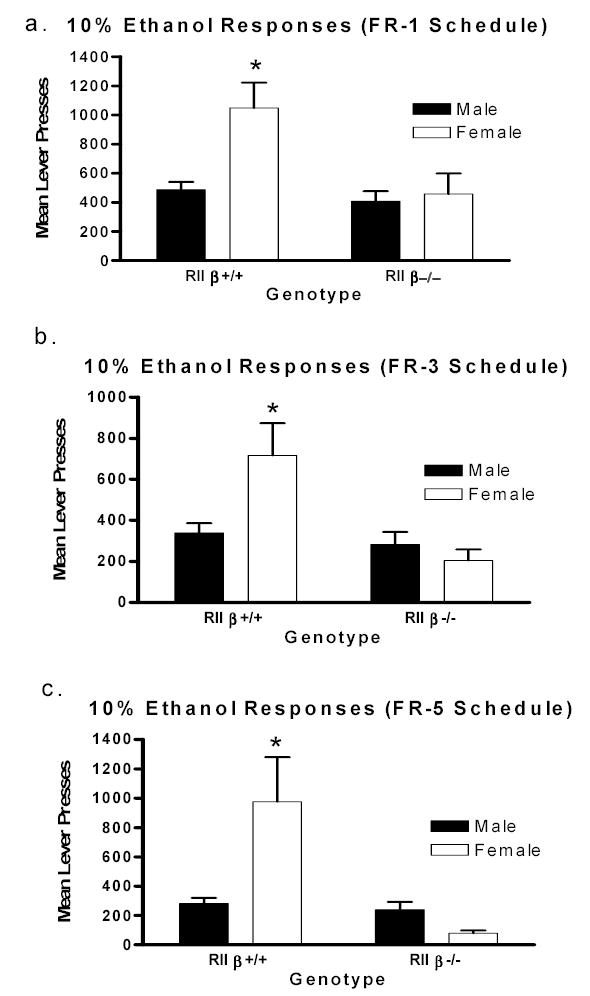
Operant self-administration of 10% ethanol (v/v), on FR-1, FR-3 and FR-5 reinforcement schedules in RIIβ +/+ and RIIβ−/− mice following homecage ethanol consumption (a–c), for all figures male mice appear as (▪) and female mice (□). All data are mean ± SEM. * p<0.05, female RIIβ +/+ mice relative to all other groups.
Ethanol responding data obtained from RIIβ +/+ and RIIβ −/− mice during FR-3 and FR-5 schedules of reinforcement are provide in Figs. 3b and 3c respectively. A significant genotype effect was found during FR-3 testing, where RIIβ−/− mice responded less for 10% ethanol compared to RIIβ +/+ mice (F1,11 = 7.42, p=0.02, ηp2= 0.40). The genotype and sex factors did not significantly interact during the FR-3 testing. There were no significant differences detected for any of the factors tested during the FR-5 sessions. Analysis of the water and food data produced no statistically significant outcomes for any of the variables under study during both FR-3 and FR-5 testing.
DISCUSSION
Contrary to expectations, we found that relative to RIIβ +/+ mice, the RIIβ −/− mice showed significantly lower operant self-administration ethanol, sucrose solution, and food. However, we are required to clarify this conclusion as significant genotype by sex interactions were consistently detected in the data analyses. Planned comparisons showed that female RIIβ +/+ mice engaged in significantly higher lever responding for ethanol compared to all other mice tested. Thus, while significant genotype effects were found that suggested RIIβ−/− mice responded and drank less ethanol than controls, these effects were driven by an unusually excessive amount of lever pressing for ethanol by female RIIβ +/+ mice. While female C57BL/6 mice have been reported to voluntarily drink and lever press more for ethanol than males (Middaugh and Kelley, 1999a; Middaugh et al., 1999b), our data indicate an excessive amount of responding that would result in uncommon levels of 10% ethanol ingestion ( 24.3 g/kg/16 hr). The average voluntary intake of 10% ethanol for female C57BL/6J mice were reported to be between 13 and 16 g/kg/24 hr (Middaugh et al., 1999b). Therefore, it is unlikely that the female RIIβ +/+ mice in the present report consumed all of the ethanol that was delivered. Importantly, the excessive responding pattern in female RIIβ +/+ mice was not selective to ethanol alone as this behavior was also observed in lever pressing for food pellets. Because female RIIβ +/+ mice did not consume all food that was delivered (as evidenced by an accumulation of food pellets at the bottom of the chamber), the act of lever pressing alone may have been reinforcing to female RIIβ +/+ mice. It should be noted that the elevated lever-pressing data (both food and ethanol) by female RIIβ +/+ mice is unlikely an artifact of small sample size as treatment effect size, estimated by the ηp2 statistic, was consistently high in all cases.
Because female RIIβ −/− mice did not show excessive responding for ethanol or food, the high level of operant lever pressing by wild-type female C57BL/6J mice is likely modulated by the RIIβ subunit of PKA. Interestingly, evidence suggests that estradiol stimulates intracellular PKA activity via a putative G protein-coupled receptor mechanism (Belcher et al., 2005; Shingo & Kito, 2005). In our experiment, the high operant responding observed in female RIIβ +/+ mice may be related to the excitatory effect estradiol has on PKA signaling. Theoretically, the effects of estradiol on PKA activity would be blunted in female RIIβ −/− mice, protecting against excessive operant responding. Thus, estradiol-induced PKA signaling in female mice may be an important mechanism for modulating reward-seeking behaviors that can be measured by operant self-administration experiments. However, it should be emphasized that if this mechanism exists, it is not specific to ethanol-seeking behaviors as female RIIβ +/+ mice also showed excessive responding for food reinforcement.
Another issue deserving attention is the rate of ethanol lever responding and ethanol intake during operant testing by male RIIβ +/+ and RIIβ −/− mice. For all ethanol concentrations tested on the FR-1 reinforcement schedule, there was a non-significant trend for the RIIβ −/− mice to lever respond more for ethanol compared to RIIβ +/+ mice. In addition, there were no statistically significant differences in ethanol consumption (across all concentrations tested) between male RIIβ +/+ and RIIβ −/− mice during operant self-administration (see Table 1). While only four animals per genotype were tested, statistical analyses showed that the power to detect significant genotype effects were always greater than 0.9. Power values of 0.8 or greater are considered desirable in behavioral research (Keppel, 1991). Despite the modest sample size, male and female RIIβ −/− mice consumed significantly more ethanol than RIIβ +/+ mice during two-bottle testing (see Fig 2a). Thus, a more conservative conclusion is that robust increases in ethanol drinking by RIIβ −/− mice are not associated with robust increases in operant self-administration of ethanol.
Samson and Chappell (2001) have recently developed an experimental procedure where rats are trained to lever-respond 30 times to gain 20 min access to a sipper tube containing ethanol. This paradigm has been called the “sipper-tube procedure” (Samson and Chappell, 2001) and is argued to distinctly separate appetitive and consummatory responses for ethanol. This argument is based on the fact that lever responding occurs without interference from the post-ingestive intoxicating effects of ethanol. Since each lever response was followed by an ethanol reward in the present study, one potential criticism is that lever responses may have been altered by the post-ingestive intoxicating effects of ethanol. However, RIIβ−/− mice are less sensitive to the sedative/hypnotic effects of ethanol (Fee et al., 2004; Thiele et al., 2000). Thus, based on the above logic RIIβ−/− mice should have showed greater lever responding relative to RIIβ +/+ mice, which they did not. It therefore seems unlikely that the absence of increased operant ethanol self-administration by the RIIβ−/− mice is related to the post-ingestive effects of ethanol.
Consistent with our previous findings, we report here that RIIβ −/− mice drink significantly more ethanol than RIIβ +/+ mice in a two-bottle paradigm. Plasma ethanol levels and consumption of non-alcohol tastants are not significantly different between RIIβ +/+ and RIIβ −/− mice (Fee et al., 2004; Thiele et al., 2000), results indicating that increased ethanol consumption by RIIβ−/− mice is not due to altered ethanol metabolism or taste preference. It is of interest to consider the possible mechanisms by which PKA signaling modulates ethanol consumption. Amygdalar infusion of a PKA inhibitor increases ethanol drinking and causes local reductions of neuropeptide Y (NPY) levels in rats. Elevated levels of ethanol drinking are rescued by amygdalar co-administration of NPY (Pandey et al., 2003). Similarly, it was recently observed that infusion of a PKA inhibitor into the shell of the nucleus accumbens increases ethanol drinking and reduces local NPY levels, and the effects of the PKA inhibitor on ethanol drinking are prevented by co-infusion of NPY (Misra and Pandey, 2005). Additionally, mutant mice lacking normal production of CREB protein have low central NPY expression and show increased ethanol drinking (Pandey et al., 2004). Because NPY has been implicated in modulating ethanol consumption (Thiele et al., 1998; 2004), and because RIIβ −/− mice show blunted PKA activity in the amygdala and nucleus accumbens, it is possible that increased ethanol drinking by RIIβ −/− mice is the result of low central NPY expression, although NPY levels in RIIβ −/− mice have yet to be determined.
Increased two-bottle ethanol consumption by RIIβ−/− mice is not associated with increased appetitive or “seeking” behavior directed at obtaining access to ethanol. It is important to note that overlapping ethanol concentrations were used during homecage bottle drinking procedures and operant testing, a strategy showing that the dissociation between ethanol consumption and operant self-administration of ethanol by RIIβ−/− mice occurs when similar concentrations of ethanol are presented. Additionally, increasing the ethanol response requirement to FR-3 and FR-5 schedules did not consistently show genotype effects (see Figs 3a & 3b). These data suggest that increased ethanol consumption by RIIβ−/− mice is the result of alterations primarily within neuronal pathways that modulate consummatory, but not appetitive behaviors. However, caution is necessary when drawing conclusions because interpretations of phenotypic data from studies with knockout mice are subject to several caveats (Gerlai, 2001). One concern is that constitutive deletion of a gene could lead to compensatory processes (up or down regulation of other genes) during development. In fact, the relative distribution of other regulatory subunits up-regulate in an apparent attempt to compensate for the loss of RIIβ in the present model (Amieux et al., 1997; Brandon et al., 1998). However, compensation is not complete in all brain regions as evidenced by reduced cAMP-stimulated PKA activity in the striatum, nucleus accumbens, amygdala, hippocampus and hypothalamus (Brandon et al., 1998; Thiele et al., 2000b). Nonetheless, it is possible that more complete compensatory alterations occurred in brain regions involved with appetitive behaviors, thus masking the potential contribution of the RIIβ subunit of PKA to such behaviors.
Several studies show a consistency between two-bottle testing and operant self-administration of ethanol, including studies with alcohol-preferring P rats (Rodd-Henricks et al., 2000) and various genetically altered mouse models (Olive et al., 2000; Risinger et al., 2000; Roberts et al., 2001; Roberts et al., 2000). However, a lack of a positive relationship between homecage ethanol consumption and operant self-administration has been reported in outbred rats (Koros et al., 1999), Lewis rats (Wilson et al., 1997), and AA and HAD rats (Files et al., 1998). A dissociation between the appetitive and consummatory components of ethanol ingestion has also been reported following pharmacological manipulations in rats (Czachowski et al., 2001a; Czachowski et al., 2001b; Czachowski et al., 2002). Antagonism of dopamine D2 receptors selectively reduces ethanol appetitive/seeking behavior while sparing ethanol consumption in rats (Czachowski et al., 2002). The nucleus accumbens (NAc) appears to be a critical neural region underpinning the dissociation of appetitive and consummatory responding as lever responding for ethanol is more sensitive to NAc infusions of raclopride (D2 antagonist) than is ethanol consumption (Czachowski et al., 2001a). On the other hand, consummatory behavior is more sensitive to alterations within the glutamatergic system. Czachowski et al. (2001) reported decreased ethanol consumption, but not appetitive lever responding for ethanol, by rats after treatment with acamprosate. This compound has been proposed to protect against hyperactive glutamatergic activity by several mechanisms including the regulation of NMDA receptor subunit composition (Rammes et al., 2001; Spanagel and Zieglgansberger, 1997), blockade of Ca2+ channels (Littleton, 1995), and antagonizing mGluR5 NMDA receptors (Backstrom et al., 2004; Harris et al., 2003; Harris et al., 2002). Because deletion of the RIIβ subunit of PKA and antagonism of glutamatergic activity with acamprosate are associated with alterations of consummatory behaviors, it is tempting to speculate that increased ethanol drinking by RIIβ−/− mice is related to altered PKA activity within neuronal circuits that involve glutamatergic signaling. Interestingly, PKA activity has been implicated in the function of mGluR5 (Domenici et al., 2004) and NMDA receptors (Maldve et al., 2002).
We did not assess blood ethanol levels in the present experiment in order to avoid possible effects of stressful procedures on ethanol self-administration. Thus we are unable to determine the blood ethanol levels that mice may have achieved during long-term (16-h or 24-h) ethanol access. Additional experiments are planned that will address more clearly the blood ethanol levels in RIIβ−/− and RIIβ+/+ mice during extended test sessions. Importantly, the amount of ethanol consumed during our 24-h measures is comparable to previously reported results. Here, wild-type C57BL/6J mice consumed approximately 10–15 g/kg/day of ethanol during two-bottle testing with 10% ethanol, and RIIβ−/− mice consumed approximately 15 g/kg/day of ethanol through concentrations of up to 18%. Male C57BL/6J mice have been reported to drink between 8–15 g/kg/day of 10% ethanol (Crabbe et al., 1999; Belknap et al., 1993), and we have previously reported similar amounts of ethanol consumption by RIIβ−/− mice (Thiele et al., 2000; Fee et al., 2004).
In summary, mutant mice lacking the RIIβ subunit of PKA consume more ethanol relative to wild-type mice in a homecage two-bottle procedure which is not associated with increased operant self-administration of ethanol. These findings indicate that increased ingestion of ethanol by RIIβ−/− mice is likely the result of altered PKA activity within neuronal pathways that control ethanol consummatory behaviors. On the other hand, the RIIβ subunit of PKA appears to not play a critical role in neuronal pathways that regulate appetitive behaviors directed at obtaining ethanol. Future studies will focus on the specific brain regions and neurotransmitter mechanisms by which the RIIβ subunit of PKA modulates ethanol consummatory behaviors. Finally, increased operant self-administration of food and ethanol by female wild-type mice was absent in female RIIβ−/− mice, suggesting that normal PKA signaling may be part of a general, and sex-dependent, mechanism involved with reinforcement-seeking behavior.
Acknowledgments
This work was supported by NIAAA grants AA13573, AA011605, AA14949, AA07573, AA015148, AA015878. We thank Ms. Lorraine Ko for her expert assistance in genotyping and maintaining mouse colonies, and Drs. Clyde Hodge and Joyce Besheer for their help with programming the operant software.
References
- Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, Idzerda RL, McKnight GS. Compensatory regulation of RIalpha protein levels in protein kinase. A mutant J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–8. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal-regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein and protein kinase A dependent mechanism and intracellular activation of protein phosphate 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998;18:3639–49. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1977) Statistical power analysis for the behavioral sciences, 2nd ed. Lawrence Erlbaum Associates, New York, NY.
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, O'Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–7. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001a;25:1431–40. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcohol Clin Exp Res. 2001b;25:344–50. [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcoholism Clinical & Experimental Research. 1999;23:1580–1586. [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Dohrman DP, Chen HM, Gordon AS, Diamond I. Ethanol-induced translocation of protein kinase A occurs in two phases: control by different molecular mechanisms. Alcohol Clin Exp Res. 2002;26:407–15. [PubMed] [Google Scholar]
- Dohrman DP, Diamond I, Gordon AS. Ethanol causes translocation of cAMP-dependent protein kinase catalytic subunit to the nucleus. Proc Natl Acad Sci U S A. 1996;93:10217–21. doi: 10.1073/pnas.93.19.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici MR, Pepponi R, Martire A, Tebano MT, Potenza RL, Popoli P. Permissive role of adenosine A2A receptors on metabotropic glutamate receptor 5 (mGluR5)-mediated effects in the striatum. J Neurochem. 2004;90:1276–9. doi: 10.1111/j.1471-4159.2004.02607.x. [DOI] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–68. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Denning CE, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. II. Operant self-administration in a continuous-access situation. Alcohol Clin Exp Res. 1998;22:2147–58. [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: Is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–81. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Goodwin SF, Del Vecchio M, Velinzon K, Hogel C, Russell SR, Tully T, Kaiser K. Defective learning in mutants of the Drosophila gene for a regulatory subunit of cAMP-dependent protein kinase. J Neurosci. 1997;17:8817–27. doi: 10.1523/JNEUROSCI.17-22-08817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart J, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-apartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, Fu MC, Hart SR, Pedigo NW, Littleton JM. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res. 2002;26:1779–1793. doi: 10.1097/01.ALC.0000042011.99580.98. [DOI] [PubMed] [Google Scholar]
- Jellinek EM. The craving for alcohol. Q J Stud Alcohol. 1955;16:35–8. [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–43. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Keppel G (1991) Design and analysis: A research's handbook, 3rd ed. Prentice Hall, Upper Saddle River, NJ.
- Kirk RE (1982) Experimental design: Procedures for the behavioral sciences, 3rd ed. Wadsworth Publishing, Pacific Grove, CA.
- Koros E, Kostowski W, Bienkowski P. Operant responding for ethanol in rats with a long-term history of free-choice ethanol drinking. Alcohol Alcohol. 1999;34:685–9. doi: 10.1093/alcalc/34.5.685. [DOI] [PubMed] [Google Scholar]
- Littleton J. Acamprosate in alcohol dependence: how does it work? Addiction. 1995;90:1179–88. doi: 10.1046/j.1360-0443.1995.90911793.x. [DOI] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippman MJ, Snyder GL, Feinberg AA, Leslie SW, Gonzales RA, Morrisett RA. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci. 2002;5:641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, George WH. Relapse prevention: introduction and overview of the model. British Journal of Addiction. 1984;79:261–273. doi: 10.1111/j.1360-0443.1984.tb00274.x. [DOI] [PubMed] [Google Scholar]
- McKnight GS. Cyclic AMP second messenger systems. Curr Opin Cell Biol. 1991;3:213–7. doi: 10.1016/0955-0674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999a;17:185–94. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999b;17:175–83. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Misra K, Pandey SC (2005) The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: A role in alcohol drinking but not anxiety-like behaviors in rats. Neuropsychopharmacology [DOI] [PubMed]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur J Neurosci. 2000;12:4131–40. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–98. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclid adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Sedore SA, Cronmiller C, Hirsh J. Type II cAMP-dependent protein kinase-deficient Drosophila are viable but show developmental, circadian, and drug response phenotypes. J Biol Chem. 2000;275:20588–96. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- Pierce CA, Block RA, Aguinis H. Cautionary note on reporting eta-squared values from multifactor ANOVA designs. Ed Psychol Meas. 2004;64:916–924. [Google Scholar]
- Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgansberger W, Schadrack J. The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacology. 2001;40:749–60. doi: 10.1016/s0028-3908(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK. Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl) 2000;152:343–50. doi: 10.1007/s002130000548. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, Koob GF. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol Clin Exp Res. 2001;25:1249–56. [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharm Exp Ther. 2000;293:1002–8. [PubMed] [Google Scholar]
- Rodan AR, Kiger JA, Jr, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcoholism Clinical & Experimental Research. 2000;24:747–753. [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, AL S, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology. 1999a;147:274–279. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Effects of alcohol deprivation on alcohol consumption using a sipper-tube procedure. Alcohol Clin Exp Res. 2001;25:680–6. [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 1999b;147:274–9. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–7. [PubMed] [Google Scholar]
- Samson HH, Hodge CW (1995) Neurobehavioral regulation of ethanol intake. In: Deitrich RA, Erwin VG (eds) Pharmacological effects of ethanol in the nervous system. CRC Press, Boca Raton, FL pp. 203–226.
- Schroeder JP, Iller KA, Hodge CW. Neuropeptide-Y Y5 receptors modulate the onset and maintenance of operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1912–1920. doi: 10.1097/01.ALC.0000098873.80433.BA. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Annu Rev Neurosci. 1995;18:463–95. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estradiol induces PKA activation through the putative membrane receptor in the living hippocampal neuron. J Neural Transm. 2005;112:1469–1473. doi: 10.1007/s00702-005-0371-8. [DOI] [PubMed] [Google Scholar]
- Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP- dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–5. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci (Online) 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Sparta DR, Hayes DM, Fee JR. A role for neuropepetide Y in neurobiological responses to ethanol and drugs of abuse. Neuropeptides. 2004;38:235–243. doi: 10.1016/j.npep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Villacres EC, Wong ST, Chavkin C, Storm DR. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J Neurosci. 1998;18:3186–94. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, Abel T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J Neurosci. 2001;21:5297–303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AW, Neill JC, Costall B. Strain differences in ethanol preference and reinforced behaviour: a comparison of two-bottle choice and operant self-administration paradigms. Behav Pharmacol. 1997;8:37–46. [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM (1991) Statistical Principles in Experimental Design, Third edn. McGraw-Hill, Inc., McGraw-Hill, Inc.
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–98. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]


