Abstract
The prefrontal cortex plays a fundamental role in the working memory functions of the cerebral cortex and is also the site of dysfunction in several neurological and psychiatric disorders, including schizophrenia. Prefrontal neurons are distinguished by their capacity for sustained activity during the time a stimulus is held in memory, and this mnemonic response is considered a substrate for a variety of cognitive functions. The neuronal basis for sustained activity in prefrontal neurons is unknown but is thought to involve recurrent excitation among pyramidal neurons. Recent studies in awake behaving monkeys have demonstrated that the persistent activity in prefrontal neurons is modulated by dopamine. To examine the mechanisms by which dopamine might modulate transmission in local excitatory circuits, we have performed dual whole-cell recordings in connected pyramidal cell pairs with and without dopamine application. We find that dopamine reduces the efficacy of unitary excitatory neurotransmission in layer V pyramidal cells by decreasing its reliability. These effects, which are reproduced by a selective D1 agonist and blocked by a D1 antagonist, are independent of voltage changes and are not attenuated by blockade of sodium and potassium channels in the postsynaptic neurons. We conclude that attenuation of local horizontal excitatory synaptic transmission in layer V pyramidal neurons by dopamine is through D1 actions at a presynaptic site.
The prefrontal cortex (PFC) plays a primary role in working memory, the mental operation critical for “online” processing of information (1, 2). Prefrontal neurons exhibit persistent neuronal firing throughout the delay interval intervening between a stimulus and a memory-guided response. Understanding the cellular and circuit basis of sustained neural activity maintained in the absence of a stimulus is considered an important quest in cognitive neuroscience (2). Previous studies in this laboratory have revealed a role for dopamine (DA) acting at D1 receptors in the modulation of a prefrontal neuron's excitatory response to its preferred stimulus (3). The sustained response of prefrontal neurons in the absence of a stimulus has generated considerable interest (4–11), but the precise pharmacological and circuit mechanisms underlying this activation remain unclear. As DA terminals and glutamatergic terminals form so-called synaptic triads with dendritic spines of pyramidal neurons (12, 13), we have proposed that DA directly modulates glutamate transmission at such triads, and thereby is a modulator of recurrent excitatory interactions between and among local pyramidal neurons that could promote persistent neural activity. To directly test this hypothesis, we have examined the synaptic effects of DA on recurrent excitatory transmission between pairs of pyramidal neurons by means of dual whole-cell patch clamp recording combined with DA application. In particular, we have examined DA's effects on unitary excitatory postsynaptic potentials (EPSPs), especially DA's modulation of glutamate release and whether pre- or postsynaptic mechanisms are involved. Our results indicate that DA directly reduces the probability of glutamate release in layer V pyramidal neurons by D1 actions at a presynaptic site. These results provide a possible neurophysiological basis for understanding the interaction of DA and glutamate in the pathophysiology and treatment of schizophrenia.
Methods
Slice Preparation and Physiological Recording.
A total of 22 ferrets ages 1.5–2 months were deeply anesthetized with sodium pentobarbital and decapitated, and their brains were immediately removed and placed in cold oxygenated Ringer's solution containing 124 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgSO4, 26 mM NaHCO3, and 10 mM dextrose, pH 7.4. The frontal lobe was separated, and horizontal sections 350–400 μm thick were cut through the medial PFC on a microslicer (Ted Pella, Redding, CA). The slices were incubated in artificial cerebrospinal fluid at 35°C for 1 h, then kept at room temperature until being transferred to the recording chamber at a temperature of 32–34°C. Neurons were visualized under infrared differential interference contrast videomicroscopy as described (14). Dual whole-cell patch clamp recordings were used for analysis of pyramid-to-pyramid monosynaptic connections. The resistance of patch pipettes was 8–12 MΩ, and pipettes were filled with a solution containing 114 mM K-gluconate, 6 mM KCl, 0.5 mM CaCl, 1 mM EGTA, 4 mM ATP-Mg, and 10 mM Hepes, pH 7.25. To block Na+ and K+ channels, 5 mM QX-314 and 125 mM Cs+-gluconate were added into the postsynaptic electrode solution in some experiments. To identify the morphology of neurons, pairs of neurons were injected with 0.2% Lucifer yellow (dipotassium salt; Sigma) and 0.5% biocytin (Molecular Probes), respectively.
Whole-cell current clamp recordings were made by using two Intracellular Electrometers IE-210 (Warner Instruments, Hamden, CT). The signals were amplified and filtered at 2 kHz in bridge-balance mode and were acquired on computer at sampling intervals of 3–33 μs by using DigiData 1200 interface and the pCLAMP 8.0 software program (Axon Instruments, Foster City, CA). To determine the pre- or postsynaptic mechanisms involved, paired-pulse stimulation was used, and the ratio of successive responses was measured. Synaptic potential amplitudes, along with the input resistance, were displayed online during the course of each experiment. Access resistance (10–25 mΩ) was monitored online at regular intervals from the setting on the bridge balance, and cells were rejected if this parameter changed by 15%.
Pharmacological Treatments.
DA (100 μM-10 mM) was pressure-ejected focally (1–2 psi pressure; 1 psi = 6.89 kPa) with 10 μM antioxidant ascorbic acid to protect the DA (Fig. 1A). Bicuculline methiodide (5–10 μM) was bath-applied to block the γ-aminobutyric acid type A (GABAA) receptors in some experiments. SKF 38393 (10–100 μM, Sigma) with 10 μM ascorbic acid was focally applied through puff pipettes, as was DA, whereas 10 μM quinpirole, 10 μM SCH 23390, 10 μM raclopride, and 10 μM CNQX (6-cyano-7-nitroquinoxaline-2,3-dion) were bath-applied (Sigma).
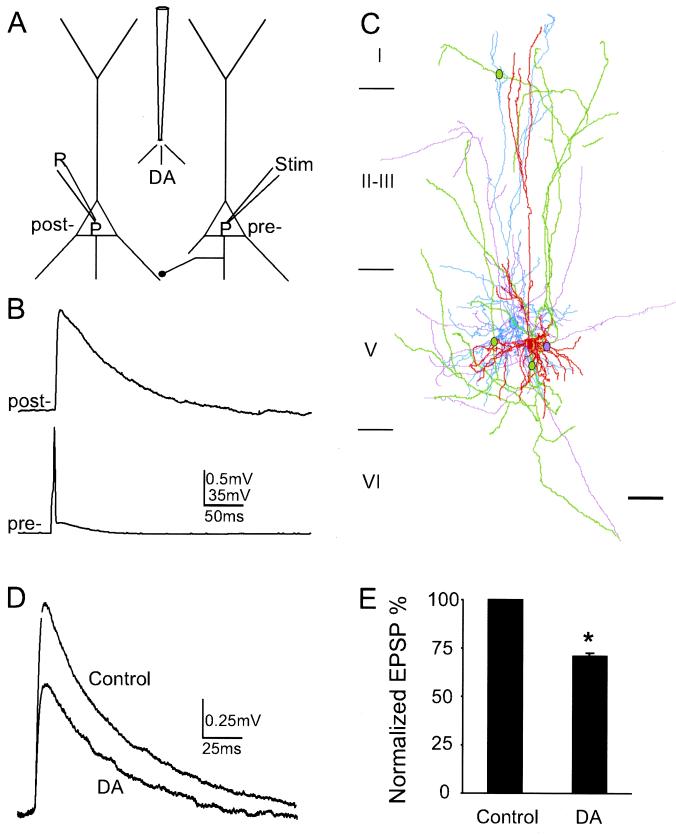
Figure 1.
DA reduces the unitary EPSP in layer V pyramidal neuronal pairs in the ferret PFC. (A) Schematic of experimental protocol used for dual whole-cell recording in current clamp mode and to show the method of DA application. The reconstructed neurons shown were simultaneously recorded and filled with Lucifer yellow and biocytin, respectively. (B) Example of a presynaptic action potential eliciting a unitary EPSP in a postsynaptic neuron. (C) Neurolucida reconstruction of a reciprocally connected pair of neurons. The dendritic arbor of one cell is drawn in light blue and its axonal arborization in purple, whereas the dendritic arbor of the other neuron is drawn in red and its axons in green. Three green dots indicate putative synaptic contacts established by green axons on light blue dendrites. The purple dot indicates the synaptic contact formed by purple axons on red dendrites. (Scale bar, 100 μm.) (D) The pressure application of 10 mM DA acutely depresses EPSP amplitude. (E) Histogram showing DA reduction of EPSP amplitude normalized to control values. EPSP amplitude is significantly reduced by 28.9 ± 0.29% from control levels (n = 16, P < 0.001).
Analysis and Statistics.
Whole-cell patch clamp typically resulted in low-noise recordings, enabling accurate detection of failures of the identified synaptic transmissions. Failure was defined as an amplitude below the limit of 1.6 × rms noise (15). The average rms noise was 0.13 ± 0.03 mV calculated from measurements of the baseline 50 ms before EPSP onset of 10 neuronal pairs. After failures were excluded, 20–100 individual sweeps were averaged, and the mean and standard deviations were determined. Measurements included latency, 20–80% rise time, and decay time constant (ι). To assess whether DA and/or its agonists and antagonists had significant effects, paired t tests were used to compare the average of all EPSPs in the 5-min baseline with the average of all other EPSP values during drug application. The data are presented as mean ± standard error. Slices were immediately fixed in cold 4% paraformaldehyde for 3–5 days after recording, and then were resectioned into 60-μm sections on a cryostat. The sections were reacted with 1% H2O2 for 4–6 h to block the endogenous peroxidase and then were placed in blocking serum with 0.5% Triton X-100 at 4°C for 12 h. They were then incubated in ABC (Vectastain, Vector Laboratories) for 4 h at room temperature and, after rinsing, transferred to a solution of anti-Lucifer yellow biotinylated rabbit IgG (Molecular Probes) for 48 h. Biocytin-labeled pyramids were developed as dark blue by using the Ni-diaminobenzidine chromogen. Lucifer yellow-labeled neurons were stained brown by using a plain diaminobenzidine reaction after additional ABC incubation. Sections were dehydrated and coverslipped with Permount (National Diagnostics). The cells were reconstructed under the ×63 oil lens plus ×2 magnification with neurolucida software (Microbrightfield, Foster, CA), and the reconstructed neurons were edited in CANVAS 5.0.2 (Deneba Systems, Miami). The presumed synaptic contacts were carefully identified and marked under the light microscope at a total magnification of ×1,250. All recorded neurons were morphologically confirmed pyramidal cells; four contacts were visualized in one fully reconstructed pair (Fig. 1C), and five appositions were observed in a second physiologically characterized pair. These numbers are in line with Markram et al. (15), who observed 4–8 contacts between pyramidal pairs.
Results
DA Depresses the Amplitude of EPSPs.
A total of 39 synaptically connected pairs of layer V pyramidal neurons were studied in ferret medial prefrontal cortical slices. Voltages were recorded in current clamp mode, and DA was pressure injected over proximal apical dendrites, soma, and basal dendrites of both cells (Fig. 1 A and C; see Methods for details). The average peak amplitude of unitary EPSPs evoked by a single presynaptic action potential in 39 pairs of neurons ranged from 0.30 to 2.01 mV with a mean of 0.8 ± 0.07 mV, and the mean failure rate was 29.2 ± 6.29% at a membrane potential of −63.7 ± 0.96 mV (Table 1 and Fig. 1B). The unitary EPSPs at this membrane potential are predominantly AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) mediated as they were almost completely blocked by the AMPA antagonist CNQX (10 μM; data not shown; ref. 15). Focal application of DA (pipette concentration of 100 μM-10 mM) reversibly reduced the amplitude of EPSPs by 28.9 ± 0.29% in 16 synaptic pairs tested (P < 0.001; Fig. 1 D and E) without significantly affecting input resistance as in agreement with previous studies (7, 16). Moreover, the depressive effects were even more obvious when failure sweeps were included in the analysis. In the synaptic pair shown in Fig. 1D, DA reduced the unitary EPSP amplitude by 39.8% (54.4% reduction if failures are included).
Table 1.
DA modulation on the unitary EPSPs between pyramidal neuronal connections
| Treatment | Amplitude, mV | Delay, ms | 20–80% rise time, ms | Decay (ι), ms | Failure rate, % |
|---|---|---|---|---|---|
| Control | 1.01 ± 0.11 | 1.13 ± 0.10 | 3.78 ± 0.26 | 81.17 ± 10.56 | 21.7 ± 5.51 |
| DA | 0.75 ± 0.08* | 1.29 ± 0.11† | 3.62 ± 0.25‡ | 80.58 ± 10.45§ | 46.6 ± 7.75* |
, P < 0.001; †, P = 0.098; ‡, P = 0.547; §, P = 0.956.
DA Reduces the Reliability of Synaptic Transmission via Presynaptic Mechanisms.
To determine whether the mechanism of DA-induced depression of excitatory transmission involves a reduction in the probability of neurotransmitter release in presynaptic terminals, a decrease in receptor sensitivity to neurotransmitter in postsynaptic neurons, or a combination of both, we examined the synaptic failure rate more closely and studied the paired-pulse ratios before and during DA application. Two lines of evidence suggest that the reduction of EPSP amplitudes by DA is based on a presynaptic mechanism. First, the failure rate, defined as failure percentage of all sweeps (14), increased an average of 25% from 21.7 ± 5.51% in the control condition to 46.6 ± 7.75% during DA application (Fig. 2A; n = 16, P < 0.001). Second, in addition to synaptic failure, we observed changes in the probability of release in response to a second stimulus (17, 18). Although both paired-pulse depression (n = 4) and facilitation (n = 4) were observed at 2- to 20-Hz stimulation, both were similarly altered by DA. Moreover, the paired-pulse ratio, defined as (EPSP2/EPSP1) × 100%, was significantly reduced from 97.5 ± 10.91% in control conditions to 72.2 ± 5.65% with DA application (Fig. 2B; n = 8, P < 0.05), decreasing about 25% at 10-Hz stimulation. This finding differs from the expected paired-pulse ratio when transmitter release is decreased (17, 18) but is consistent with the recent results of Behr et al. (19). These evidences suggest that DA might be activating a signal located in the axon terminals of presynaptic neurons to reduce the release probability of glutamate or the reliability of synaptic transmission or possibly might be activating GABAergic neurons to shunt the EPSP signal (9). We consider the latter possibility unlikely because EPSP amplitudes were unaffected by the application of 5–10 μM bicuculline, the GABAA receptor antagonist, and bicuculline had no effect on DA's depression of EPSPs. [Amplitudes of EPSPs were significantly reduced 31.4 ± 4.71% at DA alone (P < 0.05) and 38.1 ± 8.38% during coapplication of bicuculline and DA (P < 0.05; Fig. 2 C and D).]
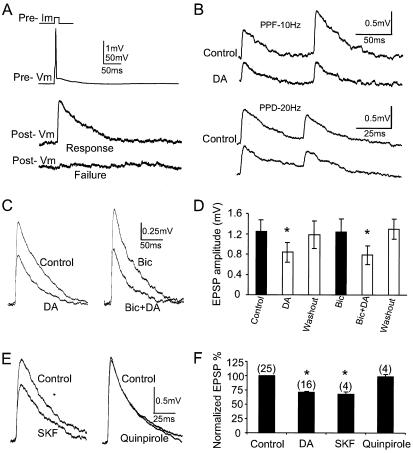
Figure 2.
The reliability of synaptic transmission is reduced via action at presynaptic D1 receptors. (A) Example of success and failure (lower trace) of unitary EPSPs evoked by a single presynaptic action potential (upper trace). DA significantly increased the failure rate up to 25%. (B) Examples of reduced EPSPs in both paired-pulse facilitation (PPF; pulse interval 100 ms, 10 Hz) and paired-pulse depression (PPD; pulse interval 50 ms, 20 Hz). The average paired-pulse ratios of EPSPs are 97.5 ± 10.91% in control and 72.2 ± 5.65% with DA application, respectively, a 25% reduction (pulse interval 100 ms; n = 8, P < 0.05). (C and D) DA modulation of EPSPs in the presence of GABAA antagonist bicuculline. Bic, bicuculline. (C) Bath application of 5 μM bicuculline alone and coapplication of bicuculline and DA on the same neuron. (D) Summary histogram showing that bicuculline has little or no effect on DA's inhibitory actions (n = 4, P < 0.05). (D and F) * indicates significant differences. (E and F) The D1 receptor agonist mimics DA effects, whereas the D2 receptor agonist had no effects. (E) The DA D1 receptor agonist, 100 μM SKF 33989, reproduced the effect of DA, whereas the D2-like receptor agonist, 10 μM quinpirole, lacked effects. (F) Summary histograms showing the normalized EPSP amplitude reduction (mean ± standard error) produced by DA and SKF but not quinpirole. The normalized values to control for DA, SKF, and quinpirole were 71.1 ± 2.90% (n = 16, P < 0.001); 68.0 ± 4.12% (n = 4, P < 0.05); and 98.8 ± 3.88% (n = 4, P = 0.633), respectively.
D1 Receptor Modulation.
DA receptors can be divided into two general classes, the D1- and D2-like receptors (20). To determine which receptor subtype is responsible for the depressant effects on unitary EPSPs, we applied the D1 receptor agonist, SKF 38393, using pressure puff ejection (due to rapid oxidization of the agent) and the D2 agonist quinpirole through bath application. In four of six pairs, SKF 38393 (pipette concentration of 100 μM with 10 μM ascorbic acid) mimicked the response of DA, whereas in the other two the reduction of unitary EPSPs observed was less than 10%. In the pair shown in Fig. 2 E and F, for example, EPSP amplitudes decreased 32%, whereas the D2 agonist, 10 μM quinpirole, had no effect on these pairs. Furthermore, 10 μM SCH 23390, a D1 antagonist, was effective in blocking the effects of DA on EPSPs when bath applied simultaneously (Fig. 3 A and B). A typical experiment in which the effects of DA alone and SCH plus DA on EPSP amplitude were examined is shown in Fig. 3A. Representative consecutive traces taken before and during DA, during SCH alone, and with SCH plus DA application demonstrate that EPSP amplitude was reversibly reduced by DA (Fig. 3A), and this depression was antagonized by SCH 23390. In these experiments, DA initially caused a depression of EPSPs by 29.7 ± 0.71% (n = 4, P < 0.01) but no significant depression (12.5 ± 3.57%) when reapplied to the same cells in the presence of 10 μM SCH 23390 (Fig. 3B; n = 4, P = 0.961). SCH 23390 itself slightly increases the amplitude of EPSPs about 9%, a nonsignificant difference (P = 0.166), suggesting little or no prevailing action of the endogenous amine. In line with these results, the depression of EPSPs by DA was not blocked by the DA D2 antagonist raclopride (1–10 μM, bath application). In these experiments, DA initially reduced the amplitude of EPSPs 34.2 ± 6.45%, and depressed them even more (43.6 ± 10.77%) when reapplied with raclopride simultaneously (Fig. 3 C and D; n = 5, P < 0.01). Raclopride itself dramatically increases the amplitude of EPSPs about 30% (P < 0.05), presumably due to reversal of the common action of D2 receptors to depress neuronal excitability (7, 21).
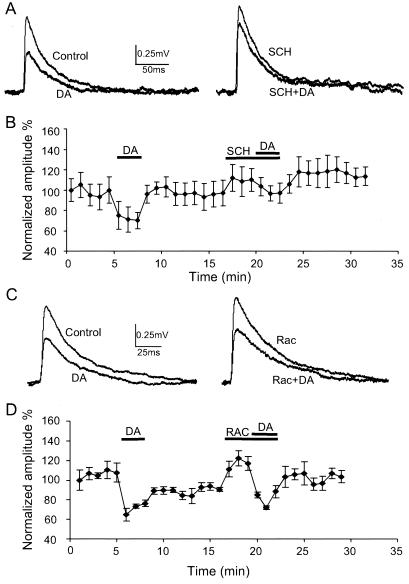
Figure 3.
The DA receptor D1 antagonist, SCH 23390, blocked DA's depressive effects, whereas the D2 antagonist, raclopride, failed to do so. (A) Sample traces of DA and SCH 23390 experiments (10 μM). In most cases, EPSPs were only slightly reduced in the presence of combined SCH 23390 and DA. (B) Summary time course of average response to DA in continuous recording (n = 4, P < 0.01). Responses are normalized against their corresponding control values. Error bars represent standard errors. The effects of DA are partially blocked by the D1 antagonist, SCH 23390. SCH 23390 itself slightly increases the amplitude of EPSPs (nonsignificantly) in three of four connected pairs tested. In the presence of SCH 23390, DA's effects were only decreased 12.5 ± 3.57% with no significant difference (P = 0.158). (C) Unlike the D1 antagonist, the D2 receptor antagonist, raclopride (10 μM, bath application), had no effects on DA's actions. Sample traces from one cell in D in which DA, raclopride, or DA and raclopride simultaneously were applied. DA reversibly reduces the EPSP amplitude but rapidly recovers to control levels. With raclopride, DA's effects remain unchanged. Moreover, raclopride itself increased the amplitude of EPSP by about 20% in this pair. (D) Summary graph showing that DA initially depresses EPSPs about 34.2 ± 6.45% (n = 5, P < 0.01) but soon recovers after washout. Bath application of raclopride significantly increases EPSP amplitude to 32.2 ± 7.86% (P < 0.05), whereas DA still significantly depresses EPSPs 43.6 ± 10.77% (P < 0.01) in the presence of raclopride, indicating that DA D2 receptor is not involved in the depressive effects of DA on EPSP.
Voltage-Independent Reduction of EPSPs by DA.
As a final step, to determine whether postsynaptic receptors or ion channels are involved in this modulation, we first examined the time course of synaptic response, including latency, rise time, and decay time of EPSPs and found no evidence that DA altered EPSP temporal dynamics (Table 1). Second, as shown in Fig. 4, synaptic responses were investigated at different holding membrane potentials to determine whether dopaminergic modulation of unitary EPSPs is voltage dependent. DC currents (−0.2–0.8n A) were injected to hold the membrane potential constant. Under these conditions, EPSP reduction was still visible over a range of membrane potentials as shown in Fig. 4A, which indicates that DA attenuated the EPSP irrespective of membrane voltage. Third, to examine whether DA modulates sodium channels and/or potassium channels located on the postsynaptic neuron, 5 mM QX-314 and 125 mM Cs+-gluconate were included in the postsynaptic recording pipette. Fig. 4B shows that DA reduces EPSP amplitude in the presence of these two channel blockers, as it does in control conditions. These findings indicate that postsynaptic sodium and/or potassium channels are not required for DA's suppression of EPSPs.
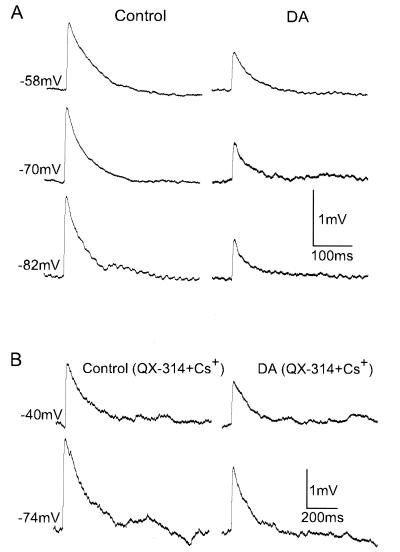
Figure 4.
DA reduces EPSP amplitudes irrespective of membrane voltage and postsynaptic Na+ and K+ channels. (A) Unitary EPSPs were reduced by focal application of 10 mM DA in the membrane potential range −58 to −82 mV. DC currents were injected into the cell to adjust the membrane potential to values given on the left. (B) To examine whether DA modulates sodium and potassium channels postsynaptically, the Na+ channel blocker QX-314 (5 mM) and K+ channel blocker Cs+-gluconate (125 mM) were included in the postsynaptic recording pipette. DA continues to reduce EPSP amplitudes in the presence of these two channel blockers, indicating that the depression effects of DA's actions do not involve the activation of postsynaptic sodium and potassium channels.
Discussion
The present study provides evidence that DA directly modulates unitary excitatory synaptic neurotransmission in local pyramidal-to-pyramidal circuits that presumably mediate recurrent excitation. Our data on failure rate and paired-pulse ratio also indicate that the direct effect on the probability of glutamate release or reliability of excitatory synaptic transmission occurs via a presynaptic mechanism acting at D1 receptors. The present findings are in line with numerous previous studies on DA modulation of synaptic properties in other brain regions using a combination of intracellular recording and extracellular stimulation (19, 22–25). The D1 receptor has also been implicated in long-term depression induced in the rat PFC (26, 27). The relationship of the present results to the long-term depression observed by Crépel and colleagues (27) is deserving of further exploration.
The presynaptic modulation of transmitter release may occur through at least one of the following mechanisms: (i) the influx of Ca2+ may be reduced by modulation of the voltage-dependent Ca2+ or K+ channels in presynaptic terminals (28); (ii) the release machinery may be altered after Ca2+ entry into presynaptic terminals (29); and (iii) the propagation of action potentials may be attenuated by modulation of Na+ channels (30–32). The first two possibilities seem unlikely because previous studies have provided evidence that DA modulates excitatory transmission by a mechanism independent of Ca2+ entry into the cell (33). In our view, the third mechanism has considerable merit because data from Cantrell et al. (30) have shown that both DA and D1 agonists can reduce Na+ currents in dissociated hippocampal pyramidal neurons.
In line with previous studies (15), several putative synaptic contacts were observed in pairs of anatomically reconstructed pyramidal neurons in this study (Fig. 1C). The site of DA's effects in local horizontal excitatory connections may occur at D1 receptors that anatomical studies have localized on the axon terminals of nondopaminergic neurons, which form excitatory asymmetric synapses onto dendritic spines (12, 13, 34). The ultrastructural data suggest that DA may act at a dendritic spine innervated by both a dopaminergic and a glutamatergic terminal (a so-called synaptic triad) in at least two ways. First, it could presynaptically modulate neurotransmitter release from axonal terminals of excitatory afferents that synapse onto dendritic spines. Second, DA could directly modulate dendritic excitability postsynaptically by modulating the response to activation of the excitatory and inhibitory amino acid receptors located on the soma and dendrites of postsynaptic neurons. Even though postsynaptic mechanisms are supported by the localization of D1 receptors in dendritic shafts and spines (12, 13, 34), by D1 enhancement of N-methyl-d-aspartate (NMDA)-mediated currents in the striatum (35, 36) and PFC (10), and by the reduction of Na+ currents in hippocampal neurons (30), these postsynaptic mechanisms would not account for our data. It remains to be determined how the presynaptic D1 receptors elucidated in this study would suppress excitatory synaptic transmission in horizontal connections and, at the same time, operate in conjunction with postsynaptic receptors and/or ion channels (7). However, the inhibition of local excitatory connections by D1 receptors indicates that the normal action of DA is to constrain neuronal activation during performance of a working memory task. Such an inhibitory role for DA is fully consistent with our previous in vivo observations in the primate (3) and is also supported by a recent in vitro study in the rodent (21) as well as by the results from D1 mutant mice in which the normal inhibition of neuronal activity by iontophoresis of DA has been demonstrated to be lacking (37). The identification of presynaptic inhibition on cortico–cortical connections provides direct evidence that DA may, through a presynaptic action, gate neurotransmitter release to control neuronal circuits in a cortical column and thereby alter working memory processes.
Acknowledgments
We thank Drs. U. Kim, W. H. Xiong, and J. Brumberg for creative discussions and generous help during the study. We thank Drs. J. Howe, G. V. Williams, and W. R. Chen for a thorough reading and critical comments of the manuscripts. We are grateful to Ms. A. Begovic and O. Krimer for technical assistance. This work was supported by National Institutes of Health Grants P50 MH44866 and R01 MH38546.
Abbreviations
- PFC
prefrontal cortex
- DA
dopamine
- GABA
γ-aminobutyric acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011524298.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011524298
References
- 1.Fuster J M. The Prefrontal Cortex. 2nd Ed. New York: Raven; 1989. p. 255. [Google Scholar]
- 2.Goldman-Rakic P S. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 3.Williams G V, Goldman-Rakic P S. Nature (London) 1995;376:572–576. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 4.Lisman J E, Fellous J M, Wang X J. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 5.Compte A, Brunel N, Goldman-Rakic P S, Wang X J. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 6.Wang X J. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C R, Seamans J K, Gorelova N. Neuropharmacology. 1999;21:161–194. doi: 10.1016/S0893-133X(98)00112-2. [DOI] [PubMed] [Google Scholar]
- 8.Seung H S, Lee D D, Reis B Y, Tank D W. Neuron. 2000;26:259–271. doi: 10.1016/s0896-6273(00)81155-1. [DOI] [PubMed] [Google Scholar]
- 9.Durstewitz D, Seamans J K, Sejnowski T J. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- 10.Seamans, J. K., Durstewitz, D., Christie, B., Sejnowski, T. J. & Stevens, C. F. (2001) Proc. Natl. Acad. Sci. USA98, in press. [DOI] [PMC free article] [PubMed]
- 11.Tanaka S. Prog Neuropsychopharmacol Biol Psychiatry. 2000;25:1–23. [Google Scholar]
- 12.Goldman-Rakic P S, Leranth C, Williams S M, Mons N, Geffard M. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smiley J F, Levey A I, Ciliax B J, Goldman-Rakic P S. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart G J, Dodt H U, Sakmann B. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- 15.Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. J Physiol (London) 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang C R, Seamans J K. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 18.Thomson A M. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- 19.Behr J, Gloveli T, Schmitz D, Heinemann U. J Neurophysiol. 2000;84:112–119. doi: 10.1152/jn.2000.84.1.112. [DOI] [PubMed] [Google Scholar]
- 20.Missale C, Nash S R, Robinson S W, Jaber M, Caron M G. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F M, Hablitz J J. J Neurophysiol. 1999;81:967–976. doi: 10.1152/jn.1999.81.3.967. [DOI] [PubMed] [Google Scholar]
- 22.Nicola S M, Kombian S B, Malenka R C. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey J, Lacey M G. J Physiol (London) 1996;492:143–154. doi: 10.1113/jphysiol.1996.sp021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pralong E, Jones R S. Eur J Neurosci. 1993;5:760–767. doi: 10.1111/j.1460-9568.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 25.Momiyama T, Sim J A, Brown D A. J Physiol (London) 1996;495:97–106. doi: 10.1113/jphysiol.1996.sp021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law-Tho D, Hirsch J C, Crépel F. Neurosci Res (NY) 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 27.Otani S, Auclair N, Desce J M, Roisin M P, Crépel F. J Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L G, Saggau P. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 29.Thompson S M, Capogna M, Scanziani M. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 30.Cantrell A R, Smith R D, Goldin A L, Scheuer T, Catterall W A. J Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prikriya M, Mennerick S. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- 32.Gorelova N A, Yang C R. J Neurophysiol. 2000;84:75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Nicola S M, Malenka R C. J Neurosci. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergson C, Mrzljak L, Smiley J F, Pappy M, Levenson R, Goldman-Rakic P S. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cepeda C, Buchwald N A, Levine M S. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cepeda C, Colwell C S, Itri J N, Chandler S H, Levine M S. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- 37.Xu M, Hu X T, Cooper D C, Moratalla R, Graybiel A M, White F J, Tonegawa S. Cell. 1994;79:945–55. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]