Abstract
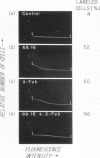
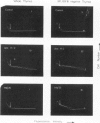
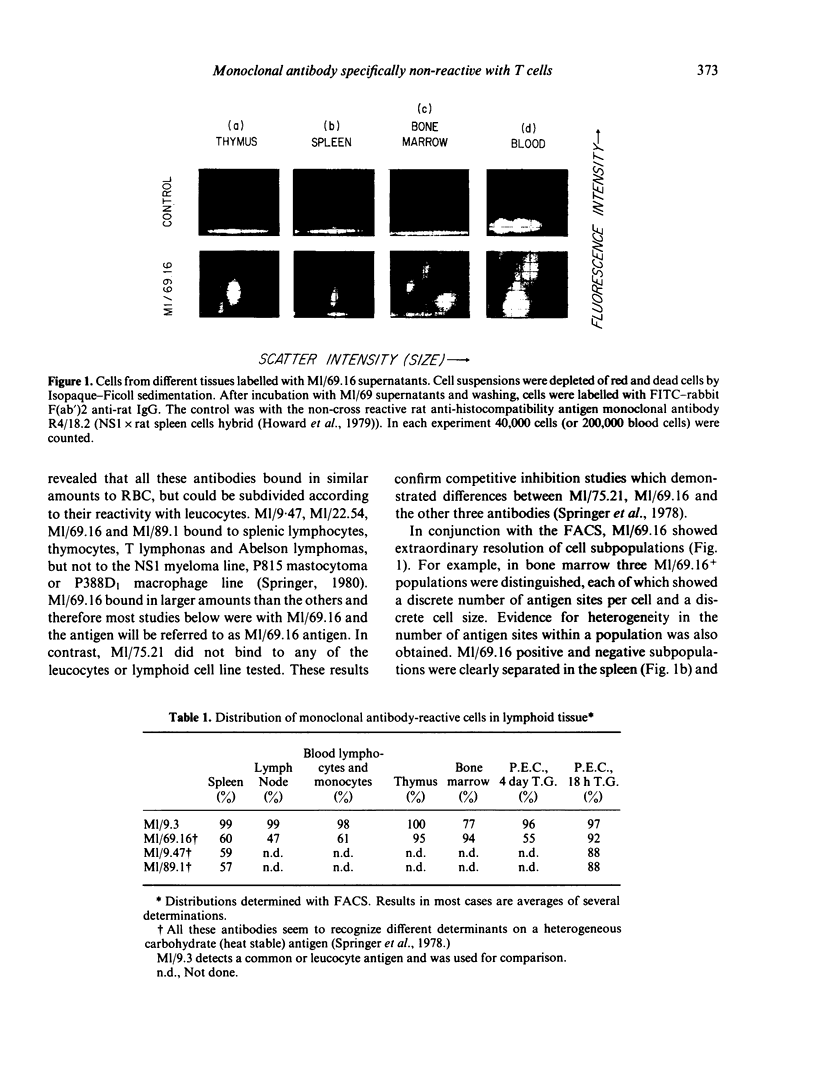
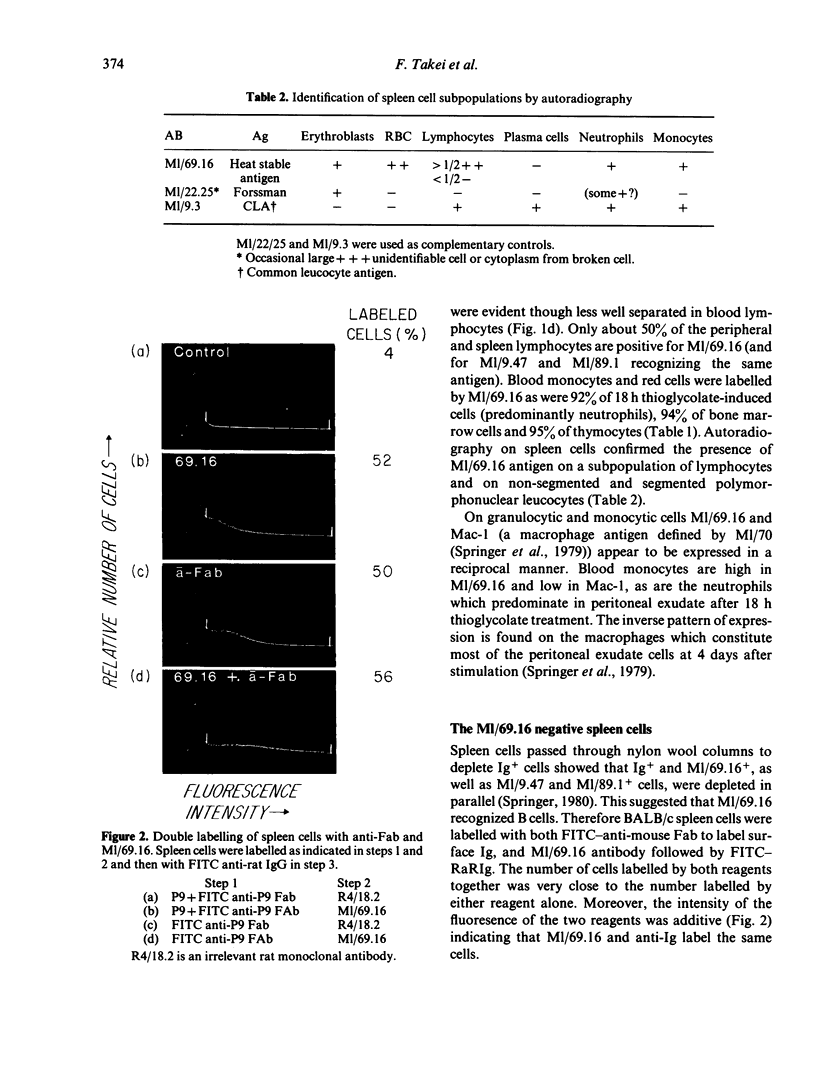
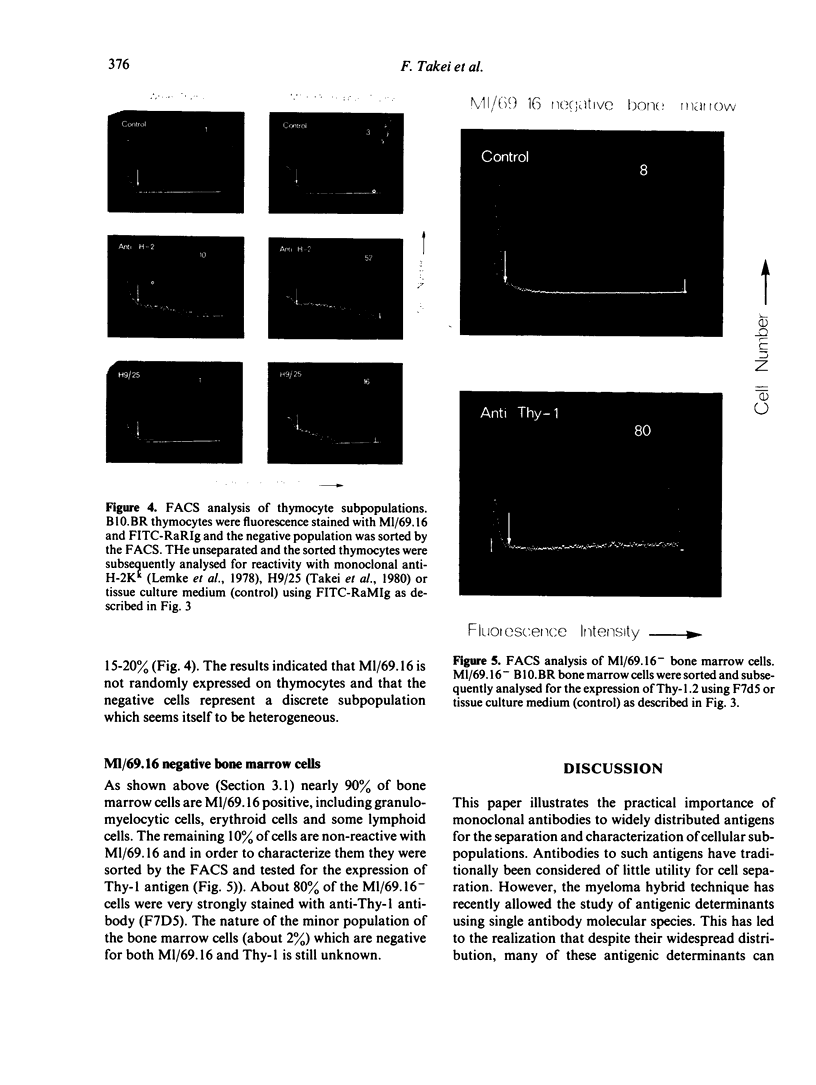
Rat monoclonal antibody M1/69.16 reacts with a heat stable antigen of mouse commonly expressed in the majority of cell types in blood, spleen, bone marrow and thymus, including cells of erythroid, myeloid and lymphoid series. However, subpopulations of cells in lymphoid tissues can be identified which are non-reactive with this antibody using the fluorescence-activated cell sorter. All surface Ig positive cells seem to react with M1/69.16 while more than 96% of Ig negative cells in spleen and lymph nodes are M1/69.16 negative. Most cells (80%-90%) in the M1/69.16 negative populations in spleen lymph nodes and bone marrow express Thy-l. Thus, peripheral T cells are specifically non-reactive with this antibody. In contrast, approximately 95% of thymocytes react with M1/69.16, leaving a minor population which is negative. The negative population (5%) is enriched in cells expressing high amounts of H-2 antigen and those bearing H9/25 antigen which is specific for lymphocyte subsets, indicating that M1/69.16 negative thymocytes represent a specific subpopulation, possibly "mature' thymocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt R., Stark R., Klein P., Müller A., Thiele H. G. Solubilization and molecular characterization of membrane-bound antigens shared by thymocytes and brain. Eur J Immunol. 1976 May;6(5):333–340. doi: 10.1002/eji.1830060506. [DOI] [PubMed] [Google Scholar]
- Brown G., Biberfeld P., Christensson B., Mason D. Y. The distribution of HLA on human lymphoid, bone marrow and peripheral blood cells. Eur J Immunol. 1979 Apr;9(4):272–275. doi: 10.1002/eji.1830090405. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Howard J. C., Butcher G. W., Galfrè G., Milstein C., Milstein C. P. Monoclonal antibodies as tools to analyze the serological and genetic complexities of major transplantation antigens. Immunol Rev. 1979;47:139–174. doi: 10.1111/j.1600-065x.1979.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Lake P., Clark E. A., Khorshidi M., Sunshine G. H. Production and characterization of cytotoxic Thy-1 antibody-secreting hybrid cell lines. Detection of T cell subsets. Eur J Immunol. 1979 Nov;9(11):875–886. doi: 10.1002/eji.1830091109. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hammerling G. J., Hohmann C., Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse. Nature. 1978 Jan 19;271(5642):249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Rouse R. V., van Ewijk W., Jones P. P., Weissman I. L. Expression of MHC antigens by mouse thymic dendritic cells. J Immunol. 1979 Jun;122(6):2508–2515. [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stern P. L., Willison K. R., Lennox E., Galfrè G., Milstein C., Secher D., Ziegler A. Monoclonal antibodies as probes for differentiation and tumor-associated antigens: a Forssman specificity on teratocarcinoma stem cells. Cell. 1978 Aug;14(4):775–783. doi: 10.1016/0092-8674(78)90333-1. [DOI] [PubMed] [Google Scholar]
- Takei F., Galfrè G., Alderson T., Lennox E. S., Milstein C. H 9/25 monoclonal antibody recognizes a new allospecificity of mouse lymphocyte subpopulations: strain and tissue distribution. Eur J Immunol. 1980 Apr;10(4):241–246. doi: 10.1002/eji.1830100404. [DOI] [PubMed] [Google Scholar]
- Takei F., Waldmann H., Lennox E. S., Milstein C. Monoclonal antibody H9/25 reacts with functional subsets of T and B cells: killer, killer precursor and plaque-forming cells. Eur J Immunol. 1980 Jul;10(7):503–509. doi: 10.1002/eji.1830100704. [DOI] [PubMed] [Google Scholar]