Abstract
Dopamine acts mainly through the D1/D5 receptor in the prefrontal cortex (PFC) to modulate neural activity and behaviors associated with working memory. To understand the mechanism of this effect, we examined the modulation of excitatory synaptic inputs onto layer V PFC pyramidal neurons by D1/D5 receptor stimulation. D1/D5 agonists increased the size of N-methyl-d-aspartate (NMDA) component of excitatory postsynaptic currents (EPSCs) through a postsynaptic mechanism. In contrast, D1/D5 agonists caused a slight reduction in the size of the non-NMDA component of EPSCs through a small decrease in release probability. With 20 Hz synaptic trains, we found that the D1/D5 agonists increased depolarization of summating the NMDA component of excitatory postsynaptic potential (EPSP). By increasing the NMDA component of EPSCs, yet slightly reducing release, D1/D5 receptor activation selectively enhanced sustained synaptic inputs and equalized the sizes of EPSPs in a 20-Hz train.
Dopamine regulates working memory processes involving the prefrontal cortex (PFC). Dopamine levels are elevated in the PFC during performance of working memory tasks (1), and task performance is generally modulated by the D1, but not D2, class of dopamine receptors (2–5). Dopamine, acting on D1 receptors, also significantly increases delay and response-related activity of PFC neurons during working memory tasks while only moderately augmenting background activity (2, 3, 6, 7). The link between the biophysical mechanisms of D1-mediated modulation and their functional consequences has not, however, been established.
Dopaminergic and glutamatergic axon terminals form “synaptic triads” on the postsynaptic dendrites of deep layer prefrontal cortex pyramidal neurons and dopamine contacts are found on somatic and dendritic regions of pyramidal and nonpyramidal neurons in the rat PFC, especially in the deeper laminae (8–10). Glutamatergic afferents from the hippocampus and dopaminergic terminals are, moreover, in direct apposition to one another in the PFC, suggesting a presynaptic site of modulation (8). The few physiological studies available to date indicate that dopamine is capable of altering excitatory synaptic responses in the PFC, although the mode of action is not known (11–15).
Computer models have suggested that the N-methyl-d-aspartate (NMDA) component of synaptic currents are critical for stabilizing sustained activity; large non-NMDA components of synaptic currents, in contrast, render delay-type activity less robust to interfering inputs and noise (16–20). Therefore, understanding how D1 receptor activation affects synaptic responses in PFC neurons is critical for understanding the functional neuromodulation of sustained activity patterns underlying working memory processes within the PFC.
Here, we characterize the neuromodulatory effects of D1 agonists on glutamatergic inputs to layer V PFC neurons. D1 receptor activation increased NMDA responses while slightly reducing non-NMDA responses. As a result, D1 agonists tended to equalize the response to a 20-Hz input train, a typical frequency observed during the delay period of working memory tasks (2, 3, 6, 7, 21–24). Exactly these modulatory effects enhanced sustained activity during delay periods in networks of simulated PFC neurons (17).
Methods
The brains of Sprague–Dawley or Long–Evans rats (14–20 days; Salk Colony) were rapidly dissected and immersed for 1 min in cold (4°C) oxygenated artificial cerebrospinal fluid (ACSF) with the following components (in mM): KCl (2.5), NaH2PO4 (1.25), NaHCO3 (25), CaCl2 (0.5), MgCl2 (6), dextrose (25), ascorbic acid (1.3), pyruvic acid (2.4), NaCl (125). Choline (110) or sucrose (200) was routinely substituted for NaCl to prevent excitotoxic damage resulting from severing of axons during slicing. After cutting, 300-μm slices containing the prelimbic/infralimbic region of the PFC were transferred to ACSF containing (in mM): NaCl (126), KCl (3), NaHCO3 (26), glucose (10), and MgCl2 (4), CaCl2 (0.7) for storage and MgCl2 (1.3), CaCl2 (2.3) for recording. The prelimbic/infralimbic region is that portion of the medial PFC flanked by the corpus callosum in coronal sections (25).
Slices were perfused by gravity-fed ACSF (maintained at 28–32°C) at a rate of 1–3 ml per min and viewed by using differential interference contrast (DIC) optics. The objective was often removed from the bath during recordings and the fluid level decreased to reduce stray pipette capacitance. Thick-walled borosilicate pipettes were filled with (in mM): K-gluconate (130), KCl (10), EGTA (1), MgCl2 (2), NaATP (2), Hepes (10) or KMeSO4 (140), Hepes (10), NaCl (4), EGTA (1), NaATP (4), TrisGTP (0.3), and phosphocreatine (14). In some experiments, KCl was omitted and CsCl (135) was substituted for KMeSO4. QX-314 (2 mM) and/or DIDS (4,4′-diisothiocyanate stilbene-2,2′-disulfonate) (2 mM) were added to pipettes in some experiments. Pipettes were connected to the headstage of an Axoclamp-2B or Axopatch-200A or B amplifier (Axon Instruments, Foster City, CA) with Ag/AgCl wire. An Ag/AgCl reference wire or pellet was placed in the bath directly or through an agar-bridge and by using offset, Vm shifts were corrected. Voltage–clamp recordings were obtained in continuous single-electrode voltage–clamp (SEVC) mode and filtered at 1 kHz. Access resistance was monitored at the start and end of the recording period, and a ±15% change was deemed acceptable. Signals were digitized by a PCI-MIO-16E1 A/D board (National Instruments, Austin, TX).
Stimulating electrodes were placed within 200 μm of the soma and constructed from sharpened epoxy insulated tungsten rods (A-M Systems, Everett, WA). Electrical stimuli consisted of a low-intensity square-wave pulse (100–150 μs) administered every 15–60 s. (−)Bicuculline methiodide, I(S),9(R) (2–20 μM) and D(−) or (±)2 amino-5-phosphonopentanoic acid (APV) (50–100 M), or bicuculline and 6,7-dinitroquinoxaline-2,3-dione (DNQX) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μM) were applied constantly throughout the entire experiment to isolate non-NMDA or NMDA excitatory postsynaptic currents (EPSCs), respectively.
In all experiments, 10 μM of the full D1/D5 agonist (±)-6-chloro-PB hydrobromide (SKF-81297) was used except for data shown in Fig. 1 where the following D1/D5 agonists were also used at varying concentrations (0.5–50 μM) (±)-SKF-38393, R(+)-SKF-81297, or R(+)-SKF-82957 (Research Biochemicals, Natick, MA). All D1 agonists were either made up fresh or stored for up to 2 days at 4°C. During application, the microscope and overhead lights were extinguished, and the drugs were delivered for 3–5 min to the bath by means of an opaque syringe. In D1 antagonist experiments, R(+) SCH-23390 was applied continuously to the slices. Statistics compared average of baseline values obtained 10–15 min before drug application to the average of all response during the 10- to 40-min period following D1 agonist application, and included all cells that showed a stable baseline response. For Figs. 1 and 2, the response at each time point was normalized to the baseline predrug average: normalized value = 100 × (raw value/baseline average value) − 100 to give a percent change for each value relative to the average baseline response at each time point. For puff experiments, l-glutamatic acid or NMDA (1–20 mM) was diluted in the bathing solution that contained combinations of tetrodotoxin (TTX, 250–500 nM), APV (100 μM), bicuculline (10 μM), or CdCl2 (200 μM) and applied by means of a patch pipette. For paired pulse experiments, non-NMDA EPSCs were isolated pharmacologically and paired pulses were delivered at 50 Hz every 30–60 s. For mini-EPSC (mEPSC) experiments, slices were bathed in TTX (0.25 to 1 μM) and bicuculline (10–20 μM) while using CsCl pipettes. mEPSCs were analyzed 5 min before D1 agonist application and for a 5-min period 10 min after the offset of the D1 agonist. For synaptic depression experiments, 10–15 pulse, 20-Hz trains were delivered every 2 min and cells were held at −58 to −65 mV. Each individual excitatory postsynaptic potential (EPSP) in the train was measured relative to the voltage just prior to each stimulus artifact (measure 1 Fig. 4A) or from the initial voltage before the train onset (measure 2 Fig. 4A). All responses in the train were then normalized to the average of the first responses in each individual train. The normalized amplitudes of each of the 15 EPSPs/time point were then averaged across the 5–12 min baseline period and the 10–30 min post D1 agonist period for each cell to generate 2 vectors of 15 values each. Repeated measures ANOVAs compared baseline and D1 condition vectors across neurons.
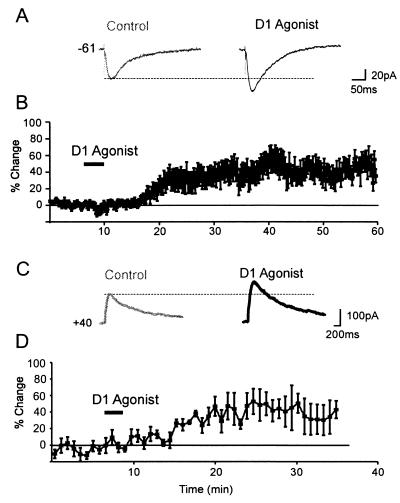
Figure 1.
D1 receptor modulation of NMDA responses in PFC neurons. (A) Representative synaptic responses. For all traces, the baseline (control) response is shown at left, the response during the period of the peak D1 agonist response (>10 min after D1 agonist offset) is on the right. A D1 agonist (0.5 μM SKF81297) enhanced the NMDA EPSC. (B) Average percentage change (and SEM) in NMDA EPSC amplitude over time. (C) Representative traces showing that the response evoked by puffing NMDA (1 mM, 8 ms) was enhanced by the D1 agonist (10 μM SKF-81297, black trace) relative to the baseline response (gray traces) at Vhold = +40 mV. (D) Average percentage change (and SEM) in postsynaptic NMDA current amplitude (n = 8).
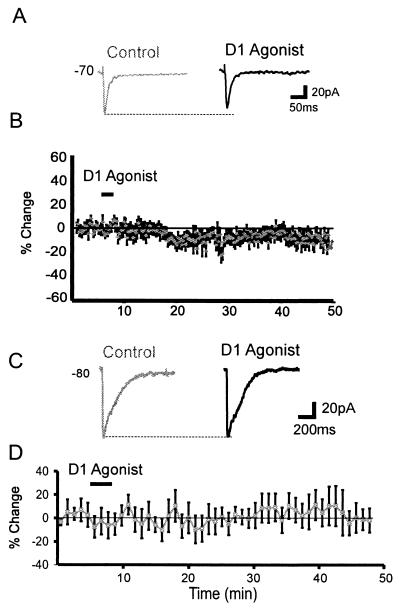
Figure 2.
D1 receptor modulation of non-NMDA responses in PFC neurons. (A) Representative traces showing that a D1 agonist (1 μM SKF81297) slightly reduced the non-NMDA EPSC. (B) Average percentage change (and SEM) in non-NMDA EPSC amplitude over time. (C) Representative traces showing the non-NMDA response evoked by puffing glutamate (1 mM, 8 ms) in the presence of APV (100 μM) was unaffected by the D1 agonist (10 μM SKF-81297, black trace) relative to the baseline response (gray traces) at Vhold = −80 mV. TTX (500 nM) was also included in the bathing solution and CsCl containing pipettes were used. (D) Average percentage change (and SEM) in postsynaptic non-NMDA current amplitude (n = 8).
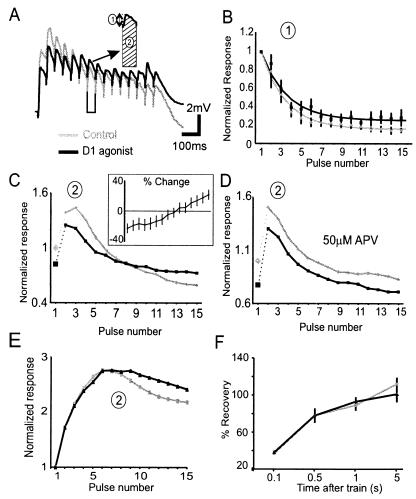
Figure 4.
Modulation of synaptic depression by D1 agonists. (A) Representative trace showing the nonisolated synaptic response to 20-Hz trains under control conditions (gray traces) and following a D1 agonist (10 μM SKF-81297, black traces). Responses were obtained by using QX-314-filled patch-pipettes in 0.5 μM bicuculline. The inset shows the procedure for measuring response size. Measurement 1 assessed the size of each individual EPSP from a baseline obtained just before each pulse in the train, whereas measurement 2 assessed the size of each response plus the concomitant depolarization on which the EPSP was riding by calculating the integral under each EPSP relative to the initial baseline. (B) Average normalized amplitude (and SEM) by using measurement 1 for the control period (gray line) and following a D1 agonist (black line). The lines represent the fit to the data by using a single exponential function. (C) Average normalized amplitude by using measurement 2 for the control period (gray line) and following a D1 agonist (black line). The D1 agonist condition was normalized to the initial averaged response for the control condition. (Inset) The same data replotted as percent change relative to the control response (n = 10). (D) In an additional group of six cells recorded in the presence of APV, no such cross over was observed following application of a D1 agonist. (C and D) The lines connecting points 1 and 2 in each figure are dotted to separate the initial facilitation from depression. (E) When NMDA receptors carried the majority of the synaptic current by conducting experiments in CNQX (3 μM) and bicuculline (10 μM). D1 agonists also caused a relative increase in later responses in the train. (F) Recovery assessed by single pulses delivered 0.1, 0.5,1, 5s after the 15-pulse/20-Hz train was unaffected by D1 agonists.
Results
D1 Agonists Selectively Increase the NMDA Component of EPSCs.
Pyramidal cells were recorded from layer V of the prelimbic cortex (25). Because the agonists we have used lack complete specificity for receptor subtypes, we refer to D1/D5 receptor agonists simply as D1 agonists throughout. D1 agonists added to the bathing solution produced a slight depression of the amplitude of nonisolated layer V postsynaptic potentials (PSPs) in PFC neurons (−25.3 ± 8.9%, n = 6; data not shown) as reported (13).
D1 agonists produced a significant increase in the size of the NMDA component of EPSCs (see Methods) [baseline = 103 ± 12pA, D1 agonist = 137 ± 17pA, 34.2 ± 8%, F(1,13)=8.1, P = 0.01, n = 15; Fig. 1 A and B]. Because the application of low (0.5–7.5 μM) and high (10–50 μM) doses of D1 agonists produced changes in NMDA EPSC size that were not significantly different [F(1,13) = 0.53, P > 0.49], the neuromodulatory effect appears to be saturated at low micromolar agonist concentrations. Recovery of initial response size was observed in 6/15 cells following agonist washout. A delayed and long-lasting action of D1 agonists on glutamatergic responses is common and has been observed previously in PFC, striatal, and hippocampal neurons (15, 26–28). The D1 agonist-induced increase in the NMDA component of EPSCs was blocked by the D1 antagonist SCH23390 (percentage change = −3.5 ± 10%, n = 4; data not shown). Thus, D1/D5 receptor activation produced a prolonged increase in the NMDA component of EPSCs.
We next sought to determine the extent to which a postsynaptic mechanism contributes to these effects. In the sub- and suprathreshold voltage range, Ca2+ currents are activated by long-lasting glutamate-mediated depolarizations (29–31) and are the targets of dopamine modulation (25). Therefore, the postsynaptic response to puff application of NMDA was recorded at +40 mV, where Ca2+ currents are expected to be inactivated. In some cells, we also used ACSF containing the voltage-gated Ca2+ channel blocker Cd2+ (plus TTX and Cs/TEA-filled pipettes). NMDA-mediated responses were evoked by pressure ejecting NMDA (1–20 mM, 5–20 ms) near the soma. For cells exhibiting a stable baseline response for >15 min, the increase in the NMDA current at a Vhold of + 40mV was 30.3 ± 11% [control = 151.9 ± 11 pA, D1 condition = 198 ± 19 pA, F(1,7) = 17, P < 0.01]. Therefore, D1 agonists selectively increase the postsynaptic sensitivity of NMDA receptors.
D1 Agonists Slightly Reduce Non-NMDA Component of EPSCs Without Affecting the Postsynaptic Response to Glutamate.
The non-NMDA component of EPSCs was slightly reduced in size—by about 10%—for 10–50 min following application of the D1 agonist [baseline = −84 ± 15 pA, D1 agonist = −74 ± 12 pA, −9 ± 3%, n = 8, F(1,7) = 6.1, P < 0.05; Fig. 1 A and B]. Non-NMDA responses have been reported to exhibit run-down in dissociated striatal neurons, which was removed by a D1 agonist (32). In the present study, although D1 agonists never increased the non-NMDA response, we did not test whether run-down was removed because cells that did not exhibit a stable baseline response for >10 min were excluded (n = 14/32). In a group of control cells, we found that responses stable initially remained unchanged in amplitude for >50 min (not shown). Therefore, in cells exhibiting a stable response, D1 agonists had a slight inhibitory influence on non-NMDA receptor-mediated synaptic currents.
Postsynaptic non-NMDA responses were examined by focally puffing glutamate (1 mM, 5–20 ms) near the soma (5–30 μm from the soma) of PFC neurons recorded by using Cs-filled pipettes in the presence of APV and TTX. Neurons were voltage-clamped to −80 mV to avoid contamination by voltage-gated Ca2+ currents (−80 mV was used rather than +40 mV to ensure there would be no contamination by NMDA currents). As shown in Fig. 2 C and D, D1 agonists had no effect on the postsynaptic response to glutamate. Therefore, D1 agonists reduced the non-NMDA component of the EPSC, but not through a postsynaptic mechanism.
D1 Agonists Produce a Minor Decrease in Release.
The reduction in the non-NMDA component of EPSC amplitude with no change in the non-NMDA receptor response to directly applied glutamate indicates that D1 agonists may act presynaptically. Changes in release were assessed by examining the effect of D1 agonists on spontaneous mEPSCs, the progressive block of synaptic responses by MK-801, paired pulse ratios and synaptic depression to 20-Hz inputs.
mEPSCs were recorded in bicuculline and TTX from 15 neurons. For all events across all neurons, the average control mEPSC amplitude was 6.9 ± 0.3 pA and 7.6 ± 0.4 pA following application of the D1 agonist (10 ± 3.8%; Fig. 3 A and B). This observation is consistent with data shown in Fig. 2 C and D, and indicates that D1 agonists did not reduce the postsynaptic non-NMDA current, and, in fact, increased it slightly. In contrast, the mEPSC frequency was reduced from 1.83 ± 0.18 events/s in the control condition to 1.58 ± 0.16 events/s in the D1 agonist condition [−14.8 ± 5%, F(1,13) = 6.6, P < 0.05]. This suggests that D1 agonists slightly reduce the release probability of glutamatergic synapses.
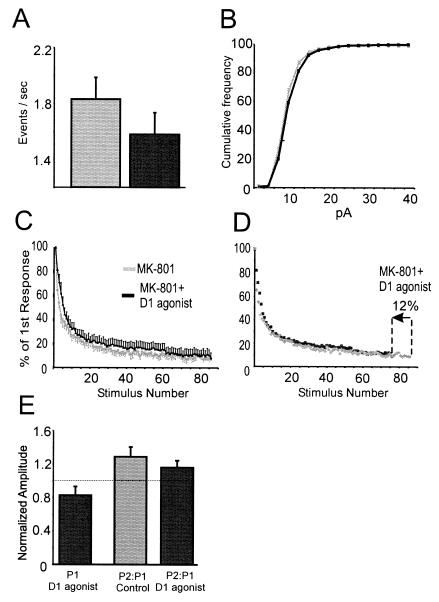
Figure 3.
Presynaptic modulation by D1 agonists. (A) Histogram of group data showing the mean mEPSC frequency for a 300-s period for the control (gray) and D1 agonist condition (black). The drop in mEPSC frequency in the D1 agonist condition was most evident for the first two bins. (B) The same data as in A replotted as a cumulative histogram. For A and B, mEPSCs were recorded at −70 mV in 0.25–1 μM TTX and 10–20 μM bicuculline by using CsCl-filled pipettes. Data were analyzed for the 5 min before D1 agonist application and 10–15 min after D1 agonist offset. (C) The MK-801 blocking function for control cells (gray boxes, n = 7) and for cells receiving the D1 agonist (▴, n = 6). (D) The D1 agonist blocking function overlaps with that of the control if the D1 agonist blocking function was accelerated by 12% by shifting the x axis. (E) Histogram representing data from paired pulse experiments. (Left, black bar) The normalized change in response 1 (P1) in the 10–40 min following application of the D1 agonist. (Middle, gray bar) The ratio of P2:P1 for the control condition. (Right, black bar) The ratio of P2:P1 for responses acquired 10–40 min following application of the D1 agonist. Pulses were separated by 20 ms, and neurons were recorded under voltage–clamp in the presence of 10 μM bicuculline and 0.5–2 μM DNQX by using CsCl/QX-314-filled pipettes.
Another, more direct, method used to assess changes in release probability over a large population of synapses is the progressive blocking function of NMDA EPSCs by open-channel blocker MK-801 (33, 34). Because MK-801 blocks NMDA receptors in a use-dependent manner, the greater the release probability, the faster the NMDA EPSC is reduced by MK-801. In these experiments, after a stable baseline was attained, stimulation was suspended while 40 μM MK-801 was bath applied for 15 min. In 6/13 cells, 10 μM SKF-81297 was coapplied for 5 of these 15 min. Although D1 agonists increased the NMDA current postsynaptically, by normalizing all responses to the first response in each condition, it was possible to compare the rate of MK-801 block in the presence and absence of D1 agonist. Despite the increase in the postsynaptic NMDA current, the MK-801 blocking function was actually slowed in cells treated with the D1 agonist by 12.7 ± 5%, which, although small, was consistent over all 86 data points (Fig. 3C). Accordingly, the curves largely overlapped if the blocking function for the D1 agonist condition was accelerated (i.e., compressed) by 12% (Fig. 3D). By this test, then, D1 agonists reduce release probability by about 12%.
Release probability sometimes may also be studied by using paired-pulse facilitation in which the second of two closely spaced responses is increased, presumably because of residual calcium remaining in the terminal from the prior stimulus. For these experiments, non-NMDA EPSCs were evoked in the presence of bicuculline. To avoid epileptiform polysynaptic activity in the absence of inhibition, submaximal concentrations of DNQX (0.5–2 μm) were applied, and stimulus intensities were kept low to produce only small monosynaptic EPSCs. D1 agonists again reduced averaged response amplitude (EPSC baseline = −6.6 ± 0.8 pA, EPSC with D1 agonist = −4.9 ± 0.7 pA, −20 ± 8%) but did not affect the ratio of second-pulse response amplitude to the first [baseline = 1.28 ± 0.11, D1 condition = 1.16 ± 0.08, F(1,9) = 0.4, P > 0.5, n = 10] (Fig. 3E). Thus, the reduction in release detected by the mEPSC and MK-801 blocking experiments was either too small to significantly affect paired pulse ratios or was limited to high probability synapses, which typically exhibit little facilitation.
D1 Modulation of 20-Hz Trains.
Unlike paired-pulse facilitation, which mainly reflects changes in low probability synapses, synaptic depression depends largely on the progressive decrease in release probability at higher probability synapses. Synaptic depression may be counteracted by postsynaptic temporal summation of EPSPs (35). Given the voltage-dependence of the NMDA receptor, this summation can be quite nonlinear (36, 37). To assess the effects of D1 agonists on synaptic depression, trains of 15 stimuli were delivered. In vivo, PFC neurons receive trains of inputs from neighboring neurons during the delay period of working memory tasks, in the 20-Hz frequency range (2, 3, 6, 7, 21–24). To explore the consequences of D1 modulation on trains of inputs in the physiological range, synaptic stimulation was therefore delivered at 20 Hz.
We measured the responses to a 20-Hz train of synaptic inputs in two ways. The first measure (measure 1 Fig. 4A) calculated the amplitude of each EPSP from a baseline obtained just before each pulse in the train. This measure controlled for residual depolarization from preceding responses in the train. Responses were normalized to first response in the train. As above, D1 agonists decreased the amplitude of the initial synaptic responses (Fig. 4A). D1 agonists did not, however, significantly affect the paired pulse ratio of the first two synaptic responses [control = 0.79 ± 1.8, D1 condition = 0.87 ± 0.08, F(1,8) = 0.52, P > 0.05] nor the time constant of depression for responses 1–15 [control = 104.9 ± 19 ms, D1 condition = 120.9 ± 17.8 ms, F(1,7) = 1, P > 0.05; Fig. 4B].
The second measure of response size (measure 2 Fig. 4A) calculated the area under each EPSP relative to the initial baseline. The data were plotted such that the averaged responses in the D1 agonist condition were normalized to the averaged initial response for the control condition. Measure 2 took into account the amount of residual depolarization because of summation of EPSPs. Despite the large drop in EPSP size by using measure 1, in most cells, there was sufficient postsynaptic summation that individual responses in the train did not drop below 50% of the initial response. In 7/10 cells, D1 agonists produced an enhancement of later pulses in the train (Fig. 4C). As shown in Fig. 4C there was a significant interaction effect [F(1,16) = 11.2, P < 0.01] with D1 agonists decreasing responses 2–5 in the train by 18 ± 7%, but increasing responses 12–15 by 13 ± 6.4%. In an additional six cells recorded in the presence of APV, D1 agonists still evoked the decrease in responses 2–5 in the train but did not increase responses 12–15 (Fig. 4D). As a result, there was no longer an interaction effect [F(1,10) = 0.02, P > 0.9] and all responses in the train were relatively decreased in the D1 agonist condition (Fig. 4D). This suggests that the D1-mediated increase in the NMDA current was responsible for the relative increase in later responses in the train. Accordingly, if the NMDA receptor was the main charge carrier (i.e., in 3–10 μM CNQX and 10 μM bicuculline) D1 agonists produced a 19 ± 10% increase in later responses in the train for 6/10 cells tested (Fig. 4E). In these 10 cells, however, D1 agonists had no effect on the time constant of depression by using measure 1 (not shown). Therefore, D1 agonists produced a modest modulation in the integrated response to trains of 20-Hz inputs.
Finally, to probe the possible effects on recovery from synaptic trains and the vesicle refilling rate, test pulses were delivered at various times after the 15-pulse 20-Hz train. In the D1 agonist condition, the relative recovery at 0.1 s (control = 37 ± 5%, D1 condition = 38 ± 7%), 0.5s (control = 78 ± 5%, D1 condition = 78 ± 4%), 1s (control = 89 ± 6%, D1 condition = 93 ± 8%), and 5s (control = 112 ± 5%, D1 condition = 101 ± 18%) was unaffected (n = 4, Fig. 4F). Therefore, D1 agonists did not appear to have a lasting effect on depression once the train was terminated.
Discussion
In the present study, D1 agonists produced an increase in the NMDA component of synaptic currents (through a postsynaptic NMDA mechanism) and a small decrease in non-NMDA-mediated responses (through a small decrease in release probability).
Dopamine has diverse and often contradictory effects in different brain regions. In dorsal striatal neurons, dopamine has been reported to depress (27) or have no effect (38) on compound PSPs. Likewise, the non-NMDA response was depressed by D2 receptor activation in some studies (27, 39), not affected in others (38) and increased by D1 receptors and protein kinase A (PKA) in others (26). Although D1 receptor activation has been reported to consistently increase the NMDA response in both striatal and cortical neurons (11, 12, 40–42), studies on dorsal striatal neurons suggest the increase is mediated by PKA (27), whereas, in ventral striatal neurons, it is mediated by protein kinase C (43). In neurons from the hippocampus or nearby cortices, dopamine has been reported to depress both NMDA and non-NMDA responses (44–47) through a PKA-dependent process (44), whereas direct application of PKA has been reported to increase non-NMDA responses (48). In PFC neurons, the effects of dopamine also depends on the dose: low concentrations (<50 μM) increase the NMDA response by means of D1 receptors, whereas, at high concentrations (>50 μM), the NMDA response was depressed by means of activation of D2 receptors (13, 15). Thus, the effects of dopamine are complex and depend on the agonist concentration, the subtype of glutamate, and/or dopamine receptor stimulated, tissue preparation, and brain region studied.
In PFC pyramidal neurons, selective D1 receptor activation produced a 10–15% decrease in the non-NMDA component of signal EPSCs and initial EPSPs in a 20-Hz train that were recorded in the presence or absence of NMDA blockade. These effects were not mediated postsynaptically (Figs. 2 B and C and 3B) but appeared to be because of reductions in release. Analyses of mEPSCs and the MK-801 blocking function revealed a small (≈12%) reduction in release probability. This effect appeared to be too small to influence paired-pulse ratios or the time course of synaptic depression. A similar dopamine-mediated reduction in release without consistent effects on paired-pulse ratios has recently been reported in subicular neurons (47). One possibility is that because D1 agonists reduce high threshold Ca2+ currents (25), they may limit presynaptic Ca2+ entry and hence release. The postsynaptic increase in NMDA currents overcame the small reductions in release, making NMDA EPSCs larger in the D1 agonist condition. D1 agonists increased later responses to 20-Hz trains by means of an APV-sensitive mechanism. Because of the slow decay time of NMDA responses, an increase in NMDA currents would tend to enhance EPSP summation. This effect would become more relevant during prolonged depolarizations from high frequency trains (49). Although slight, the D1/D5-induced reduction of initial responses, and increase in later responses acted together to equalize EPSPs throughout the train.
Functional Considerations.
During delay periods of working memory tasks, deep layer PFC neurons show sustained activity thought to represent the short-term retention of information to plan and organize forthcoming action (23, 50). Recently, computer models have been used to explore the mechanisms responsible for producing sustained firing modes (16–20). Simulations suggest that NMDA currents are crucial for producing sustained activity. Because of their slow decay time constant, these currents produce a nearly constant synaptic drive that could maintain recurrent activity at rates around 20 Hz. Moreover, the voltage-dependence of the NMDA conductance contributes to the selectivity and robustness of delay-period activity because it especially enhances active and depolarized subpopulations of neurons compared with neurons that are hyperpolarized or firing at low rates.
On the other hand, non-NMDA conductances are voltage-independent and evoke only transient depolarizations, thus contributing much less to sustained activity. In fact, strong non-NMDA activated currents actually reduce the robustness of delay activity because they tend to induce synchronous oscillations and increase the impact of brief, interfering inputs and noise (17, 20). Slightly reducing non-NMDA responses and increasing NMDA component of currents biases inputs to act as constant current sources. However, strong non-NMDA mediated currents may be useful in situations that require the integration of new information or fast switching between different representations or response options. Hence, dopamine by means of D1 receptors might switch PFC networks from a mode that favors integration of new information and exploration to a mode that favors robustly maintained recurrent activity in the context of goal-directed behavior over extended periods of time. It is through these mechanisms that dopamine may enhance sustained activity on working memory tasks (2, 3, 6, 7), which is thought to underlie the trial-unique active retention of information within the PFC (50).
Acknowledgments
We thank Lee Campbell for technical support. We are also grateful to Jane Sullivan, Charles Yang, Stan Floresco, German Barrioneuvo, and Guillermo Gonzales-Burgos for their comments. This work was supported by a grant from the Howard Hughes Medical Institute. J.K.S. was supported by a grant from the National Sciences and Engineering Research Council of Canada and the Howard Hughes Medical Institute. D.D. was supported by a research stipend from the Deutsche Forschungsgemeinschaft.
Abbreviations
- PFC
prefrontal cortex
- NMDA
N-methyl-d-aspartate
- ACSF
artificial cerebrospinal fluid
- EPSC
excitatory postsynaptic current
- mEPSC
mini-EPSC
- EPSP
excitatory postsynaptic potential
- APV
2-amino-5-phosphonovaleric acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011518798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011518798
References
- 1.Watanabe M, Kodama T, Hikosaka K. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- 2.Sawaguchi T, Matsumura M, Kubota K. J Neurophysiol. 1990;63:1385–1400. doi: 10.1152/jn.1990.63.6.1385. [DOI] [PubMed] [Google Scholar]
- 3.Sawaguchi T, Matsumura M, Kubota K. J Neurophysiol. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- 4.Williams G V, Goldman-Rakic P S. Nature (London) 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 5.Seamans J K, Floresco S B, Phillips A G. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawaguchi T, Matsumura M, Kubota K. Neurosci Res. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- 7.Sawaguchi T, Matsumura M, Kubota K. Brain Res. 1986;371:404–408. doi: 10.1016/0006-8993(86)90385-9. [DOI] [PubMed] [Google Scholar]
- 8.Carr D B, Sesack S R. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Goldman-Rakic P S, Leranth C, Williams S M, Mons N, Geffard M. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krimer L S, Jakab R L, Goldman-Rakic P S. J Neurosci. 1997;17:7450–7461. doi: 10.1523/JNEUROSCI.17-19-07450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cepeda C, Li Z, Cromwell H C, Altemus K L, Crawford C A, Nansen E A, Ariano M A, Sibley D R, Peacock W J, Mathern G W, Levine M S. Dev Neurosci. 1999;21:223–235. doi: 10.1159/000017402. [DOI] [PubMed] [Google Scholar]
- 12.Cepeda C, Radisavljevic Z, Peacock W, Levine M S, Buchwald N A. Synapse. 1992;11:330–341. doi: 10.1002/syn.890110408. [DOI] [PubMed] [Google Scholar]
- 13.Law-Tho D, Hirsch J C, Crepel F. Neurosci Res. 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 14.Yang X-D, Faber D S. Proc Natl Acad Sci USA. 1991;88:4299–4303. doi: 10.1073/pnas.88.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng P, Zhang X X, Bunney B S, Shi W X. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]
- 16.Durstewitz D, Kelc M, Güntürkün O. J Neurosci. 1999;19:2807–2822. doi: 10.1523/JNEUROSCI.19-07-02807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durstewitz D, Seamans J K, Sejnowski T J. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- 18.Lisman J E, Fellous J M, Wang X J. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 19.Seamans J K, Durstewitz D, Sejnowski T J. Proc 6th Joint Symp Neural Comput. 1999;9:128–135. [Google Scholar]
- 20.Wang X J. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batuev A S, Kursina N P, Shutov A P. Behav Brain Res. 1990;41:95–102. doi: 10.1016/0166-4328(90)90145-5. [DOI] [PubMed] [Google Scholar]
- 22.Funahashi S, Bruce C J, Goldman-Rakic P S. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 23.Fuster J M. J Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Miller E K, Erickson C A, Desimone R. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C R, Seamans J K. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colwell C S, Levine M S. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umemiya M, Raymond L A. J Neurophysiol. 1997;78:1248–1255. doi: 10.1152/jn.1997.78.3.1248. [DOI] [PubMed] [Google Scholar]
- 28.Yang S N. Hippocampus. 2000;10:57–63. doi: 10.1002/(SICI)1098-1063(2000)10:1<57::AID-HIPO6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Calton J L, Kang M H, Wilson W A, Moore S D. J Neurophysiol. 2000;83:685–692. doi: 10.1152/jn.2000.83.2.685. [DOI] [PubMed] [Google Scholar]
- 30.Mermelstein P G, Bito H, Deisseroth K, Tsien R W. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seamans J K, Gorelova N A, Yang C R. J Neurosci. 1997;17:5936–5948. doi: 10.1523/JNEUROSCI.17-15-05936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen P B, Fienberg A A, Nairn A C, Greengard P. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 33.Hessler N A, Shirke A M, Malinow R. Nature (London) 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 34.Rosenmund C, Clements J D, Westbrook G L. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 35.Thomson A M. J Physiol (London) 1997;502:131–147. doi: 10.1111/j.1469-7793.1997.131bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collingridge G L, Herron C E, Lester R A. J Physiol (London) 1988;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herron C E, Lester R A, Coan E J, Collingridge G L. Nature (London) 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- 38.Nicola S M, Malenka R C. J Neurophysiol. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- 39.Levine M S, Cepeda C. Adv Pharmacol. 1998;42:724–729. doi: 10.1016/s1054-3589(08)60850-9. [DOI] [PubMed] [Google Scholar]
- 40.Cepeda C, Levine M S. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- 41.Levine M S, Li Z, Cepeda C, Cromwell H C, Altemus K L. Synapse. 1996;24:65–78. doi: 10.1002/syn.890240102. [DOI] [PubMed] [Google Scholar]
- 42.Levine M S, Altemus K L, Cepeda C, Cromwell H C, Crawford C, Ariano M A, Drago J, Sibley D R, Westphal H. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chergui K, Lacey M G. Neuropharmacology. 1999;38:223–231. doi: 10.1016/s0028-3908(98)00187-7. [DOI] [PubMed] [Google Scholar]
- 44.Hsu K S. J Neurophysiol. 1996;76:1887–1895. doi: 10.1152/jn.1996.76.3.1887. [DOI] [PubMed] [Google Scholar]
- 45.Otmakhova N A, Lisman J E. J Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pralong E, Jones R S. Eur J Neurosci. 1993;5:760–767. doi: 10.1111/j.1460-9568.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 47.Behr J, Gloveli T, Schmitz D, Heinemann U. J Neurophysiol. 2000;84:112–119. doi: 10.1152/jn.2000.84.1.112. [DOI] [PubMed] [Google Scholar]
- 48.Greengard P, Jen J, Nairn A C, Stevens C F. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- 49.Otani S, Blond O, Desce J M, Crepel F. Neuroscience. 1998;85:669–676. doi: 10.1016/s0306-4522(97)00677-5. [DOI] [PubMed] [Google Scholar]
- 50.Goldman-Rakic P S. Proc Natl Acad Sci USA. 1996;93:13473–80. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]