Abstract
We report the cloning and characterization of a novel epidermal growth factor (EGF) domain gene that was identified in a retroviral gene entrapment screen and is expressed in endothelial cells. This gene encodes a protein of 278 amino acids with an amino-terminal signal peptide and two centrally located EGF-like domains. We have named this novel gene in accordance with the guidelines of the Mouse Genome Informatics group Egfl7, for EGF-like domain 7. Egfl7 mRNA is expressed in highly vascularized adult tissues such as the lung, heart, uterus, and ovary. In addition, Egfl7 is expressed early during mouse embryogenesis and in undifferentiated murine embryonic stem cells. The analysis of Egfl7 expression in embryonic day 9.5 embryos by in situ hybridization indicates that Egfl7 is expressed in vascular structures in both the embryo proper and the yolk sac and at sites of mesodermal precursors of angioblasts. Within the cell, EGFL7 protein is localized to the endoplasmic reticulum and Golgi apparatus, suggesting that the protein is targeted for secretion. Indeed, recombinant EGFL7 is readily detectable in the supernatant media of transiently transfected HEK293 cells. We also report the identification of an Egfl7 paralog, Egfl8, and show that EGFL8 protein shares similar domains and molecular weight with EGFL7.
Keywords: Egfl7, Egfl8, gene trap screen, EGF-domain, secreted protein, mouse development, endothelial cells
INTRODUCTION
Organogenesis during vertebrate embryogenesis is dependent on the development of a functional vascular system. The first vascular structures in mammals arise in the visceral yolk sac by aggregation of mesodermal cells into angiogenic clusters. These clusters subsequently differentiate into blood islands that consist of primitive endothelial cells lining a lumen that is filled with embryonic hematopoietic cells. Two distinguishable processes occur during the subsequent formation of the vascular system during embryogenesis. Vasculogenesis refers to the initial differentiation of mesoderm-derived angioblasts into endothelial cells and their subsequent coalescence into primitive blood vessels. Angiogenesis involves the subsequent growth and remodeling of this primary capillary plexus into a mature vasculature through a process of clipping, branching, sprouting of new vessels, and the recruitment of pericytes (Risau, 1997). The processes of vasculogenesis and angiogenesis are controlled by a complex system of growth factors and their cognate receptors. These include the endothelial-restricted vascular epidermal growth factor (VEGF)/VEGF receptor (VEGFR) and Angiopoietin/Tie signaling pathways (reviewed in Rossant and Howard, 2002; Yancopoulos et al., 2000), as well as ephrins, transforming growth factor-β, and basic fibroblast growth factor, and their receptors (Tallquist et al., 1999), and signaling through Notch receptors (reviewed in Iso et al., 2003; Gridley, 2001; Rossant and Hirashima, 2003).
While the importance of these pathways for vascular development has clearly been demonstrated, it is likely that many other critical factors remain unidentified. In an effort to identify genes involved in cardiovascular development in a mouse model, we have devised a retroviral-based gene trap screen that exploited the ability of embryonic stem (ES) cells to differentiate in vitro into embryoid bodies comprising all three germ layers. This genetic screen provided us with a method to identify genes that are differentially expressed in a spatial and temporal manner during murine embryogenesis (Xiong et al., 1998, 1999; Leahy et al., 1999). One of the genes identified in the screen, Vezf1, is essential for vascular development, and Vezf1 mutant embryos display vascular remodeling defects, hemorrhaging, and abnormal lymphangiogenesis (Kuhnert et al., in press).
Here, we report the characterization of a novel, early endothelial gene that we identified in the screen, called epidermal growth factor (EGF)-like domain 7 (Egfl7). EGFL7 is a secreted protein that contains two EGF-like repeats in the central region of the protein and a putative signal peptide at the amino terminus. Of interest, Egfl7 is restricted during vascular system development to vascular endothelial cells and their mesodermal precursors. In adults, Egfl7 is expressed in highly vascularized tissues such as lung, heart, and kidney. Database searches using the Mus musculus Egfl7 cDNA revealed the existence of orthologs in the human, Xenopus laevis, and rat genome. In addition, a second mouse open reading frame that shares both EGF-like domains and is of similar molecular weight has been identified and represents a paralog of EGFL7. We have named this second gene Egfl8.
RESULTS AND DISCUSSION
Identification of Egfl7 in a Gene Trap Screen
We recently have performed a retroviral entrapment vector screen for genes with restricted expression during in vitro differentiation of ES cells and embryogenesis (Xiong et al., 1998). One of the retroviral insertions, 1-13, displayed strong alkaline phosphatase (AP) reporter gene expression in the extraembryonic mesoderm and in vascular structures of the embryo proper. Using genomic sequences that flank the retroviral integration site to probe an embryonic library, a cDNA clone corresponding to 3′ sequences of Vezf1 was identified (Xiong et al., 1999). We discovered subsequently that this clone comprised a chimeric cDNA that contained Vezf1 sequences at its 5′ end and a second open reading frame corresponding to a novel murine cDNA found in the GenBank database. The cDNA was originally named Zneu1 or Notch4-like protein (GenBank accession no. AF184973). Further comparison of genomic and cDNA sequences revealed that the retroviral insertion in the ES cell clone 1-13 had occurred in the Zneu1/Notch4-like gene (F. Kuhnert and H. Stuhlmann, unpublished observations). This finding suggested that the endothelial-specific expression pattern of the AP reporter gene observed in embryos derived from clone 1-13 (Xiong et al., 1998) reflects the expression of the endogenous Zneu1 gene rather than Vezf1.
Cloning of Egfl7 cDNA
To isolate a full-length cDNA of the novel gene, total RNA from E11.5 mouse embryos was reverse transcribed, and a fragment of approximately 1.4-kb length was amplified by polymerase chain reaction (PCR) with primers corresponding to the GenBank Zneu1 cDNA sequence. The 5′ and 3′ ends of the transcript were determined by 5′ and 3′ rapid amplification of cDNA ends (RACE), respectively. The full-length cDNA sequence aligned with the GenBank sequence of Zneu1 but extended further 5′ to mark the transcriptional start site. Thus, the 1373-bp cDNA contains 283 bp of 5′ untranslated region (UTR), an open reading frame (ORF) of 837-bp length, and 253 bp of 3′UTR (GenBank accession no. AY309459). The ORF encodes a putative 278 amino acid, 29-kDa protein with a predicted N-terminal signal peptide and two centrally located EGF-like domains (Fig. 1), with the second domain belonging to a subclass of EGF-like domains that bind Ca2+. Furthermore, a region with similarity to the DSL domain that is conserved in ligands of Notch receptors (Lindsell et al., 1995) was detected within the first EGF-like domain. The presence of a signal peptide sequence suggests that the protein is secreted. Based on its structural hallmarks and in accordance with the Mouse Genome Committee at the Jackson Laboratory and the HUGO Genomic Nomenclature Committee, the gene identified in our screen was renamed Egfl7 for epidermal growth factor-like domain 7.
Fig. 1.
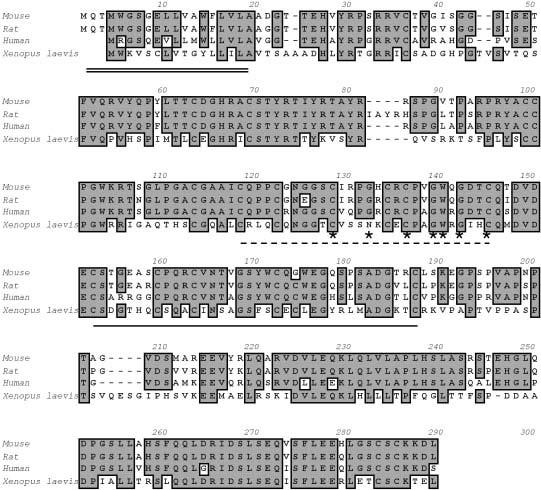
Alignment of the predicted EGFL7 proteins from mouse, rat, human, and Xenopus. The signal peptide is underlined with a double line (amino acids [aa] 1–19 in mouse and rat), the epidermal growth factor (EGF)-like domain with a dashed line (aa 111-138 in mouse), the Ca2+ EGF-like domain with a single, solid line (aa 145-180 in mouse), and the amino acids similar to the DSL domain are marked with asterisks.
EGF-like domains consist of 30 to 40 amino acids with significant identity to EGF (Gray et al., 1983). The EGF domain is found in many vertebrate proteins and occurs in various numbers of repeats from 1, in the case of prostaglandin H2 synthase 2, to greater than 30 repeats in the case of Notch proteins and fibrillin. In general, EGF domain proteins are secreted or transmembrane proteins or they are components of the extracellular matrix. One notable exception is prostaglandin G/H synthase, which is localized to the endoplasmic reticulum (van der Weiden et al., 1996). While the function of the EGF domain in each protein can vary, it generally is involved in protein–protein binding. For example, a subset of the EGF-like domains in Notch is required for the binding of the receptor to its ligand on an adjacent cell (Kojika and Griffin, 2001), and in a similar manner for the binding of laminin to nidogen (Gerl et al., 1991; Mayer et al., 1993). An EGF-like domain subfamily is believed to require the binding of Ca2+ for its function. For example, structural analysis of fibrillin revealed that Ca2+ binding to a particular EGF domain caused a structural change in the protein (Rao et al., 1995; Downing et al., 1996; Stenflo et al., 2000). In addition, mutations in amino acids important for Ca2+ binding in factor IX cause hemophilia B (Handford et al., 1991), and in fibrillin-2,they are associated with Marfan's syndrome (Putnam et al., 1995; Wu et al., 1995; Reinhardt et al., 2000).
A database search using the murine EGFL7 amino acid sequence revealed the existence of closely related sequences in the genomes of human, rat, and Xenopus laevis. Alignment of these sequences (Fig. 1) showed that mouse EGFL7 is 73% identical to the human, 88% identical to rat, and 45% identical to X. laevis orthologs, respectively. All three orthologs share the predicted signal peptide, the EGF-like domains, and the similarity with the DSL domain with EGFL7, and they also share significant sequence homologies outside these domains (Fig. 1). A search of the mouse genome database maintained by the European Molecular Biology Laboratory (EMBL) and the Sanger Institute (http://www.ensembl.org) showed that the Egfl7 gene resides on mouse chromosome 2. Alignment of Egfl7 cDNA and genomic sequences revealed that the Egfl7 gene spans an 11.5-kb region and consists of eight coding exons and two upstream noncoding exons (data not shown).
Egfl7 Is Expressed in Adult Mouse Tissues and in Endothelial Cell Lines
Northern blot analysis was performed on total RNA from adult mouse tissues and from murine and human cell lines. High levels of a 1.4-kb Egfl7-specific transcript were detected in the lung, and lower levels in heart, ovary, uterus, and kidney (Fig. 2A). Low levels of Egfl7 transcript could be detected in all other adult tissues by using the more-sensitive reverse transcriptase-PCR (RT-PCR) method (Fig. 2B). Intriguingly, the Egfl7 expression pattern in adult tissues is similar to that previously reported for two other EGF domain-containing proteins, Notch4 and its ligand Dll4 (Uyttendaele et al., 1996; Shutter et al., 2000).
Fig. 2.
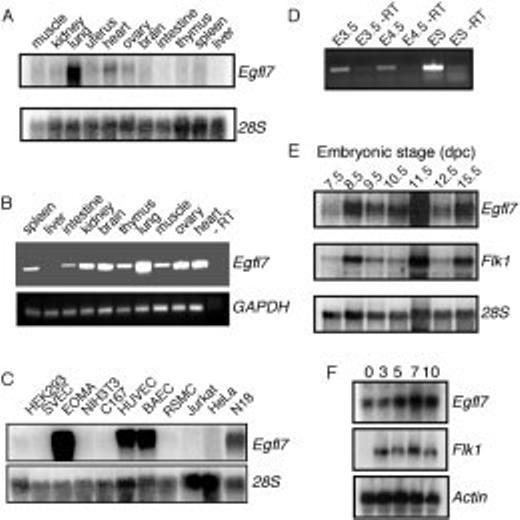
Analysis of Egfl7 expression in adult tissues, cell lines, and during mouse embryogenesis. Northern blots were hybridized with a riboprobe transcribed from the 3′ end of Egfl7. As a loading control, blots were stripped and hybridized with either a 28S riboprobe or an actin riboprobe as labeled. A: Adult mouse tissue expression of Egfl7. B: Reverse transcriptase-polymerase chain reaction (RT-PCR) amplification using the same total RNA from (A) with specific primers to Egfl7 or a loading control, GAPDH. An RT-PCR reaction without the RT is shown in the rightmost lane. C: Egfl7 expression in various mammalian cell lines. D: RT-PCR analysis of Egfl7 expression in embryonic day (E) 3.5 preimplantation and E4.5 peri-implantation mouse blastocysts and in undifferentiated embryonic stem (ES) cells. As a control, duplicate samples of total RNA were amplified without the addition of reverse transcriptase (-RT). E: Egfl7 expression during embryogenesis from E7.5 to E15.5. After hybridization to an Egfl7-specific riboprobe, the blot was stripped and probed with a flk1 riboprobe. F: Total RNA extracted during embryoid body differentiation at 0-, 3-, 5-, 7-, and 10-day time points after differentiation.
High levels of Egfl7 transcript were also detected in the adult hemangioendothelioma-derived line EOMA (Obeso et al., 1990) and in primary endothelial cells HUVEC and BAEC (Fig. 2C). In contrast, only low to background levels of Egfl7 transcript were detected in two transformed endothelial lines, the fps/fes transformed yolk sac-derived endothelial cell line C167 (Wang et al., 1996) and the SV40-transformed, peripheral lymph node-derived endothelial cell line SVEC (O'Connell and Edidin, 1990). Whereas low or no Egfl7 transcripts were detected in nonendothelial cell lines, including rat smooth muscle cells, a human T-cell leukemia cell line (Jurkat), HeLa, NIH3T3, and HEK293, detectable levels of Egfl7 transcript were present in the mouse neuroblastoma cell line N18TG2 (Fig. 2C).
Egfl7 Is Expressed Early During Mouse Embryogenesis
Total RNA was isolated from whole blastocysts and postimplantation embryos between E7.5 and E15.5 of development. Egfl7-specific transcripts were readily detectable by RT-PCR in E3.5 preimplantation and in E4.5 peri-implantation blastocysts, and in ES cells that are derived from the inner cell mass of blastocysts (Fig. 2D), indicating that Egfl7 is expressed early during embryonic development in the mouse. Northern blot analysis demonstrated that Egfl7 expression increased from E7.5 to E8.5 and then remained relatively constant (Fig. 2E). A similar expression profile in postimplantation embryos was observed for flk-1/VEGFR-2 (Fig. 2E). flk-1, an early marker for angioblasts and endothelial cells, is expressed as early as E6.5–E7.0 of embryogenesis (Yamaguchi et al., 1993; Drake and Fleming, 2000), and its function is required for the development of the endothelial and hematopoietic cell lineages (Shalaby et al., 1995, 1997). The expression profiles of Egfl7 and flk-1 were further compared by using the in vitro model system of ES cell differentiation into embryoid bodies (Wiles and Keller, 1991; Keller, 1995; Leahy et al., 1999). Overall, expression of Egfl7 and flk-1 appeared similar, with a slight increase from day 3 to a peak of expression at day 7 (Fig. 2F). However, Egfl7 but not flk-1 transcripts were detected in undifferentiated ES cells, consistent with its expression at the blastocyst stage (Fig. 2D). Thus, Egfl7 is an early embryonic gene and its expression precedes that of flk-1.
Egfl7 Expression During Embryogenesis Is Localized to the Yolk Sac Mesoderm and to the Developing Vascular System
The temporal and spatial distribution of Egfl7 transcripts during development was determined by RNA in situ hybridization on sections of staged embryos. In primitive streak stage embryos, Egfl7 was predominantly expressed in the extra-embryonic mesoderm of the visceral yolk sac at sites where blood islands originate (Fig. 3A). In addition, Egfl7 mRNA was detected in the most anterior and posterior regions of the embryo proper, corresponding to sites of precardiac mesoderm, and mesodermal cell clusters migrating from the posterior primitive streak, respectively (Fig. 3A). Immunostaining of close-by sections revealed an overlapping expression for the pan-endothelial marker PECAM-1 and the Egfl7 mRNA (compare Fig. 3A with B). Significant levels of Egfl7 transcripts were also detected in the small vessels and capillaries of the uterine wall at the site of implantation (Fig. 3A). Analysis of E8.5 embryos undergoing the turning process displayed restricted Egfl7 expression in vascular structures that arise by vasculogenesis, e.g., the paired dorsal aorta, the endocardial lining of the primitive heart, and the head vein (compare Fig. 3C with D). Egfl7 transcript levels were maximal levels at E9.5. Thus, strong punctuated in situ signals were detected in the developing vasculature such as the aortic sac, arterial chamber of the heart, endocardium, meningeal plexus of the brain, putative capillaries in the head mesenchyme, and the yolk sac blood islands (Fig. 3E,F). At this stage, the inner cells of the blood islands have differentiated into primitive nucleated erythrocytes, and the outer layer into elongated spindle-shaped endothelial cells (Fig. 3H, high magnification of the yolk sac membrane). High levels of Egfl7 transcripts were detected in the endothelial layer of the blood islands, whereas only background levels were present in the primitive erythrocytes and extra-embryonic endoderm (Fig. 3G). During later stages of organogenesis, Egfl7 transcripts appear still associated with cardiovascular structures, albeit at overall decreased levels (Fig. 3I,L). However, high levels of expression were detected in the inter-alveolar compartments of lung buds that contain a large number of small capillaries as evidenced by the strong immunoreactivity for PE-CAM-1 (compare Fig. 3M with N). No signal above the background level was detected on the epithelium of the segmented bronchi (Fig. 3M). Egfl7 expression was also detected in the endocardium, although at lower levels compared with the lung (Fig. 3O,P). In summary, the observed early onset and restricted expression of Egfl7 at the blastocyst stage and in vascular endothelial cells and their mesodermal progenitors suggest that Egfl7 is an early marker of the endothelial cell lineage. The high levels of Egfl7 transcripts detected in the lung buds, as well in the adult lung (L. Campagnolo and H. Stuhlmann, manuscript in preparation), may point to a critical role of Egfl7 in the developing lung vasculature.
Fig. 3.
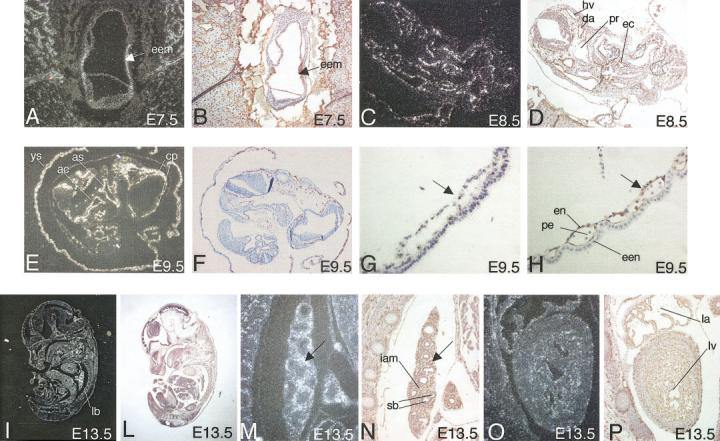
Localization of Egfl7 transcripts in mouse embryos. Staged CD-1 embryos were isolated, embedded in paraffin, and sectioned. A,C,E,G,I,M,O: Sections were processed for RNA in situ hybridization, using an antisense riboprobe corresponding to the full-length Egfl7 cDNA. B,D,F,H,L,N,P: Adjacent sections were immunostained using anti-PECAM1 antibodies. G,H: High-power magnification of yolk sacs of an embryonic day (E) 9.5 embryo shown in E,F. I,L–P: High-power magnification of lung bud (M,N) and high-power magnification of heart (O,P) of an E13.5 embryo shown in I,L. Photographs were taken under a Zeiss Axioplan microscope using darkfield optics and brightfield optics. Arrows in A,B indicate Egfl7 signal in the extra-embryonic mesoderm. Arrows in G,H indicate Egfl7 signal in the endothelial layer of the yolk sac blood islands. Arrows in M,N indicate Egfl7 signal in the capillaries of the inter-alveolar mesenchyme in lung buds. ac, atrial chamber; as, aortic sac; cp, choroid plexus within the head mesenchyme; da, dorsal aorta; ec, endocardial lining of primitive heart; eem, extra-embryonic mesoderm; een, extra-embryonic endoderm; en, endothelium of the blood island; hv, head vein; iam, inter-alveolar mesenchyme; la, lumen of left atrium; lb, lung bud; lv, lumen of left ventricle; pe, primitive erythrocytes; pr, pharyngeal region; sb, segmental bronchi.
EGFL7 Is Targeted for Secretion Through the Endoplasmic Reticulum and the Golgi Apparatus
HEK293 cells transiently transfected with a Egfl7–EGFP fusion construct displayed punctate, perinuclear EGFL7–EGFP fluorescence with a pronounced focal point to one side of the nucleus (Fig. 4A, middle column). No staining was detected in the nucleus or at the cell surface. EGFL7 signal colocalized with that of the ER-specific protein Calnexin (Bergeron et al., 1994) and the Golgi apparatus protein p58 (Bloom and Brashear, 1989; Donaldson et al., 1990; Ktistakis et al., 1991) but not with the early endosomal marker EEA1 (Fig. 4A). EGFL7 was detected by an affinity-purified polyclonal anti-EGFL7 antibody as a single band of approximately 30–32 kDa on a Western blot (Fig. 4B). Indirect immunofluorescence studies using the anti-EGFL7 antibody showed a perinuclear staining pattern of endogenous EGFL7 in EOMA cells similar to that of EGFL7–EGFP in transfected HEK293 cells and overlapping with Calnexin immunofluorescence (compare Fig. 4C with A).
Fig. 4.
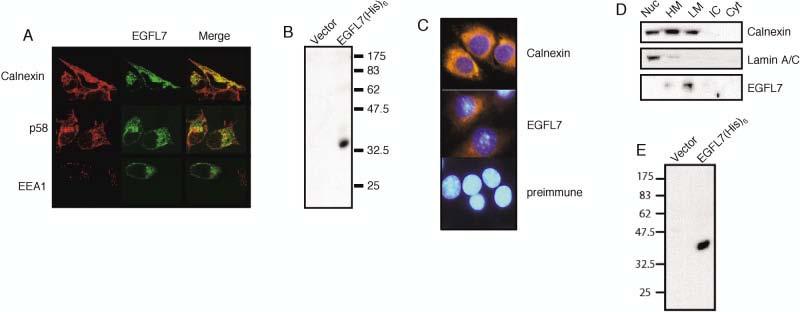
Subcellular localization of EGFL7 in HEK293 and EOMA cells. A: HEK293 cells were stably transfected with an EGFL7–EGFP fusion construct, followed by staining with either Calnexin (endoplasmic reticulum) or p58 (Golgi) specific antibodies. The double-labeled cells were analyzed by confocal microscopy. The rightmost column is an overlay of the green (enhanced green fluorescent protein [EGFP]) and red (Calnexin or p58) channels showing the yellow regions of signal overlap. B: An EGFL7 polyclonal antibody recognizes a single band in transiently transfected HEK293 cells. HEK293 cells were transiently transfected with either empty vector or EGFL7-(His)6 under the control of the CMV promoter, followed by Western blot analysis using an affinity purified polyclonal EGFL7 antibody. C: Indirect immunofluorescence analysis of Calnexin and EGFL7 in EOMA cells. Nuclear staining was achieved by staining with Hoechst 33342. D: HEK293 cells were stably transfected with an EGFL7–(His)6 fusion and fractionated by differential centrifugation. Protein samples from each cellular fraction were separated in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blot with EGFL7, Calnexin, or Lamin A/C specific antibodies. Nuc, nuclear; HM, heavy microsome; LM, light microsome; IC, insoluble cytoplasmic; Cyt, soluble cytoplasmic. E: HEK293 EBNA1 cells were transiently transfected with an EGFL7–(His)6 fusion construct under the control of the CMV promoter. Samples from the media of mock-transfected and EGFL7–(His)6-transfected cells were harvested 2 days post-transfection and analyzed by Western blot using an HRP-conjugated anti-(His)6 antibody.
The subcellular localization of EGFL7 in stably transformed HEK293 cells was further analyzed by biochemical fractionation (Feng et al., 1997), followed by Western blot analysis using antibodies against EGFL7, Calnexin (marking microsomal fractions), and lamin A/C (marking nuclear fractions; Fig. 4D). EGFL7 was readily detected in the light microsome and, to a lesser degree, in the heavy microsome fraction but was not detected in the nuclear fraction or in either the soluble or insoluble cytoplasmic fractions (Fig. 4D). Together, these results suggest that intracellular EGFL7 is being predominantly targeted to organelles of the secretory pathway.
Stable transfection of HEK293 EBNA1 cells with an EGFL7-(His)6 containing episomal expression vector resulted in high levels of stable expression (not shown; Kohfeldt et al., 1997). Recombinant EGFL7 was readily detectable by anti-His antibodies in the supernatant from cells transfected with pCEP4–Pu/AC7–EGFL7 but not the empty vector control (Fig. 4E). The apparent 41-kDa molecular mass of the recombinant EGFL7 suggests that it is post-translationally modified during the secretion process, consistent with several potential N- and O-linked glycosylation sites detected in the protein. Together, our results suggest that the EGFL7 protein is targeted through the ER and Golgi for secretion. Future experiments will examine if EGFL7 functions as a growth factor or cytokine through specific receptor interactions and if it acts in an autocrine or paracrine manner. Alternatively, EGFL7 may interact with proteins of the endothelial extracellular matrix, possibly by means of the EGF domains.
Identification of a Mouse Paralog, EGFL8
A BLAST search of the mouse genome using the EGFL7 amino acid sequence revealed the existence of a paralog gene that encodes a putative protein of 293 amino acids and is located on mouse chromosome 17 (GenBank accession no. NM 152922). In agreement with the Mouse Nomenclature Committee, we have named this gene Egfl8, for EGF-like domain 8. Although the overall amino acid identity between EGFL7 and EGFL8 is relatively low (35%; Fig. 5A), the two proteins share the same overall domain structure, including an EGF-like domain, a Ca2+ binding EGF-like domain, and a N-terminal signal peptide. Of interest, the respective chromosomal regions at the Egfl7 locus on chromosome 2 and the Egfl8 locus on chromosome 17 are similar. They share other paralogous genes, including Notch1/Notch4 and Pbx3/Pbx2, and they are synthenic to the MHC regions on human chromosomes 9 and 6, respectively. A third region that contains additional paralogs of Notch and Pbx, but apparently no Egfl7/8 paralog, has been identified on mouse chromosome 1 and human chromosome 1. Recent paralogy mapping suggested that these three regions arose by triplication events from a common ancestor before the human–mouse divergence (Katsanis et al., 1996; Yu et al., 2000).
Fig. 5.
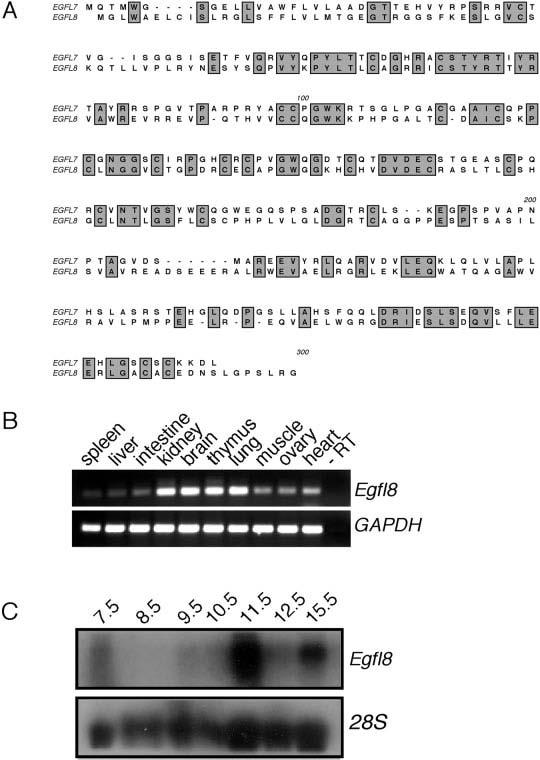
Cloning and expression of the EGFL7 mouse paralog EGFL8. A: Alignment of the mouse EGFL7 and EGFL8 proteins. Regions of identity are indicated by grey shading. B: Reverse transcriptase (RT)-polymerase chain reaction analysis of Egfl7 and Egfl8 expression in adult mouse tissues. Specific intron-spanning primers that amplify Egfl7, Egfl8, or GAPDH were used for polymerase chain reaction amplification. C: Northern blot analysis of Egfl8 expression during embryogenesis from embryonic day (E) 7.5 to E15.5. As a loading control, blots were stripped and hybridized to an 28S riboprobe.
The similarity in protein domain architecture between EGFL7 and EGFL8, as well as the possibility that they arose through a chromosomal duplication event, led us to investigate if they show similar expression patterns. The expression profiles of Egfl8 and Egfl7 in adult organs are similar, with the highest levels of expression in the kidney, brain, thymus, and lung (Fig. 5B; compare with Fig. 2B). However, embryonic expression is not detected before E11.5, and transcript levels vary between E11.5 and E15.5 (Fig. 5C; compare with Fig. 2E). Thus, although structurally related, EGFL7 and EGFL8 may not overlap in their function during embryonic development.
In summary, our results identified a novel endothelial gene, Egfl7, and its paralog Egfl8. Egfl7 is expressed early during embryogenesis and is restricted to the vascular endothelium and their mesodermal progenitors in the extraembryonic mesoderm. Egfl7 encodes a protein with an N-terminal signal peptide and two internal EGF-like domains that is targeted for secretion through the ER and Golgi apparatus. These studies suggest a distinct role for Egfl7 during the development of the murine vasculature, and possibly in the mature vasculature of the adult mouse.
The GenBank accession number for the full-length murine Egfl7 cDNA is AY309459.
EXPERIMENTAL PROCEDURES
Cloning of Egfl7 cDNA
Total RNA from E11.5 CD-1 mouse embryos (morning of vaginal plug was counted as E0.5) was used as a template for RT-PCR (Superscript II kit, Invitrogen, Carlsbad, CA). PCR primers used for Egfl7 amplification were 5′-GTAGGGCTCTGCCGGGA-3′ (forward primer), and 5′-TCAAGTCTCAGCTTTATTATGACAAAGA-3′ (reverse primer). The 5′ and 3′ RACE reactions (First Choice RACE-ready cDNA from E9.5 mouse embryos, Ambion, Inc., Austin, TX) were carried out according to the manufacturer's instructions. The nested primers used for the 5′ RACE reaction were 5′-TCTTCTTTTTGTGGCCTGTGGT-3′ (outer primer) and 5′-TGCCGGGGGCTTCCCACAGG-3′ (inner primer). The single primer used for the 3′ RACE was 5′-CTGTCTGGTGGTGCCTATGA-3′.
Construction of Egfl7 Expression Plasmids
The Enhanced Green Fluorescent Protein (EGFP) fused to the C-terminus of EGFL7 was generated by cloning the Egfl7 ORF into the pEGFP-N1 vector (Clontech). An 834-bp Egfl7 fragment was amplified by PCR with 5′-CTGCAAGCTTGCCACCATGCAGACCATGTGGGGCTCC-3′ (forward primer) and 5′-CTGCGGATCCCCCAGATCTTTTTTGCAGGAGCA-3′ (reverse primer). An EGFL7–(His)6 fusion was generated by cloning the PCR-amplified Egfl7 ORF into the pET40b(+) vector (Novagen, Madison, WI), using 5′-CTGCGGATCCCATGCAGACCATGTGGGGCTCC-3′ (forward primer) and 5′-CTGCCTCGAGCAGATCTTTTTTGCAGGA-3′ (reverse primer). The His-tagged EGFL7 construct was then subcloned into pCDNA3 (Invitrogen, Carlsbad, CA) and the episomal vector pCEP-PU/AC7 (Kohfeldt et al., 1997), respectively.
Cell Culture and Transfections
HEK293, HEK293 EBNA1, and EOMA cells were grown using standard condition. HUVEC cells were maintained in EGM-2 endothelial cell medium (Cambrex, East Rutherford, NJ). Transfections (500 μg of plasmid DNA each) were carried out in duplicates in six-well dishes using Effectene transfection reagent (Qiagen, Valencia, CA). HEK293 EBNA1 cells were transfected with the pCEP–PU/AC7–EGFL7–(His)6 construct and selected by using 3-μg/ml puromycin for 3 days.
RNA Preparation, RT-PCR, and Northern Blot Analysis
Total RNA was prepared from staged postimplantation embryos, tissues, and cell monolayers by using guanidinium–CsCl gradients. Blastocysts (15 and 20 each, respectively) were collected by flushing uterine tubes from pregnant mice with DMEM containing 1% bovine serum albumin, and RNA was prepared using Trizol (Invitrogen, Carlsbad, CA).
RT-PCR was performed by using the Superscript II kit (Invitrogen) using the following primers: 5′-CCACAAAAAGAAGAAGGCTACCC-3′ (Egfl7 forward primer), 5′-TCCAAGAAGGACCCTGCTCACTC-3′ (Egfl7 reverse primer); 5′-AAGCAGACAGCGAAGAGGAG-3′ (Egfl8 forward primer), 5′-TTCCTCCAGTAGCAGCACCT-3′ (Egfl8 reverse primer); 5′-ACCCAGAAGACTGTGGATGG-3′ (GAPDH forward primer), 5′-GAGACAACCTGGTCCTCAG-3′ (GAPDH reverse primer).
Samples (10 μg) of total RNA were analyzed on Northern blots using standard procedures. [α-32P]UTP-labeled riboprobes were synthesized from an Egfl7-specific PCR template (nucleotides 908–1104), an Egfl8-specific template (nucleotides 869–997 of the Ensembl database-predicted cDNA sequence), and a flk-1-specific template (nucleotides 1039–1279 of the flk-1 cDNA), respectively, using a T7 RNA polymerase kit (Strip-EZ RNA, Ambion). Riboprobes specific for 28S rRNA, or actin mRNA, respectively, were used to control for RNA loading.
RNA In Situ Hybridization and PECAM-1 Immunohistochemistry of Mouse Embryos
Staged CD-1 mouse embryos were dissected from their deciduas, fixed, and embedded in paraffin. Full-length Egfl7 cDNA, subcloned in both orientations into the pCRII-TOPO vector (Invitrogen), was used as a template for the synthesis of riboprobes. Sense and antisense probes were linearized with BamHI and transcribed from the T7 promoter. RNA in situ hybridization was performed as described (Leahy et al., 1999). Darkfield photographs were taken by using a Zeiss Axioplan 2 microscope.
Deparaffinized slide sections were treated with Proteinase K (10 mg/ml in 0.2 M Tris HCl, pH 7.2, 20 min at 37°C), incubated in TNB blocking solution (TSA-Indirect Kit, NEN), and immunostained with anti-PECAM1 antibody (2.5 μg/ml, overnight at 4°C, B-D Pharmingen). PECAM-1 antibody was revealed with a biotinylated rabbit anti-rat antibody (1:200 dilution, Vector Labs, Burlingame, CA) and the TSA-Indirect Kit (NEN Life Sciences).
EGFL7 Polyclonal Antibody Production
Rabbit polyclonal antibodies were raised (Covance, Inc., Princeton, NJ) against full-length EGFL7 that was fused in frame to glutathione S-transferase and expressed in Escherichia coli strain BL21(DE3) as inclusion bodies. Affinity-purified EGFL7 antibodies were prepared using a carboxyl-terminal fragment (amino acids 167–278) and used at a 1/1,000–1/2,000 dilution in Western blot experiments, and at a 1/100 dilution for immunofluorescence.
Western Blot Analyses and Antibodies
Denatured protein samples were resolved on 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Bedford, MA) by using standard procedures. Specific antibodies against EGFL7 (1/2,000 dilution), Calnexin (1/2,000 dilution), and lamin A/C (1/1,000 dilution), HRP-conjugated anti-(His)6 (1:2,500; Invitrogen, Carlsbad, CA) and horseradish peroxidase-conjugated goat anti-mouse or donkey anti-rabbit IgG (Amersham Biosciences Corp, Piscataway, NJ) were used. Specific bands were detected with ECL Plus Western Blot Reagent (Amersham Biosciences Corp).
Immunofluorescence
HEK293 cells stably transfected with Egfl7–EGFP were grown on coverslips in six-well dishes. The following antibody dilutions in phosphate buffered saline/5% normal goat serum were used: 1/500 for anti-Calnexin, 1/100 for anti-p58, and 1/100 for anti-EGFL7. Cy3-conjugated secondary antibodies (Chemicon International, Temecula, CA) were used at 1/2,000 dilution. The mounted coverslips were analyzed by confocal microscopy.
Subcellular Fractionation
Subcellular fractionation was performed essentially as described (Feng et al., 1997). Four 10-cm plates of HEK293 cells stably transfected with a pCDNA3-EGFL7-(His)6 construct grown to 90% confluence were used as a starting material. The resulting nuclear, light microsome, heavy microsome, soluble cytoplasmic, and insoluble cytoplasmic fractions were analyzed by Western blot analysis.
ACKNOWLEDGMENTS
We thank Dr. Gregorio Siracusa for help with collecting mouse blastocysts and Dr. Bill Kiosses for help with confocal microscopy. We also thank Wendy LeVine for expert mouse husbandry and histology. We thank our colleagues at The Scripps Research Institute, Drs. Fan-Li Chou and Mark Ginsberg for lamin antibodies, and Drs. Oliver Pertz and Klaus Hahn for the pCEP-PU/AC7 plasmid and HEK293 EBNA1 cells. Finally, we thank members of the Stuhlmann lab and the Vascular Biology Division at The Scripps Research Institute for suggestions on the manuscript. While this manuscript was under review, a study with similar results was published (Soncin et al. [2003], EMBO J. 22: 5700).
Footnotes
Grant sponsor: National Institutes of Health; Grant numbers: R01 HL65738; R21 HL72270.
REFERENCES
- Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Brashear TA. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- Donaldson JG, Lippincott-Schwartz J, Bloom GS, Kreis TE, Klausner RD. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990;111:2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing AK, Knott V, Werner JM, Cardy CM, Campbell ID, Handford PA. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell. 1996;85:597–605. doi: 10.1016/s0092-8674(00)81259-3. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl M, Mann K, Aumailley M, Timpl R. Localization of a major nidogen-binding site to domain III of laminin B2 chain. Eur J Biochem. 1991;202:167–174. doi: 10.1111/j.1432-1033.1991.tb16358.x. [DOI] [PubMed] [Google Scholar]
- Gray A, Dull TJ, Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983;303:722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Gridley T. Notch signaling during vascular development. Proc Natl Acad Sci U S A. 2001;98:5377–5378. doi: 10.1073/pnas.101138098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991;351:164–167. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Fitzgibbon J, Fisher EM. Paralogy mapping: identification of a region in the human MHC triplicated onto human chromosomes 1 and 9 allows the prediction and isolation of novel PBX and NOTCH loci. Genomics. 1996;35:101–108. doi: 10.1006/geno.1996.0328. [DOI] [PubMed] [Google Scholar]
- Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Kohfeldt E, Maurer P, Vannahme C, Timpl R. Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett. 1997;414:557–561. doi: 10.1016/s0014-5793(97)01070-3. [DOI] [PubMed] [Google Scholar]
- Kojika S, Griffin JD. Notch receptors and hematopoiesis. Exp Hematol. 2001;29:1041–1052. doi: 10.1016/s0301-472x(01)00676-2. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Roth MG, Bloom GS. PtK1 cells contain a nondiffusible, dominant factor that makes the Golgi apparatus resistant to brefeldin A. J Cell Biol. 1991;113:1009–1023. doi: 10.1083/jcb.113.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy A, Xiong JW, Kuhnert F, Stuhlmann H. Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J Exp Zool. 1999;284:67–81. doi: 10.1002/(sici)1097-010x(19990615)284:1<67::aid-jez10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Shawber CJ, Boulter J, Weinmaster G. Jagged: a mammalian ligand that activates Notch1. Cell. 1995;80:909–917. doi: 10.1016/0092-8674(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Mayer U, Nischt R, Poschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 1993;12:1879–1885. doi: 10.1002/j.1460-2075.1993.tb05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J, Weber J, Auerbach R. A hemangioendothelioma-derived cell line: its use as a model for the study of endothelial cell biology. Lab Invest. 1990;63:259–269. [PubMed] [Google Scholar]
- O'Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 1995;11:456–458. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- Rao Z, Handford P, Mayhew M, Knott V, Brownlee GG, Stuart D. The structure of a Ca(2+)-binding epidermal growth factor-like domain: its role in protein-protein interactions. Cell. 1995;82:131–141. doi: 10.1016/0092-8674(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Ono RN, Notbohm H, Muller PK, Bachinger HP, Sakai LY. Mutations in calcium-binding epidermal growth factor modules render fibrillin-1 susceptible to proteolysis. A potential disease-causing mechanism in Marfan syndrome. J Biol Chem. 2000;275:12339–12345. doi: 10.1074/jbc.275.16.12339. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rossant J, Hirashima M. Vascular development and patterning: making the right choices. Curr Opin Genet Dev. 2003;13:408–412. doi: 10.1016/s0959-437x(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- Stenflo J, Stenberg Y, Muranyi A. Calcium-binding EGF-like modules in coagulation proteinases: function of the calcium ion in module interactions. Biochim Biophys Acta. 2000;1477:51–63. doi: 10.1016/s0167-4838(99)00262-9. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P, Klinghoffer RA. Growth factor signaling pathways in vascular development. Oncogene. 1999;18:7917–7932. doi: 10.1038/sj.onc.1203216. [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- van der Weiden RM, Wisse LJ, Helmerhorst FM, Keirse MJ, Poelmann RE. Immunohistochemical and ultrastructural localization of prostaglandin H synthase in the preimplantation mouse embryo. J Reprod Fertil. 1996;107:161–166. doi: 10.1530/jrf.0.1070161. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Greer P, Auerbach R. Isolation and propagation of yolk-sac-derived endothelial cells from a hypervascular transgenic mouse expressing a gain-of-function fps/fes proto-oncogene. In Vitro Cell Dev Biol Anim. 1996;32:292–299. doi: 10.1007/BF02723062. [DOI] [PubMed] [Google Scholar]
- Wiles MV, Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wu YS, Bevilacqua VL, Berg JM. Fibrillin domain folding and calcium binding: significance to Marfan syndrome. Chem Biol. 1995;2:91–97. doi: 10.1016/1074-5521(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Xiong JW, Battaglino R, Leahy A, Stuhlmann H. Large-scale screening for developmental genes in embryonic stem cells and embryoid bodies using retroviral entrapment vectors. Dev Dyn. 1998;212:181–197. doi: 10.1002/(SICI)1097-0177(199806)212:2<181::AID-AJA4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Xiong JW, Leahy A, Lee HH, Stuhlmann H. Vezf1: a Zn finger transcription factor restricted to endothelial cells and their precursors. Dev Biol. 1999;206:123–141. doi: 10.1006/dbio.1998.9144. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yu YC, Yang Z, Blanchong CA, Miller W. The human and mouse MHC class III region: a parade of 21 genes at the centromeric segment. Immunol Today. 2000;21:320–328. doi: 10.1016/s0167-5699(00)01664-9. [DOI] [PubMed] [Google Scholar]


