Abstract
Classic work by Huggins and Hodges demonstrated that human prostate cancer regresses dramatically during antihormonal therapy but recurs frequently with androgen independence. Perturbations in the androgen receptor (AR) and PTEN–AKT signaling axes are significantly correlated with the progression of prostate cancer. Genetic alterations of the AR cause receptor hypersensitivity, promiscuity, and androgen-independent receptor transactivation. Prostate cancers maintain an elevated AKT activity through the loss of PTEN function or the establishment of autocrine signaling by growth factors and cytokines. We used an in vivo prostate regeneration system to investigate the biological potency of the potential crosstalk between these two signal transduction pathways. We demonstrate a direct synergy between AKT and AR signaling that is sufficient to initiate and progress naïve adult murine prostatic epithelium to frank carcinoma and override the effect of androgen ablation. Both genotropic and nongenotropic signals mediated by AR are essential for this synergistic effect. However, phosphorylation of AR by AKT at Ser-213 and Ser-791 is not critical for this synergy. These results suggest that more efficient therapeutics for advanced prostate cancer may need to target simultaneously AR signaling and AKT or the growth factor receptor tyrosine kinases that activate AKT.
Keywords: prostate regeneration, tissue structure
Fundamental changes take place in androgen receptor (AR) signaling during prostate cancer progression (1, 2). AR signaling mediates cell differentiation and growth inhibition in the normal adult human and murine prostate (3–6). For example, AR can induce the expression of the luminal cell differentiation marker prostate-specific antigen (PSA) and the cell-cycle inhibitor p21. Transgenic mice harboring a prostate-specific AR transgene develop normal prostate tissues or show mild hyperplasia after a long latency period (7, 8). However, cell autonomous androgen signals stimulate the proliferation of human or rodent AR-positive cancer cells (9). AR signaling also plays a critical role in the transition of prostate cancer from the androgen-dependent to -independent stage. Increased transcriptional level of AR mRNA has been demonstrated as the most common molecular determinant of resistance to antiandrogen therapy in a xenograft castration model (1).
Loss of function of PTEN and activation of AKT are significantly correlated with the progression of prostate cancer (10). In vivo studies showed that murine prostatic intraepithelial neoplasia (mPIN) develops in transgenic mice expressing activated AKT1 specifically in the prostate (11). Previous in vitro studies using long-term passaged cancer cell lines have demonstrated that PTEN and AKT are, respectively, negative and positive modulators of AR transcriptional activity (12–15). Some papers have reported that AKT negatively regulates AR protein levels and transcriptional activity at the posttranscriptional (16) and posttranslational (17, 18) levels.
We have modified and improved a prostate cell regeneration assay in which dissociated adult murine prostate epithelial cells are combined with embryonic urogenital sinus mesenchymal (UGSM) cells and engrafted under the kidney capsule of immunodeficient host CB.17SCID/SCID mice to regenerate murine prostate tissue (19, 20). The Sca-1 surface antigen can enrich for prostatic stem cell activity, and a single stem/progenitor cell is capable of regenerating a whole tubule containing both basal and luminal cells in such regeneration experiments (21, 22). Single genetic perturbations in the PTEN–AKT signaling axis introduced into the dissociated prostate epithelial cells by lentivirus are sufficient to initiate mPIN lesions in this regeneration system within 6 weeks (21). We used this assay to evaluate the biological consequences of combined AKT and AR signaling in murine prostate epithelia. Our data demonstrate a direct synergy between AKT and AR signaling that is sufficient to initiate and progress naïve adult murine prostatic epithelium to frank carcinoma and override the effect of androgen ablation. Both genotropic and nongenotropic signals mediated by the AR are essential for this synergistic effect.
Results
The AKT and AR Signaling Pathways Synergize to Promote the Progression of Prostate Cancer.
Lentiviral vectors were designed to express GFP, AR, or activated AKT. Dissociated adult murine prostate cells were infected with the GFP lentivirus alone, AKT lentivirus alone, AR lentivirus alone, or both the AKT and AR lentivirus. A mixture of prostate epithelial cells that were separately infected with either the AKT or the AR lentivirus was included to investigate whether the AKT and AR signaling pathways interact in a cell-autonomous or trans manner. The prostate cells were then combined with urogenital sinus mesenchymal cells and engrafted under the kidney capsules of CB.17SCID/SCID mice (21). Mice were killed 6 weeks later, and grafts were collected. Two and a half hours before the mice were killed, BrdU was injected i.p. (80 mg/kg) to label proliferating cells.
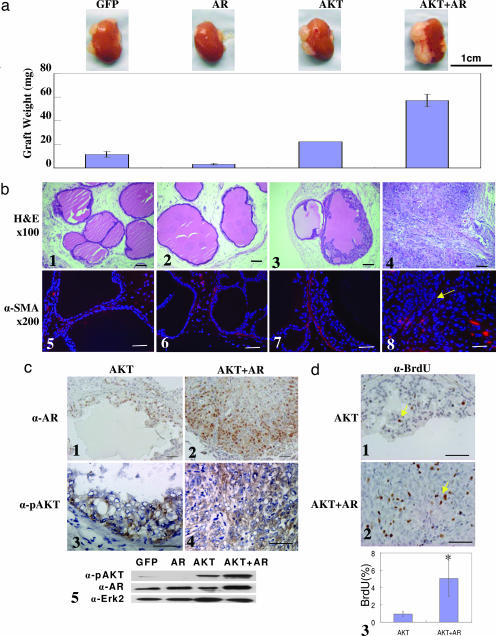
Fig. 1a shows that regenerated tissues derived from cells infected by AKT weighed approximately twice as much as the GFP control grafts, but tissues regenerated from cells infected with AR weighed significantly less than controls. This finding suggests that increased expression of AR alone impairs the regenerative capacity of the dissociated prostate cells, which is consistent with previous in vitro data showing that high-level expression of AR attenuates prostate cell growth and induces cell differentiation (5, 6). Strikingly, tissues regenerated from the cells infected with AKT and AR weighed three times more than did those in the AKT group. However, tissues regenerated from the mixed sample of cells that were infected with AKT or AR separately were similar in size to those in the AKT group (data not shown). These data suggest that signals mediated by AKT and AR can strongly synergize in a cell-autonomous manner but not in trans.
Fig. 1.
AKT and AR synergize and progress naïve prostatic epithelium to invasive adenocarcinoma in a dissociated prostate cell regeneration method within 6–8 weeks. (a) Weight (± SEM) of regenerated tissues derived from prostatic epithelial cells infected by the indicated lentivirus combinations (n ≥ 4). The micrograph above each bar shows a representative picture of the regenerated tissues attached to the murine kidneys. (Scale bar: 1 cm.) (b) H&E staining of the regenerated tissues (b1–b4) and IHC analysis for SMA in the regenerated tissues (b5–b8). The yellow arrow indicates the absence of the SMA-positive stromal layer. (c) IHC staining for AR (c1 and c2) and phospho-AKT (c3 and c4) in regenerated tissues in the AKT and AKT-plus-AR groups, respectively, and Western blot analysis of the AR and phospho-AKT (c5). Erk2 is used as a loading control. (d) IHC analysis for BrdU in regenerated tissues in the AKT (d1) and AKT-plus-AR (d2) groups. (d3) Quantification of BrdU-positive cells in the AKT and AKT-plus-AR groups (∗, P < 0.001). (Original magnification: ×200. Scale bars: 100 μm.)
Histological analysis (Fig. 1b Upper) shows that regenerated tissues in the GFP group and the AR group contain normal tubular structures. Regenerated tissues in the AKT group contained mPIN lesions defined by stratifications of epithelial cells displaying nuclear atypia. Grafts regenerated from the mixture of cells infected with either AKT or AR lentivirus also contained mPIN lesions (data not shown). In contrast, tissues in the AKT-plus-AR group were composed of sheets of carcinoma cells without glandular structures. Immunohistochemical (IHC) staining for smooth muscle actin (SMA) (Fig. 1b Lower) shows that stromal cells form a continuous layer, surrounding the regenerated tubules in the GFP, AR, and AKT groups. However, the stromal layers were discontinuous or absent in the AKT-plus-AR group (Fig. 1b, yellow arrow). IHC and Western blot analyses (Fig. 1c) demonstrate that the AR and phospho-AKT were expressed as expected in the regenerated mPIN and carcinoma tissues. BrdU staining demonstrated a 4- to 5-fold increase in the cellular proliferation index in the regenerated carcinoma (Fig. 1d).
Synergy Between AKT and AR Signaling Causes Androgen-Insensitive Prostate Cancer.
Dissociated prostate epithelial cells from wild-type C57BL/6 mice were combined with urogenital sinus mesenchymal cells and engrafted under the kidney capsule of the immunodeficient CB.17SCID/SCID mice for 6 weeks. Host mice were then castrated, and grafts were collected 4 weeks after castration. Hematoxylin/eosin (H&E) staining showed that the epithelial cell layers are disrupted after androgen ablation, and the columnar epithelial cells appear flat. IHC analysis showed that the AR was exported from the nucleus to the cytoplasm, and basal cells are enriched in regenerated tissues as shown by immunostaining for the basal cell marker p63 (23). Tissues generated from wild-type adult murine prostate cells regress after androgen ablation similar to normal prostate (data not shown).
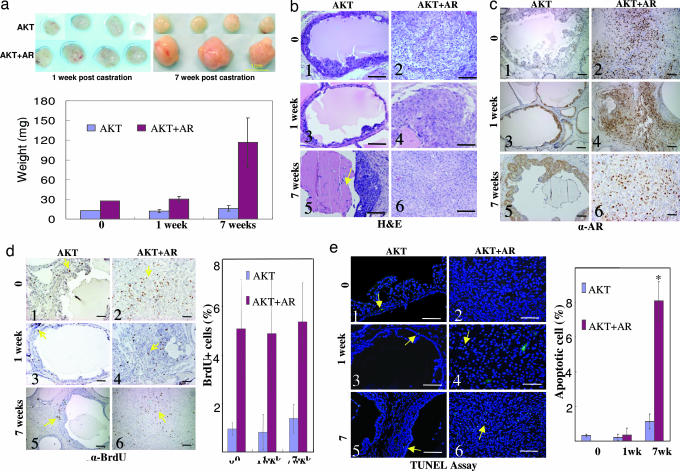
Dissociated prostate cells were infected with AKT alone or AKT plus AR and engrafted into two groups of mice. Five weeks after engraftment, tissues generated in the AKT-plus-AR group consistently grew larger than those in the AKT group (Fig. 2a). Seven weeks after castration, the weight of the grafts in the AKT group barely changed, whereas the grafts in the AKT-plus-AR group continued to grow and weighed approximately three times more. H&E staining demonstrates that mPIN lesions and carcinoma were generated in the AKT-alone group and the AKT-plus-AR group before castration (Fig. 2b Top). After castration, mPIN lesions generated in the AKT group were reduced from cell shrinkage, and increased apoptotic debris was observed inside the lumens (Fig. 2 b3 and b5, yellow arrow). No change in histology was observed in the carcinoma in the AKT-plus-AR group, except that there were occasional necrotic foci (Fig. 2 b4 and b6, and additional data not shown). AR was exported from the nucleus and was predominantly localized cytoplasmically in the generated mPIN lesions after castration (Fig. 2c Left). However, clear nuclear localization of AR was observed in the adenocarcinoma (Fig. 2c Right), perhaps because excess AR regulated by the non-androgen-responsive human ubiquitin promoter is hypersensitive to residual adrenal androgen. The cellular proliferation index was 4-fold higher in the adenocarcinoma than in the mPIN lesions by BrdU analysis (Fig. 2d). However, there was no significant change in the proliferation index in each group before and after castration, suggesting that androgen ablation does not attenuate cellular proliferation induced by AKT. The TUNEL assay demonstrated that the apoptotic index in the regenerated tissues was low in both groups before and 1 week after castration (<0.5%) but increased significantly in both the AKT group (1%) and the AKT-plus-AR group (8%) 7 weeks after castration (Fig. 2e). We conclude that the proproliferative synergistic effects of AKT and AR override the effects of androgen ablation.
Fig. 2.
Synergy of AKT and AR overrides the effect of androgen ablation. (a) Regenerated tissues in the AKT (Upper) and AKT-plus-AR (Lower) group 1 week (Left) and 7 weeks (Right) after castration. Three mice were included in each group, and four grafts were implanted in each mouse. (Scale bar: 1 cm.) The Bottom chart shows the weight (± SEM) of the regenerated tissue (n ≥ 3). (b) H&E staining of regenerated tissues before and after castration. (c) IHC staining for AR in the tissues before and after castration. (d) IHC staining of the BrdU in the regenerated tissues before and after castration. Yellow arrows indicate the BrdU-positive staining. The chart on the right shows quantification. (e) TUNEL assay of the regenerated tissues before and after castration. Yellow arrows indicate green apoptotic cells. (Original magnification: ×200. Scale bars: 100 μm.) The chart on the right shows quantification (∗, P < 0.01 compared with before castration and 1 week after castration).
Synergy of AKT and AR Is Dependent on both the Genotropic and Nongenotropic Signaling of the AR.
Binding of androgen induces receptor nuclear translocation, dimerization, binding to DNA, and recruitment of transcriptional machinery to activate downstream signaling. Additionally, in a nongenotropic signaling pathway, AR is also capable of binding and activating c-Src kinase through a polyproline region to stimulate prostate cancer cell proliferation and bone growth (24–26). The stability of AR is also reported to be modulated by AKT through phosphorylation (13, 14, 17, 18). In the ARS213A/S791A mutant, the two putative AKT phosphorylation serines (Ser-213 and Ser-791) are mutated to alanines (12, 13, 18). This mutant acts similarly to wild-type AR in an in vitro transcriptional reporter assay (12). The N705S (27), ΔNLS (1), and V581F (28) mutants are defective in AR ligand binding, nuclear translocation, and DNA binding, respectively. The ΔPro mutant (deletion of the polyproline region) has been shown to act similarly to the wild-type AR in promoting androgen-independent growth of LNCaP human prostate cancer cells, but it lacks activity in the AR nongenotropic signaling pathway (1, 25, 29).
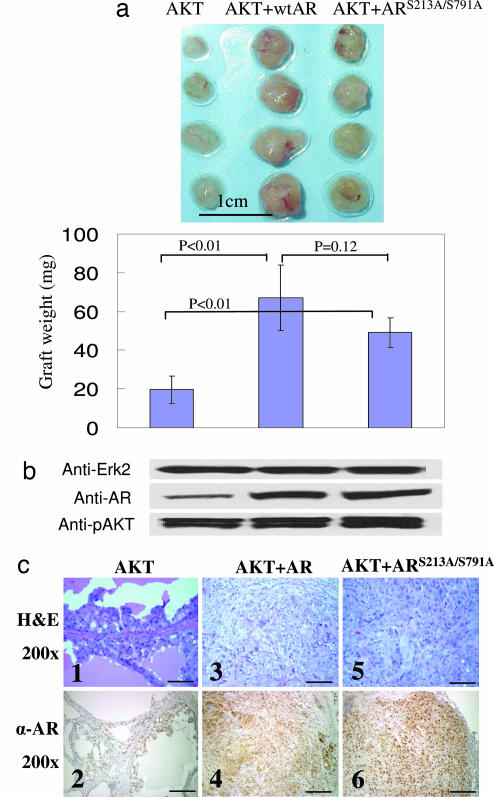
As shown previously, tissues regenerated from cells infected with AKT and wild-type AR grew larger than those derived from cells infected with AKT alone (Fig. 3a). The grafts in the AKT-plus-ARS213A/S791A group were also significantly larger than the control AKT grafts. Western blot analysis shows that the expression levels of phospho-AKT and AR were similar (Fig. 3b), but invasive carcinoma was found only in the AKT-plus-AR and AKT-plus-ARS213A/S791A groups (Fig. 3c). These data suggest that phosphorylation of AR at Ser-213 and Ser-791 by AKT is not required for the synergy of AKT and AR, consistent with previous in vitro work showing that AKT does not have an effect on AR transcription by direct phosphorylation (12).
Fig. 3.
The putative AKT phosphorylation sites, Ser-213 and Ser-791, in the AR are not critical for the synergy of AKT and AR. (a Upper) The images are of regenerated tissues derived from cells infected with AKT lentivirus alone and AKT lentivirus in combination with AR or ARS213A/S791A mutant lentivirus. (Scale bar: 1 cm.) (a Lower) The chart shows the quantification of the weight (± SEM) of the grafts (n = 4). Regenerated tissues in the AKT-plus-AR group and AKT-plus-ARS213A/S791A weighed significantly more than those in the AKT group (P < 0.01). However, there was no significant difference between the weight of the tissues in the AKT-plus-AR group and AKT-plus-ARS213A/S791A group (P = 0.12). (b) Western blot analysis of phospho-AKT and AR. Erk2 was used as a loading control. (c) H&E staining (Upper) and IHC analysis of the AR (Lower) of the regenerated tissues. (Original magnification: ×200. Scale bars: 100 μm.)
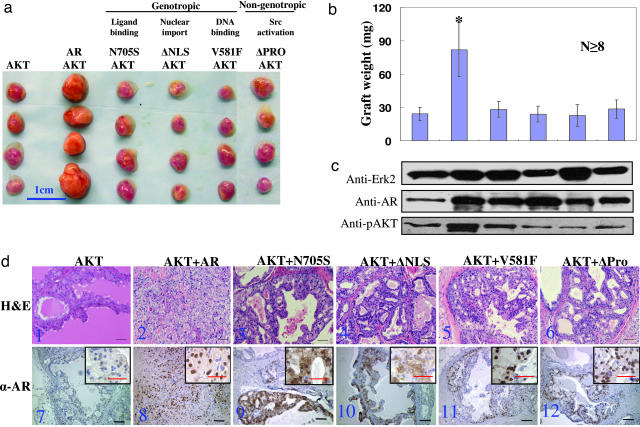
However, when the N705S, V581F, ΔNLS, and ΔPro mutants of AR were used in combination with AKT, the weights of the regenerated tissues were similar to those regenerated from cells infected with AKT alone (Fig. 4a and b). Western blot analysis demonstrated that the lentiviral driven expression level of wild-type AR and the different AR mutants was similar in each group (Fig. 4c). H&E staining showed that whereas tissues regenerated from cells infected with AKT plus wild-type AR contained carcinoma, only mPIN lesions were observed in tissues in all of the other groups (Fig. 4d Upper). IHC analysis confirmed the behavior of the AR mutants by their cellular localization (Fig. 4d Lower). These combined data show that both the genotropic and nongenotropic signaling pathways mediated by the AR are critical for the biological synergy of AKT and AR.
Fig. 4.
Both the genotropic and nongenotropic signaling of the AR are critical for the synergy of AKT and AR. (a) A series of AR mutants and their defects in signaling. Shown is a representative picture of regenerated tissues derived from cells infected with AKT lentivirus alone and AKT lentivirus in combination with AR or AR mutant lentivirus. (Scale bar: 1 cm.) (b) Quantification of the weight (± SEM) of the regenerated grafts (n ≥ 8; ∗, P < 0.0001 compared with other groups). (c) Western blot analysis of phospho-AKT and AR or AR mutants. Erk2 is used as a loading control. (d) H&E staining (d1–d6) and IHC staining (d7–d12) of AR in the regenerated tissues. (Original magnification: ×200. Magnification of Insets: ×400. Scale bars: 100 μm.)
Discussion
We have investigated the consequence of the dual activation of AR and AKT signaling in a biological context. Previous work using cell-culture models has generated alternative views about the potential crosstalk between AKT and AR signaling by using reporter assays that measure AR transcriptional activity. AKT has been claimed either to activate or to suppress AR transcriptional activity, depending on the cell types and passage number of the cells used in the assays (14, 17, 18). In this paper we demonstrate a direct synergy between AKT and AR signaling that can transform naïve prostatic epithelium into androgen-insensitive, but AR-dependent, carcinoma. Phosphorylation of AR at Ser-213 and Ser-791 by AKT can cause AR degradation when coexpressed in COS-1 cells (17). However, we do not see any change in the expression level of the wild-type AR and the ARS213A/S791A mutant in the presence of AKT in the regenerated carcinomas. On the contrary, we noticed a slight increase in the expression level of the endogenous AR in the AKT-induced mPIN lesions compared with the control normal tissues by immunostaining (data not shown). These studies suggest that AKT regulates the expression and stability of the AR at multiple levels ranging from transcriptional to posttranslational modulation.
Dissociated prostate epithelial cells or Sca-1 surface antigen-enriched prostate stem cells expressing AKT are capable of generating mPIN lesions, whereas the simultaneous expression of AR and activated AKT in adult murine prostate cells leads to the generation of adenocarcinoma without distinctive glandular structures. It is possible that the carcinoma progressed from mPIN lesions. AKT and AR may target terminally differentiated cells that lack the ability to initiate glandular morphogenesis.
We showed that both the AR nuclear signaling and nongenotropic signaling through c-Src are required for the synergy of AKT and AR. It has been shown previously that the AR-mediated nongenotropic signal through the Src kinase may provide a positive feedback on the traditional AR nuclear signal transduction pathway. Mitogen-activated protein kinase, a downstream target of the Src kinase, has been shown to phosphorylate AR and AR coactivators directly and increase androgen-dependent AR transcriptional activity in vitro (30–32). In addition, the association of AR and c-Src is also required for the AR-mediated activation of phosphatidylinositol 3-kinase–AKT signaling by direct interaction of AR and the p85α regulatory subunit of phosphatidylinositol 3-kinase (29, 33). Therefore, therapeutic targeting of AR signaling might block genotropic signaling as AR antagonists do or inhibit nongenotropic signaling of AR, for example, with Src kinase inhibitors as their specificity and availability are improved (34–37).
Expression of several other Src family kinases, including Lyn, Lck, and Yes, have also been identified in normal and cancerous prostate tissue as well as some prostate cancer cell lines (34, 38). Despite the fact that they all share a highly conserved Src homology 3 domain with Src, it has not yet been reported whether similar interactions exist between AR and these other Src family kinases. Interestingly, a Lyn knockout mouse develops a smaller prostate with a much lower degree of branching of the ductal networks (39). Because AR signaling plays a critical role in prostate morphogenesis, it is interesting to postulate that Lyn is one of the effectors downstream of AR signaling that stimulates prostatic branching.
Methods
DNA Constructs.
Lentiviral vectors that mediate the expression of the myristoylated human AKT1 and the GFP were described in ref. 21. FUGW lentiviral vector was modified by replacing the internal ribosomal entry site with the CMV promoter, resulting in the FUCGW vector. cDNAs encoding the wild-type AR and ARS213A/S791A were kind gifts from Charles Sawyers (University of California, Los Angeles) and were subcloned into the lentiviral vector FUCGW with XbaI enzyme. Lentiviral vectors mediating the expression of N705S, V581F, ΔNLS, and ΔPro were described in ref. 1.
Prostate Regeneration.
Dissociated prostate epithelial cells were prepared from 6- to 10-week-old C57BL/6 mice as described in refs. 20 and 21. Dissociated prostate epithelial cells (1–2 × 105) and 1 × 105 urogenital sinus mesenchymal cells were used in each experiment. Lentivirus preparation, titering, and infection of dissociated prostate cells were performed as described in refs. 20 and 21. Procedures of prostate regeneration were as described in ref. 20.
Western Blot Analysis.
Rabbit polyclonal anti-phospho-AKTser-473 (9277, 1:1,000; Cell Signaling Technology, Beverly, MA), anti-AR (N-20, 1:1,000; Santa Cruz Biotechnology), and anti-extracellular signal-regulated kinase 2 (Erk2) (1:1,000; Santa Cruz Biotechnology) antibodies were used for Western blot analysis. Erk2 was used as a loading control.
Histology and IHC and Immunofluorescent Analyses.
Histological and IHC analyses were performed as described in ref. 20. Formalin-fixed and paraffin-embedded (PFPE) sections were stained with H&E or biotinylated anti-BrdU (1:500; Pharmingen), polyclonal rabbit anti-AR (1:200; Santa Cruz Biotechnology), monoclonal antibody against p63 (4A4, 1:150; Santa Cruz Biotechnology), and rabbit polyclonal anti-phospho-AKTser-473 (9277, 1:200; Cell Signaling Technology). For visualization of SMA, PFPE sections were stained with murine monoclonal anti-SMA (1:1,000; Sigma) and incubated with Alexa Fluor 594 goat anti-mouse IgG (H+L) (1:1,000; Molecular Probes). Sections were counterstained with DAPI in mounting medium (Vector Laboratories) and analyzed by fluorescence microscopy. For TUNEL, the In Situ Cell Death Detection kit (Roche Applied Science, Indianapolis) was used according to the detailed protocol supplied with the kit.
Acknowledgments
We thank Barbara Anderson for preparation of the manuscript. This work was supported by funds from the Prostate Cancer Foundation, University of California, Los Angeles Specialized Program of Research Excellence in Prostate Cancer, National Cancer Institute, National Institutes of Health Grant P50 CA92131 (primary investigator, Jean B. deKernion), and funds from the Department of Urology. O.N.W. is an Investigator of the Howard Hughes Medical Institute. L.X. is supported by the Prostate Cancer Foundation. M.A.T. is supported by National Institutes of Health Grant R01 CA107300 and is a Scholar of the Leukemia and Lymphoma Society. D.A.L. is supported by National Institutes of Health/Public Health Service Tumor Cell Biology Training Grant T32 CA09056. I.K.M. is supported by a Department of Defense Physician Research Training Award.
Abbreviations
- AR
androgen receptor
- Erk2
extracellular signal-regulated kinase 2
- H&E
hematoxylin/eosin
- IHC
immunohistochemical
- mPIN
murine prostatic intraepithelial neoplasia
- SMA
smooth muscle actin.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. Nat. Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 2.Isaacs J. T., Isaacs W. B. Nat. Med. 2004;10:26–27. doi: 10.1038/nm0104-26. [DOI] [PubMed] [Google Scholar]
- 3.Lu S., Liu M., Epner D. E., Tsai S. Y., Tsai M. J. Mol. Endocrinol. 1999;13:376–384. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 4.Xu L. L., Srikantan V., Sesterhenn I. A., Augustus M., Dean R., Moul J. W., Carter K. C., Srivastava S. J. Urol. 2000;163:972–979. [PubMed] [Google Scholar]
- 5.Garraway L. A., Lin D., Signoretti S., Waltregny D., Dilks J., Bhattacharya N., Loda M. Prostate. 2003;55:206–218. doi: 10.1002/pros.10244. [DOI] [PubMed] [Google Scholar]
- 6.Berger R., Febbo P. G., Majumder P. K., Zhao J. J., Mukherjee S., Signoretti S., Campbell K. T., Sellers W. R., Roberts T. M., Loda M., et al. Cancer Res. 2004;64:8867–8875. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- 7.Stanbrough M., Leav I., Kwan P. W., Bubley G. J., Balk S. P. Proc. Natl. Acad. Sci. USA. 2001;98:10823–10828. doi: 10.1073/pnas.191235898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han G., Buchanan G., Ittmann M., Harris J. M., Yu X., Demayo F. J., Tilley W., Greenberg N. M. Proc. Natl. Acad. Sci. USA. 2005;102:1151–1156. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J., Arnold J. T., Isaacs J. T. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 10.Sellers W. R., Sawyers C. L. In: Somatic Genetics of Prostate Cancer: Oncogenes and Tumor Suppressors. Kantoff P, editor. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 11.Majumder P. K., Yeh J. J., George D. J., Febbo P. G., Kum J., Xue Q., Bikoff R., Ma H., Kantoff P. W., Golub T. R., et al. Proc. Natl. Acad. Sci. USA. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nan B., Snabboon T., Unni E., Yuan X. J., Whang Y. E., Marcelli M. J. Mol. Endocrinol. 2003;31:169–183. doi: 10.1677/jme.0.0310169. [DOI] [PubMed] [Google Scholar]
- 13.Wen Y., Hu M. C., Makino K., Spohn B., Bartholomeusz G., Yan D. H., Hung M. C. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 14.Lin H. K., Hu Y. C., Yang L., Altuwaijri S., Chen Y. T., Kang H. Y., Chang C. J. Biol. Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 15.Gregory C. W., Whang Y. E., McCall W., Fei X., Liu Y., Ponguta L. A., French F. S., Wilson E. M., Earp H. S., III Clin. Cancer Res. 2005;11:1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- 16.Cinar B., De Benedetti A., Freeman M. R. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 17.Lin H. K., Yeh S., Kang H. Y., Chang C. Proc. Natl. Acad. Sci. USA. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taneja S. S., Ha S., Swenson N. K., Huang H. Y., Lee P., Melamed J., Shapiro E., Garabedian M. J., Logan S. K. J. Biol. Chem. 2005;280:40916–40924. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- 19.Cunha G. R., Lung B. J. Exp. Zool. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 20.Xin L., Ide H., Kim Y., Dubey P., Witte O. N. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl. 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin L., Lawson D. A., Witte O. N. Proc. Natl. Acad. Sci. USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burger P. E., Xiong X., Coetzee S., Salm S. N., Moscatelli D., Goto K., Wilson E. L. Proc. Natl. Acad. Sci. USA. 2005;102:7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Signoretti S., Waltregny D., Dilks J., Isaac B., Lin D., Garraway L., Yang A., Montironi R., McKeon F., Loda M. Am. J. Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kousteni S., Bellido T., Plotkin L. I., O'Brien C. A., Bodenner D. L., Han L., Han K., DiGregorio G. B., Katzenellenbogen J. A., Katzenellenbogen B. S., et al. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 25.Migliaccio A., Castoria G., Di Domenico M., de Falco A., Bilancio A., Lombardi M., Barone M. V., Ametrano D., Zannini M. S., Abbondanza C., Auricchio F. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolagas S. C., Kousteni S., Jilka R. L. Recent Prog. Horm. Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 27.Matias P. M., Donner P., Coelho R., Thomaz M., Peixoto C., Macedo S., Otto N., Joschko S., Scholz P., Wegg A., et al. J. Biol. Chem. 2000;275:26164–26171. doi: 10.1074/jbc.M004571200. [DOI] [PubMed] [Google Scholar]
- 28.Lobaccaro J. M., Poujol N., Chiche L., Lumbroso S., Brown T. R., Sultan C. Mol. Cell. Endocrinol. 1996;116:137–147. doi: 10.1016/0303-7207(95)03709-8. [DOI] [PubMed] [Google Scholar]
- 29.Sun M., Yang L., Feldman R. I., Sun X. M., Bhalla K. N., Jove R., Nicosia S. V., Cheng J. Q. J. Biol. Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 30.Yeh S., Lin H. K., Kang H. Y., Thin T. H., Lin M. F., Chang C. Proc. Natl. Acad. Sci. USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowan B. G., Weigel N. L., O'Malley B. W. J. Biol. Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 32.Gregory C. W., Fei X., Ponguta L. A., He B., Bill H. M., French F. S., Wilson E. M. J. Biol. Chem. 2004;279:7119–7130. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- 33.Baron S., Manin M., Beaudoin C., Leotoing L., Communal Y., Veyssiere G., Morel L. J. Biol. Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 34.Nam S., Kim D., Cheng J. Q., Zhang S., Lee J. H., Buettner R., Mirosevich J., Lee F. Y., Jove R. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 35.Copland M., Hamilton A., Elrick L. J., Baird J. W., Allan E. K., Jordanides N., Barow M., Mountford J. C., Holyoake T. L. Blood. 2006 Feb; doi: 10.1182/blood-2005-07-2947. 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 36.Coluccia A. M., Benati D., Dekhil H., De Filippo A., Lan C., Gambacorti-Passerini C. Cancer Res. 2006;66:2279–2286. doi: 10.1158/0008-5472.CAN-05-2057. [DOI] [PubMed] [Google Scholar]
- 37.Moasser M. M., Srethapakdi M., Sachar K. S., Kraker A. J., Rosen N. Cancer Res. 1999;59:6145–6152. [PubMed] [Google Scholar]
- 38.Robinson D., He F., Pretlow T., Kung H. J. Proc. Natl. Acad. Sci. USA. 1996;93:5958–5962. doi: 10.1073/pnas.93.12.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldenberg-Furmanov M., Stein I., Pikarsky E., Rubin H., Kasem S., Wygoda M., Weinstein I., Reuveni H., Ben-Sasson S. A. Cancer Res. 2004;64:1058–1066. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]