Funding for medical research comes from three sources: government, charities, and industry. Research funded by industry should benefit the public, but as an aside to commercial interests. Patsopoulos et al compared the proportion of the most frequently cited articles in the Institute for Scientific Information database that were funded by public or industry sources over the past decade.1 They found a significant trend towards funding by industry, despite the continued dominance of academics as authors. If we take this as a robust finding, three questions arise: why is this happening; what are its implications; and what, if anything, should be done about it?
Clinical academic medicine has long had ties with industry—academics are funded to speak at conferences, provide consultancy, or help design and conduct studies. In many countries, academia is increasingly adopting a commercial approach, as universities seek alternative sources of income in response to declining public investment. The rise in the influence of industry may be as much pull as push, especially where science parks, commercial spin-offs, and intellectual property rights are concerned. However, this increasing “privatisation” of public life has implications for society as a whole.
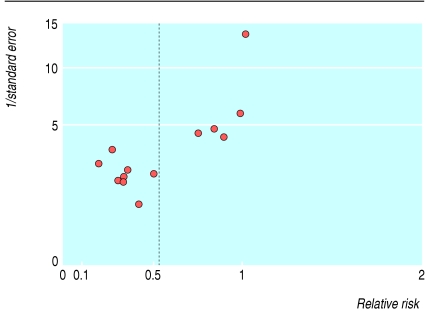
The production of national guidelines that are both evidence based and cost effective may pitch society against the interests of industry. For example, most of the evidence on which the 2004 NICE (National Institute for Health and Clinical Excellence) guidelines on dyspepsia are based came from randomised controlled trials funded by industry.2 This created several distortions in the evidence base. Firstly, evidence from trials of proton pump inhibitors was abundant compared with data for off-patent treatments such as metoclopramide or lifestyle interventions. Secondly, placebo was chosen as a comparator when “current” treatment would have been better. Thirdly, in one instance (cisapride in non-ulcer dyspepsia) a large number of poor quality studies funded by industry led to a result that was later discounted as potentially biased (figure).3
Figure 1.
Funnel plot4 showing asymmetry in meta-analysis of randomised controlled trials of cisapride versus placebo in non-ulcer dyspepsia. If relative risk (effect size) is plotted against 1/standard error, small negative trials should balance small positive trials. This plot indicates an absence of negative trials, which biases the pooled effect.
The issues raised in the paper by Patsopoulos et al are pertinent to many areas of life, not just medicine. When she was Britain's prime minister, Margaret Thatcher said, “There is no such thing as society.”5 Although we cannot ignore or shun industry, there is such a thing as civic society, and effective mechanisms are needed to protect its interests against those of private individuals and corporations. Absolute transparency of declaration of interests is one mechanism, and investment in detailed analysis of potential bias and evidence gaps in the production of guidelines is another. Public funding for research needs to concentrate on these gaps—for example, by comparing new drugs with cheaper and older ones and complex and behavioural interventions that cannot be patented. Industry should also be more transparent over trial registration and public access to data that affect patient care. A decline in public funding for high quality research is worrying and would ultimately harm patients. However, recent funding announcements in the United Kingdom indicate that government recognises this threat, and some correction of the balance should take place in the coming decade.6
This article was posted on bmj.com on 17 March 2006: http://bmj.com/cgi/doi/10.1136/bmj.38771.471563.80
Competing interests: BD has been paid a speaker's honorarium and travel expenses by Astra-Zeneca, Wyeth, Reckitt Benkiser, AxCan Pharma, and Takeda; he has also been paid for advice on research by Astra-Zeneca, Wyeth, and Merck. He has received small project grants from Astra-Zeneca and Wyeth and major grants and fellowship funding from the NHS, Medical Research Council, and National Institutes of Health (USA). He is a part time principal general practitioner and part time academic funded by Higher Education Funding Council for England. He receives travel funding from Oxford University Press as editor of Family Practice. He is not a member of a political party. He coauthored the technical report for the National Institute for Health and Clinical Excellence 2004 dyspepsia guideline.
References
- 1.Patsopoulos NA, Analatos AA, Ioannidis JPA. Origin and funding of the most frequently cited papers in medicine: database analysis. BMJ 2006;332: 1061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Health and Clinical Excellence. The management of dyspepsia in adult patients in primary care. London, UK: NICE, 2004.
- 3.Soo S, Moayyedi P, Deeks J, Delaney B, Harris A, Innes M, Bennett C, Forman F. Pharmacological interventions for non-ulcer dyspepsia (full Cochrane review). In: The Cochrane Library. Oxford: Update Software, 2001; issue 3.
- 4.Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323: 101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas K. Margaret Thatcher interviewed in “Woman's Own”, 23 Sep 1987. www.margaretthatcher.org/speeches/displaydocument.asp?docid=106689 (accessed 9 Feb 2006).
- 6.Department of Health. Best research for best health. London: DOH, 2006. www.dh.gov.uk/PublicationsAndStatistics/Publications/PublicationsPolicyAndGuidance/PublicationsPolicyAndGuidanceArticle/fs/en?CONTENT_ID=4127127&chk=uSh6qN (accessed 9 Feb 2006).