Abstract
Neostigmine is used to antagonize neoromuscluar blocker-induced residual neuromuscular paralysis. Despite a previous meta-analysis, the effect of neostigmine on postoperative nausea and vomiting (PONV) remains unresolved. We reevaluated the effect of neostigmine on PONV while considering the different anticholinergics as potentially confounding factors. We performed a systematic literature search using Medline, Embase, Cochrane library, reference listings, and hand searching with no language restriction through December 2004 and identified 10 clinical, randomized, controlled trials evaluating neostigmine's effect on PONV. Data on nausea or vomiting from 933 patients were extracted for the early (0-6 h), delayed (6-24 h), and overall postoperative periods (0-24 h) and analyzed with RevMan 4.2 (Cochrane Collaboration, Oxford, UK) and multiple logistic regression analysis. The combination of neostigmine with either atropine or glycopyrrolate did not significantly increase the incidence of overall (0-24 h) vomiting (relative risk (RR) 0.91 [0.70-1.18], P=0.48) or nausea (RR 1.24 [95% CI: 0.98-1.59], P=0.08). Multiple logistic regression analysis indicated that that there was not a significant increase in the risk of vomiting with large compared with small doses of neostigmine. In contrast to a previous analysis, we conclude that there is insufficient evidence to conclude that neostigmine increases the risk of PONV.
Keywords: Vomiting: postoperative, nausea; Statistics: meta analysis; Antagonists (Neuromuscular relaxants): neostigmine; Pharmacology: atropine, glycopyrrolate
Introduction
Nausea and vomiting remain among the most common perioperative complications, occurring in about 30% of postoperative patients (1-3). While the origin is generally believed to be multifactorial, there is increasing evidence that patient-specific risk factors play a major role (4). However, drugs specific to anesthesia, including volatile anesthetics, nitrous oxide, and postoperative opioids, are at least as important and — in contrast to the patient-specific risk factors — under the control of anesthesiologists (5,6).
Neostigmine is often used to antagonize residual neuromuscular block. Because anticholinesterases such as neostigmine have cholinergic effects on the gastrointestinal tract (increased motility and gastric acid secretion) and on the heart (bradycardia, cardiac arrest), they are co-administered with anticholinergics, such as atropine or glycopyrrolate (7). Atropine is a tertiary amine and can cross the blood-brain barrier to cause central effects. In contrast, glycopyrrolate is a quaternary amine that does not easily cross the blood-brain barrier and thus has no important central effects (8).
Intrathecal neostigmine has been shown to cause severe nausea and vomiting in a dose-dependent manner, probably via action on the brain stem (9). The effect of IV neostigmine on postoperative nausea and vomiting (PONV) remains controversial. Tramér et al. concluded in their meta-analysis (10) that neostigmine in doses ≥ 2.5 mg increases the incidence of PONV. However, a later study (11), not included in Tramér et al.'s (10) systemic review, was unable to confirm neostigmine's emetogenic effect. Furthermore, the incidence of PONV appears to be reduced when neostigmine is combined with atropine as opposed to glycopyrrolate (12).
If neostigmine were truly emetogenic, it would be reasonable to reconsider its routine use in antagonizing neuromuscular paralysis, especially for patients at increased risk for PONV. Our goal was thus to determine whether neostigmine administration produces a clinically relevant increase in the risk of PONV, and the extent to which risk depends on the co-administration of the anticholinergics.
Methods
We searched for all published full reports of randomized, controlled trials that compared patients given neuromuscular blocking antagonists (intervention) with those allowed to recover spontaneously from neuromuscular block (control group, i.e., placebo or no added anticholinesterase). Included trials were required to have dichotomous outcomes (presence or absence) for postoperative nausea (PON), postoperative vomiting (POV), or adverse events.
We searched systematically for relevant reports without any language restrictions in MEDLINE (1966-2004), EMBASE (1980-2004), and Cochrane Library (2004, Issue 4); the date of our last electronic search was December 8, 2004. We used the free text terms “nausea, vomiting, emesis, neostigmine, prostigmine, edrophonium, antagonism, and neuromuscular block” in any combination for the search. A manual scan was performed through the reference lists of all studies in the search results until no further relevant references could be identified.
Authors of the original publication were contacted if analyses endpoints were insufficiently reported. We sought to obtain separate data for nausea and vomiting and for the early, delayed, and overall study period of 24 hours (13). Two authors (CC and CA) independently read each retrieved report to assess the adequacy of randomization and blinding. The 5-point Oxford score was used to assess the quality of the study design (14) and differences were resolved by consensus.
We obtained information on patients, anesthetics, type and dose of anticholinergics, type and dose of anticholinesterases, and intervention-related adverse effects from each included report. Dichotomous data on harm and efficacy were extracted from the published reports. Extracted outcome data were early nausea, early vomiting (0-6 hr postoperative cumulative incidence), delayed nausea, delayed vomiting (6-24 hr postoperative cumulative incidence), overall postoperative nausea, and overall vomiting (0-24 hr postoperative cumulative incidence), as well as data on clinically diagnosed adverse events.
Data extracted from the relevant studies were entered into RevMan 4.2 (Review Manager, Cochrane Collaboration, UK) and analyzed. The relative risk (RR) with the corresponding 95% confidence intervals (CI) was calculated for each study. The results were pooled together using Mantel-Haenszel method for combining trials. The individual effect sizes were weighted according to the reciprocal of their variance. A random effect model was used and heterogeneity was determined under the assumption (null hypothesis) that there were no differences in treatment effect between trials. Results are presented as relative risks (RR) [95% confidence intervals]. The comparisons of neostigmine versus control were also divided into subgroups based on whether atropine or glycopyrrolate was given.
Multiple logistic regression analysis was used to investigate the relationship of the dose of neostigmine and POV. The analysis was corrected by center, with the largest center being the reference group (SPSS version 12.0 for windows, SPSS Inc., U.S.A). An absolute or relative increase of 10% or 25%, respectively, is considered to be clinically important.
Results
We found 15 reports that met our criteria. Five of these were subsequently excluded: two did not have control groups (15,16); another did not report PONV results (17); the data of one other study were insufficient to be considered in the analysis, despite getting more information from the authors (18); and in the last excluded study, only the combination of edrophonium and atropine was compared to placebo (19). In one trial, although only data on PONV were published, the authors generously provided the detailed data so that they could be included in the analysis (11). We therefore performed meta-analyses on 10 comparisons of neostigmine versus an inactive control with 933 study patients (Table 1) (11,20-28). All trials were randomized, except one that had a pseudo-randomization; we only included those patients who received standard anesthesia (n=41) or no reversal (n=40) after cholecystectomy from this study (28).
Table 1.
Characteristics of analyzed studies
| Study ID | Score (Random, Blind, Dropouts) | N | Interventions | Outcomes | Notes |
|---|---|---|---|---|---|
| Boeke, 1994 (20) | (1, 0, 1) | 79 adults | Vecuronium; Neostigmine 1.5 mg and atropine vs. no treatment | PON, POV in day care; first and second day nausea, vomiting | Early: day care stage |
| Ding, 1994 (21) | (1, 0, 1) | 69 women | Succinylcholine + mivacurium + no reversal vs. mivacurium + mivacurium + no reversal vs. mivacurium + mivacurium + neostigmine 2.5 mg and glycopyyrolate 0.5 mg | PON, POV in PACU; 24 h PON, POV | Early: PACU stay |
| Hovorka, 1997 (22) | (1, 2, 1) | 160 women | Mivacurium; Neostigmine 2.0 mg and glycopyrrolate vs. placebo | PON and POV in 0-1 h; 2-3 h; 3-9 h; 9-15 h; 15-21h; 21-27 h total (0-27h) | Early: 0-3 h |
| Janhunen, 1972 (28) | (0 to 1,0,0) | 81 cholecystectomy patients | Tubocurarine; Neostigmine 2.0 mg + atropine 1.0 mg vs. No reversal | POV (0-24 h) | Only included standard vs. no reversal patients |
| Joshi, 1999 (23) | (2, 2, 0) | 100 adults | Mivacurium vs. rocuronium; Residual block reversal with neostigmine 2.5mg + glycopyrrolate 0.5 mg | PACU, Phase II, 24 h PON and POV | Early: PACU and Phase II stage |
| King, 1988 (24) | (1, 0, 0) | 38 adults | Tubocurarine; Neostigmine 2.5 mg vs. No treatment | PON, POV in 24 h | |
| Lovstad, 2001 (25) | (2, 2, 1) | 90 women | Mivacurium; Neostigmine 2.5 mg + glycopyyrolate 0.5 mg vs. placebo | 0-6 h; 6-24 h; 0-24 h PON, POV; Satisfaction score | Pretreated with Ondansetron |
| Nelskyla, 1998 (11) | (2, 2, 1) | 100 women | Mivacurium; Neostigmine 2.0 mg + glycopyrrolate 0.4 mg vs. No treatment | PON, POV in PACU, ward, on the way home, at home, 24 h | Only PONV, no PON, POV data Recount data after got original data |
| Walsh, 1988 (26) | (2, 0, 1) | 120 children | Pancuronium; Neostigmine (60 μg/kg) + atropine(20 μg/kg) vs. No treatment | Early POV | |
| Watcha, 1995 (27) | (1, 2, 0) | 113 children | Mivacurium; Placebo vs. Edrophonium (1 mg/kg) + atropine (10 μg/kg) vs. Neostigmine (70 μg/kg) + Glycopyrrolate (10 μg/kg) | Early POV, POV in 24 h |
Early (0-6 h) postoperative nausea was reported as an outcome in six trials (Table 2) (11,20-23,25): five with glycopyrrolate (11,21-23,25) and one with atropine (20). The relative risk (RR) of suffering postoperative nausea in this early period was 1.24 [0.86-1.80; P = 0.25]. Early postoperative vomiting (0-6 h) was reported in eight studies. Patients in six of them received glycopyrrolate (11,21-23,25,27); patients in the other two received atropine (20,26). The RR for patients vomiting in the early postoperative period was 1.05 [0.72-1.55;P = 0.79].
Table 2.
Early and delayed postoperative nausea and vomiting with of neostigmine versus control; results of the meta-analyses
| Outcome | Anticholinergics | Number of Studies | Number of Participants | Relative risk [95% CI] |
|---|---|---|---|---|
| Early Nausea (0-6 h) | Atropine and Glycopyrrolate | 6 | 584 | 1.24 [0.86, 1.80] |
| Atropine | 1 | 79 | 0.67 [0.36, 1.26] | |
| Glycopyrrolate | 5 | 505 | 1.39 [0.97, 1.99] | |
| Early Vomiting (0-6 h) | Atropine and Glycopyrrolate | 8 | 768 | 1.05 [0.72, 1.55] |
| Atropine | 2 | 199 | 0.75 [0.52, 1.08] | |
| Glycopyrrolate | 6 | 568 | 1.35 [0.88, 2.06] | |
| Delayed Nausea (6-24 h) | Glycopyrrolate | 4 | 337 | 1.09 [0.76, 1.57] |
| Delayed Vomiting (6-24 h) | Glycopyrrolate | 4 | 337 | 1.01 [0.58, 1.78] |
Delayed (6-24 h) postoperative nausea as an outcome was included in four studies with a RR of 1.09 [0.76-1.57; P = 0.64] (11,21,23,25). All were with neostigmine combined with glycopyrrolate versus control. Delayed postoperative vomiting was an outcome in four studies; all were with glycopyrrolate. The RR was 1.01 [0.58-1.78; P = 0.96] (11,21,23,25).
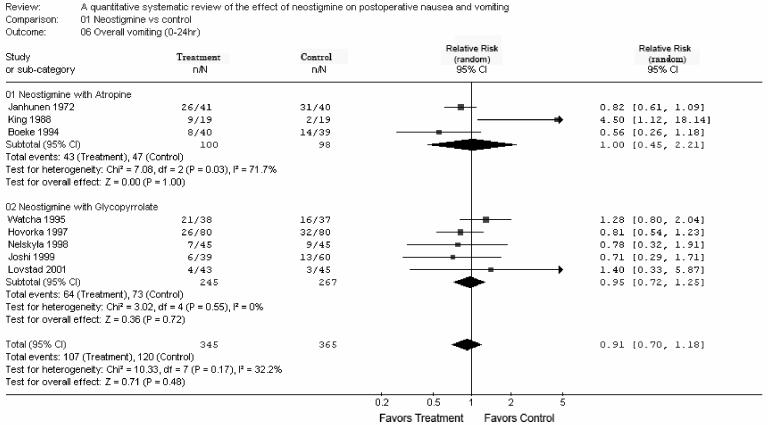
Overall (0-24 h) postoperative nausea was reported in six studies with a RR of 1.24 [0.98-1.59; P = 0.08] (Fig. 1) (11,20,22-25). Overall postoperative vomiting (0-24 h) was reported in eight studies with co-administration of atropine or glycopyrrolate with a RR of 0.91 [0.70-1.18; P = 0.48] (Fig. 2) (11,20,22-25,27,28). Thus, neostigmine was not associated with a significant increase in PON or POV in any of the above-mentioned analyses.
Fig. 1.
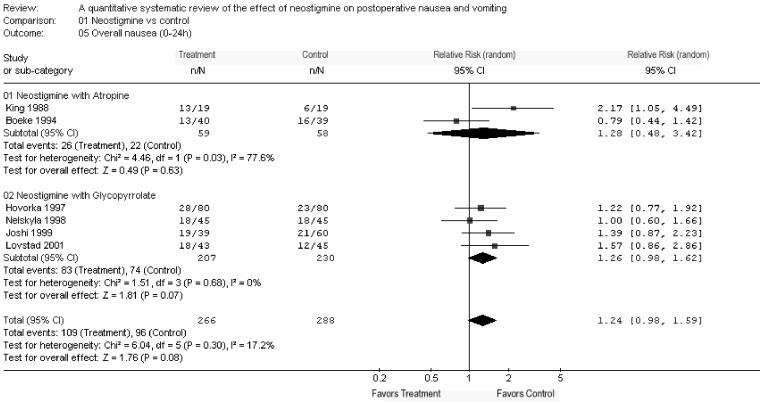
Overall postoperative nausea (0-24 h)
Fig. 2.
Overall postoperative vomiting (0-24 h)
We performed multiple logistic regression analysis of overall vomiting on nine studies with 800 patients (Table 3) (11,12,20,22-25,27,28). The average dose of neostigmine when given with glycopyrrolate was 3.02 mg/70 kg; the average dose when given with atropine was 2.59 mg/70 kg. We used the coefficients in Table 3 to calculate the odds ratio for a combination of interventions by “odds ratio = e(dose*α+β)” where “e” is the natural logarithm (2.71), “dose” is the neostigmine dose in mg, “α” is the coefficient for the neostigmine (ln 1.32 = 0.278), and “β” is the coefficient for the concomitant anticholinergic drug, -0.73 (ln 0.482) for glycopyrrolate or -1.14 (ln 0.32) for atropine. Thus, patients who received an average of 3.02 mg neostigmine and glycopyrrolate had an odds ratio for developing POV of 1.11 (= e(3.02*0.278-0.73)) while those receiving an average of 2.59 mg neostigmine with atropine had an odd ratio of 0.66 (= e(2.59*0.278-1.14)). For comparison, the effect of the center, i.e. where the study was conducted, had odds ratios ranging from 0.12 to 5.24. Thus, logistic regression analysis suggested that neostigmine does not significantly increase overall vomiting.
Table 3.
Multiple logistic regression analysis of overall postoperative vomiting
| Binary Outcome of 24-h Postoperative Vomiting | β (Standard error) | P-value | Odds ratio (=eβ) [95% CI] |
|---|---|---|---|
| Neostigmine dose (per mg) | 0.277 (0.175) | 0.110 | 1.32 [0.94-1.86] |
| Anticholinergic | 0.030 | 1.00 | |
| Glycopyrrolate | -0.729 (0.507) | 0.150 | 0.48 [0.18-1.30] |
| Atropine | -1.140 (0.464) | 0.010 | 0.32 [0.13-0.79] |
| Study-(Hovorka 1997 (22) as reference) | - | 1.00 | |
| Study-Janhunen 1972 (28) | 1.657(0.339) | 0.000 | 5.24 [2.70-10.19] |
| Study-King 1988 (24) | -0.207 (0.427) | 0.630 | 0.81 [0.35-1.88] |
| Study-Boeke 1994 (20) | -0.136 (0.330) | 0.680 | 0.87 [0.46-1.67] |
| Study-Watcha 1995(27) | 0.132 (0.383) | 0.730 | 1.14 [0.54-2.42] |
| Study-Nelskyla 1998 (11) | -0.968 (0.321) | 0.003 | 0.38 [0.20-0.71] |
| Study-Joshi 1999 (23) | -0.945 (0.308) | 0.002 | 0.39 [0.21-0.71] |
| Study-Lovstad 2001 (25) | -2.094 (0.448) | 0.000 | 0.12 [0.05-0.30] |
| Study-Chhibber 1999 (12). | 1.042 (0.486) | 0.030 | 2.84 [1.09-7.35] |
| Constant | -0.478 (0.199) | 0.020 | 0.62 |
Two studies described inadequate muscle strength in their control groups: One patient was excluded from a 40-patient control group due to failure to regain muscle strength (20), and two of 50 control patients were excluded from the efficacy analysis of another study because they needed muscle reversal due to muscle weakness (19). There were no other reports of other side effects, either in patients given neostigmine or in the control patients.
Discussion
We found insufficient evidence that the use of neostigmine, accompanied by either atropine or glycopyrrolate, increases the relative risk of early, delayed, or overall postoperative nausea or vomiting. This finding contrasts with a previous meta-analysis in which the authors concluded that neostigmine in doses of 2.5 mg or greater increases PONV (10). Several differences might account for this discrepancy. For example, a limitation of the previous meta-analysis (10) is its treatment of a study by Janhunen and Tammisto (28) in which five different modes of general anesthesia in patients undergoing cholecystectomy or vein stripping were compared. In Janhunen and Tammisto's pseudo-randomized study, two groups of patients (I and II) were given meperidine and reversed with neostigmine and atropine; however, the second group also received halothane (group II). Tramér et al. (10) combined the data from groups I and II and compared them with those of patients in another group (IV) who received meperidine but not neostigmine or halothane. Considering that volatile anesthetics are a major cause of postoperative vomiting (5), by including the data from patients of group II who received halothane, Tramér et al. (10) introduced a substantial bias. And because the trial was not randomized, the proportion of patients undergoing cholecystectomy or vein stripping was dissimilar, which again may have introduced bias. We avoided this problem by limiting our comparison to cholecystectomy patients from Janhunen and Tammisto's group I (meperidine and neostigmine) with those of group IV (meperidine without neostigmine). Thus, in spite of including two additional studies in our meta-analysis, it incorporates only 933 patients as opposed to 1134 patients in the analysis by Tramer et al. (10).
Tramér et al. also identified a dose-dependent relationship between neostigmine and PONV, which we were unable to confirm. A closer look at their Fig. 2 reveals that the label of the Y-axis should probably be risk reduction rather than number-needed-to-treat (NNT) as printed. But even then, the “1” at the top of the dotted line should be “0” and values for the 1.5-mg neostigmine were less than with no antagonism. This would represent a negative effect at low dose and this would be inconsistent with a classical pharmacological dose-response relationship. Furthermore, since dose cannot be considered as a covariate in RevMan, we subjected the data to logistic regression analysis, which showed that the dose of neostigmine did not exert a statistically significant effect on the rate of PONV. Furthermore, center effects (i.e., where the study was performed) were an order of magnitude greater than the dose dependence, suggesting that the non-significant effect of neostigmine dose is considerably smaller than other influences.
A further limitation of the previous meta-analysis is that it did not include atropine or glycopyrrolate as potential confounders; instead, the authors argued that the choice of the anticholinergic partner drug does not affect PONV. Our logistic regression analysis revealed that atropine was associated with a statistically significant lower risk for postoperative vomiting whereas glycopyrrolate was not. This result is supported by the study from Chhibber et al. (12) in which atropine was associated with significantly less postoperative emesis when compared directly with glycopyrrolate. Thus, it could be hypothesized that atropine is a better anti-emetic than glycopyrrolate because of its known central anti-cholinergic effects. Since patients given atropine received about 0.5 mg less neostigmine than those given glycopyrrolate, only a multivariate analysis could correct for those confounders. Ignoring the different anti-emetic effects of the anti-cholinergic partner drugs may thus have contributed to the appearance of a dose-response relationship for neostigmine in the previous meta-analysis. However, since there is only one true head-to-head comparison of atropine versus glycopyrrolate (12), these results should be interpreted with some caution.
It is possible that the rate of PONV is dependent on the ratio between neostigmine and the co-administered anticholinergic drug. However, the ratio of neostigmine to glycopyrrolate was 5:1 in almost all of the studies included in our meta-analysis and we only had 2 studies in which atropine was the anticholinergic. This consistency in the ratio of neostigmine to glycopyrrolate precluded introducing this factor into the multiple regression analysis (co-linearity problem).
There were only three cases of residual muscle weakness noted in the control groups. Among the 10 studies we used for efficacy analysis, only 1 of 933 patients was reported to have residual muscle relaxation requiring treatment [0.1%]. Although this low incidence of residual paralysis suggests that antagonism of neuromuscular block may not be necessary, numerous complications have been reported when patients are inadequately antagonized (29).
Search strategies of systematic reviews are designed to locate all relevant studies pertinent to the question. To achieve the highest level of evidence, meta-analyses provide an objective approach to quantify the effects of all data available from trials. It is, therefore, conceivable that the point estimates from meta-analyses have more external validity than single studies. However, the best approach to weighing the relative impact of studies remains in dispute. For example, we used the standard inversed standard error to weight the studies, although this approach can lend too much weight to small studies (30,31). Point estimates regarding the effectiveness should, therefore, be interpreted with some caution. Another problem of meta-analyses is the heterogeneity of reported end-points (e.g. nausea and vomiting) and when they are measured (early, delayed, or overall period). A consequence is that, as in our analysis, point estimates for different outcomes are not necessarily derived from the same trials. Thus, while analysis of the early postoperative period suggests that neostigmine might increase early nausea but not early vomiting, this result should be interpreted with a high degree of skepticism.
Our meta-analysis failed to demonstrate that neostigmine leads to a clinically important increase in the risk of PONV. This might be due to the limited number of patients available for analysis. For example, the incidences of overall nausea for the neostigmine and the control group were 41% and 33%, respectively, so that the average group size of 280 patients only provided a 43% power to detect this absolute difference of 8% (which would be of limited clinical importance in any case). Thus, only a large, well-designed, randomized controlled trial can fully resolve this issue (32). Such a trial should also address whether there is a dose-response relationship between neostigmine and PONV and the effect of atropine versus glycopyrrolate, possibly by a using a factorial design which has been proven to be a powerful tool (6,33,34). Defining a clinically important decrease in PONV of about 25% for the omission of neostigmine would require at least 372 patients if an incidence of 60% were to be studied; 524 patients for an incidence of 50%; and 744 for 40% (13).
In conclusion, our meta-analysis suggested that neostigmine does not increase the risk of vomiting in the early, delayed, or overall postoperative period and that there is insufficient evidence to conclude that neostigmine leads to a clinically important increase in the risk of postoperative nausea. Thus, concerns about the effect of neostigmine on postoperative nausea and vomiting should probably not influence the clinician's decision to antagonize neuromuscular block.
Acknowledgements
We thank Nancy Alsip (Outcomes Research Institute, University of Louisville) for her valuable help in reviewing and editing this manuscript. We are grateful to Kaisa Nelskyla and Kari Korttila (Helsinki, Finland) for providing the detailed results of their study, and to Peter Kranke (Wuerzburg, Germany) for his constructive comments.
Footnotes
Received from the Outcomes Research Institute and Department of Anesthesiology and Perioperative Medicine, University of Louisville, Louisville, KY, USA.
Supported by NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY).
Implication statement: Neostigmine does not increase postoperative vomiting. However, data on the effect on postoperative nausea remain conflicting and there is insufficient evidence upon which to draw strong conclusions.
References
- 1.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia and Analgesia. 2003;97:62–71. doi: 10.1213/01.ane.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 4.Apfel CC, Kranke P, Eberhart LHJ, et al. A comparison of predicting models for postoperative nausea and vomiting. British Journal of Anaesthesia. 2002;88:234–40. doi: 10.1093/bja/88.2.234. [DOI] [PubMed] [Google Scholar]
- 5.Apfel CC, Kranke P, Katz MH, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–68. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- 6.Apfel C, Korttila K, Abdalla M, et al. A Factorial Trial of Six Interventions for the Prevention of Postoperative Nausea and Vomiting. New Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner DA, Smith G. Evaluation of the combined effects of atropine and neostigmine on the lower oesophageal sphincter. Br J Anaesth. 1985;57:956–9. doi: 10.1093/bja/57.10.956. [DOI] [PubMed] [Google Scholar]
- 8.Proakis AG, Harris GB. Comparative penetration of glycopyrrolate and atropine across the blood--brain and placental barriers in anesthetized dogs. Anesthesiology. 1978;48:339–44. doi: 10.1097/00000542-197805000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tramér MR, Fuchs-Buder T. Omitting antagonism of neuromuscular block: effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. Br J Anaesth. 1999;82:379–86. doi: 10.1093/bja/82.3.379. [DOI] [PubMed] [Google Scholar]
- 11.Chhibber AK, Lustik SJ, Thakur R, et al. Effects of anticholinergics on postoperative vomiting, recovery, and hospital stay in children undergoing tonsillectomy with or without adenoidectomy. Anesthesiology. 1999;90:697–700. doi: 10.1097/00000542-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Nelskyla K, Yli-Hankala A, Soikkeli A, Korttila K. Neostigmine with glycopyrrolate does not increase the incidence or severity of postoperative nausea and vomiting in outpatients undergoing gynaecological laparoscopy. Br J Anaesth. 1998;81:757–60. doi: 10.1093/bja/81.5.757. [DOI] [PubMed] [Google Scholar]
- 13.Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002;46:921–8. doi: 10.1034/j.1399-6576.2002.460801.x. [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Huang CH, Wang MJ, Susetio L, et al. Comparison of the combined effects of atropine and neostigmine with atropine and edrophonium on the occurrence of postoperative nausea and vomiting. Ma Zui Xue Za Zhi. 1993;31:113–6. [PubMed] [Google Scholar]
- 16.Naguib M, Abdulatif M, al-Ghamdi A. Dose-response relationships for edrophonium and neostigmine antagonism of rocuronium bromide (ORG 9426)-induced neuromuscular blockade. Anesthesiology. 1993;79:739–45. doi: 10.1097/00000542-199310000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Devcic A, Munshi CA, Gandhi SK, Kampine JP. Antagonism of mivacurium neuromuscular block: neostigmine versus edrophonium. Anesth Analg. 1995;81:1005–9. doi: 10.1097/00000539-199511000-00019. [DOI] [PubMed] [Google Scholar]
- 18.McCourt KC, Mirakhur RK, Kerr CM. Dosage of neostigmine for reversal of rocuronium block from two levels of spontaneous recovery. Anaesthesia. 1999;54:651–5. doi: 10.1046/j.1365-2044.1999.00893.x. [DOI] [PubMed] [Google Scholar]
- 19.Bevan DR, Kahwaji R, Ansermino JM, et al. Residual block after mivacurium with or without edrophonium reversal in adults and children. Anesthesiology. 1996;84:362–7. doi: 10.1097/00000542-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Boeke AJ, de Lange JJ, van Druenen B, Langemeijer JJ. Effect of antagonizing residual neuromuscular block by neostigmine and atropine on postoperative vomiting. Br J Anaesth. 1994;72:654–6. doi: 10.1093/bja/72.6.654. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Fredman B, White PF. Use of mivacurium during laparoscopic surgery: effect of reversal drugs on postoperative recovery. Anesth Analg. 1994;78:450–4. doi: 10.1213/00000539-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hovorka J, Korttila K, Nelskyla K, et al. Reversal of neuromuscular blockade with neostigmine has no effect on the incidence or severity of postoperative nausea and vomiting. Anesth Analg. 1997;85:1359–61. doi: 10.1097/00000539-199712000-00032. [DOI] [PubMed] [Google Scholar]
- 23.Joshi GP, Garg SA, Hailey A, Yu SY. The effects of antagonizing residual neuromuscular blockade by neostigmine and glycopyrrolate on nausea and vomiting after ambulatory surgery. Anesth Analg. 1999;89:628–31. doi: 10.1097/00000539-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 24.King MJ, Milazkiewicz R, Carli F, Deacock AR. Influence of neostigmine on postoperative vomiting. Br J Anaesth. 1988;61:403–6. doi: 10.1093/bja/61.4.403. [DOI] [PubMed] [Google Scholar]
- 25.Lovstad RZ, Thagaard KS, Berner NS, Raeder JC. Neostigmine 50 microg kg(-1) with glycopyrrolate increases postoperative nausea in women after laparoscopic gynaecological surgery. Acta Anaesthesiol Scand. 2001;45:495–500. doi: 10.1034/j.1399-6576.2001.045004495.x. [DOI] [PubMed] [Google Scholar]
- 26.Walsh C, Smith CE, Ryan B, et al. Postoperative vomiting following strabismus surgery in paediatric outpatients: spontaneous versus controlled ventilation. Can J Anaesth. 1988;35:31–5. doi: 10.1007/BF03010541. [DOI] [PubMed] [Google Scholar]
- 27.Watcha MF, Safavi FZ, Mcculloch DA, et al. Effect of antagonism of mivacurium-induced neuromuscular block on postoperative emesis in children. Anesth Analg. 1995;80:713–7. doi: 10.1097/00000539-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Janhunen L, Tammisto T. Postoperative vomiting after different modes of general anaesthesia. Ann Chir Gynaecol Fenn. 1972;61:152–9. [PubMed] [Google Scholar]
- 29.Berg H, Roed J, Viby-Mogensen J, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–103. doi: 10.1111/j.1399-6576.1997.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs-Buder T, Mencke T. Use of reversal agents in day care procedures (with special reference to postoperative nausea and vomiting) Eur J Anaesthesiol Suppl. 2001;23:53–9. [PubMed] [Google Scholar]
- 31.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 32.Cappelleri JC, Ioannidis JP, Schmid CH, et al. Large trials vs meta-analysis of smaller trials: how do their results compare? JAMA. 1996;276:1332–8. [PubMed] [Google Scholar]
- 33.Apfel CC, Korttila K, Abdalla M, et al. An international multicenter protocol to assess the single and combined benefits of antiemetic interventions in a controlled clinical trial of a 2x2x2x2x2x2 factorial design (IMPACT) Control Clin Trials. 2003;24:736–51. doi: 10.1016/s0197-2456(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 34.McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA. 2003;289:2545–53. doi: 10.1001/jama.289.19.2545. [DOI] [PubMed] [Google Scholar]