Abstract
Human embryonic stem cells (hESCs) are pluripotent cells that have the potential to differentiate into any tissue in the human body; therefore, they are a valuable resource for regenerative medicine, drug screening, and developmental studies. However, the clinical application of hESCs is hampered by the difficulties of eliminating animal products in the culture medium and/or the complexity of conditions required to support hESC growth. We have developed a simple medium [termed hESC Cocktail (HESCO)] containing basic fibroblast growth factor, Wnt3a, April (a proliferation-inducing ligand)/BAFF (B cell-activating factor belonging to TNF), albumin, cholesterol, insulin, and transferrin, which is sufficient for hESC self-renewal and proliferation. Cells grown in HESCO were maintained in an undifferentiated state as determined by using six different stem cell markers, and their genomic integrity was confirmed by karyotyping. Cells cultured in HESCO readily form embryoid bodies in tissue culture and teratomas in mice. In both cases, the cells differentiated into each of the three cell lineages, ectoderm, endoderm, and mesoderm, indicating that they maintained their pluripotency. The use of a minimal medium sufficient for hESC growth is expected to greatly facilitate clinical application and developmental studies of hESCs.
Keywords: April/BAFF, fibroblast growth factor, serum free culture, wnt
Human embryonic stem cells (hESCs) are pluripotent cells that have the potential to differentiate into the three germ layers and possibly all tissues of the human body (1–6). hESCs were originally isolated from the inner cell mass of human embryos and can be passaged through >100 divisions in vitro (7, 8). Differentiation protocols of hESCs have been successfully established in vitro for many cell types (5, 8–13), including neuronal cells (9), hematopoietic cells (10), insulin-producing cells (11), endothelial cells (12), and cardiomyocytes (13).
The ability of hESCs to differentiate into many cell types distinguishes them from adult stem cells, which can only differentiate into limited range of cell types (7, 8). Thus, hESCs have enormous therapeutic value and provide a useful system for studies of development. To make hESCs compatible for clinical therapy, banks of hESC lines with different HLA are being established (14). In addition, other technologies, such as nuclear transfer, may allow the generation of autologous embryonic stem cells in the future (15). Thus, hESCs are expected to provide a great resource for regenerative medicine (16).
Until recently, hESC lines were derived in medium containing animal products. The presence of xenograft or allograft animal products in hESC culture media has four problems. First, it may contain toxic proteins or immunogens that evoke an immune response and thus lead to rejection upon transplantation (17). Second, the use of animal products increases the risk of hESC contamination by the animal pathogens, such as viruses or prions (18). Third, separating animal products, such as feeder cells, from hESCs is time- and labor-intensive. Finally, the use of medium with undefined factors greatly complicates developmental studies. Therefore, it is important to grow hESCs in a defined medium without animal products.
Currently several components required for hESC growth have been identified. Basic fibroblast growth factor (bFGF) has been shown to be essential for hESC self-renewal (19, 20). Three other requirements are (i) feeder cells, conditioned medium, or cytokines, such as TGF (21, 22) or Wnt3a (23); (ii) matrix; and (iii) FBS or serum replacement (24, 25).
For hESC culture, several types of matrices have been used to coat the culture dish surface. Matrigel secreted by mouse Engelbreth Holm–Swarm sarcoma cells is able to support the hESC growth (25). It contains multiple extracellular matrix components, such as laminin, collagen type IV, heparan sulfate, proteoglycan, and entactin (26). Human serum can substitute for Matrigel (27). However, both Matrigel and human serum are mixtures with undefined components. Other defined matrices, such as fibronectin, laminin, and collagen can support feeder-free hESC growth, but the efficacy varies among laboratories and some reagents have disparities between different lots (25, 28, 29).
In addition to the matrix requirement, serum or serum replacement is essential for hESC culture. “Knockout serum” from Invitrogen is a serum replacement frequently used in hESC culture that contains animal derived-products (20). One animal-free product, X-vivo, which was optimized for hematopoietic cell culture, supports hESC growth (29). Knockout serum and X-vivo are both proprietary and contain multiple components. Moreover, in feeder-free culture, hESCs grown in medium containing these serum replacements form differentiated cells around the hESC colonies, indicating that optimal conditions have not been achieved (29). Thus, further efforts are required to define a culture condition with minimal components that reproducibly supports robust growth of hESCs.
In pursuit of this goal, we defined a simple mixture containing only recombinant, chemically synthesized, or human source-purified factors that support hESC growth [termed hESC Cocktail (HESCO)]. Cells incubated in HESCO are easy to grow in an undifferentiated state and can be readily induced to differentiate into a variety of cell lineages. HESCO provides a simple and defined culture environment to support hESC growth and will greatly facilitate the use of hESCs in therapeutic applications and developmental studies.
Results
A Medium Containing Minimal Components Supports hESC Growth.
The presence of Wnt3a and bFGF alone in standard DMEM/F12 medium cannot support hESC growth in the absence of a feeder layer and serum (Table 1). We found that the presence of insulin, transferrin, albumin, and a proliferation-inducing ligand (April)/B cell-activating factor belonging to TNF (BAFF) in the medium can support hESC proliferation for more than three passages (Table 1 and J.L., R.H., Y.-H. Liu, C.J.B., F. L. Lu, and M.S., unpublished data). To further optimize the hESC culture conditions, we tested a variety of different components and found that the addition of chemically defined cholesterol to the medium improved hESC growth (Table 1). Thus, the final mixture, HESCO, contains Wnt3a, FGF, insulin, transferrin, April/BAFF, cholesterol, and albumin and can actively support hESC self-renewal. hESCs grown in feeder cell-conditioned medium can be directly shifted to HESCO and vice versa without gradual adaptation steps in the culture, which suggests that the signals supporting the hESC growth may be similar in these two conditions.
Table 1.
Summary of cell growth with different cytokine cocktail combinations
| Components | Growth |
|||
|---|---|---|---|---|
| − | + | ++ | ++ | |
| April/BAFF | × | × | ||
| bFGF | × | × | × | |
| Wnt | × | × | × | |
| Insulin | × | × | ||
| Transferrin | × | × | ||
| Albumin | × | × | ||
| Cholesterol | × | |||
| Conditioned medium | × | |||
All cells were cultured with Matrigel-coated tissue culture plate. The relative amounts of cells with undifferentiated morphology after three passages are indicated. The × symbols indicate the component(s) present in the medium.
We also tested several matrices in combination with the HESCO culture medium. Among them, fibronectin consistently supported hESC growth (Table 2). The presence of collagen along with fibronectin further improves the survival of hESCs (Table 2). To define the minimal components for hESC growth, we used fibronectin in the absence of collagen as the matrix in the experiments described below. The final hESC growth conditions using HESCO and fibronectin are defined.
Table 2.
Summary of cell growth using different coating matrix
| Matrix | Growth |
||||
|---|---|---|---|---|---|
| + | ++ | +++ | ++++ | ++++ | |
| Fibronectin | × | × | |||
| Collagen | × | × | |||
| Laminin | × | ||||
| Matrigel | × | ||||
The relative amounts of cells with undifferentiated morphology after three passages are indicated. The × symbols indicate the matrix coated on the plate.
hESCs Cultured in HESCO Exhibit Normal Cell Morphologies.
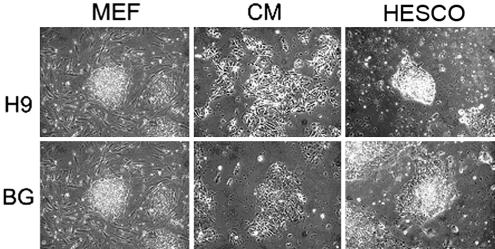
To determine whether hESCs grown in HESCO were maintained in an undifferentiated state, a variety of tests were used. The morphology of two hESC lines, H9 and BG01, cultured in HESCO or conditioned medium for >2 months (eight passages) was examined. Fibronectin and Matrigel from at least six different lots were tested, and the results were consistent. Unlike the elongated cells observed in conditioned medium, hESCs cultured in HESCO were more condensed and had a high nucleus/cytoplasm ratio similar to cells cultured on feeder cells (Fig. 1). Importantly, compared with most of the feeder-free culture conditions currently used, hESCs cultured in HESCO did not have the differentiated cells surrounding the hESC colonies (Figs. 1 and 2) (20, 29). Thus, hESCs cultured in HESCO medium remain in an undifferentiated state.
Fig. 1.
The morphology of hESCs cultured in the presence of feeder cells (MEF), conditioned medium (CM), or HESCO. The morphology of H9 (Upper) and BG01 (Lower) cells cultured on the feeder cells (Left) and in HESCO (Right) are more condensed with a high nucleus/cytoplasm ratio, whereas the cells cultured in the conditioned medium (Center) are elongated with a low nucleus/cytoplasm ratio. (Original magnification, ×40.)
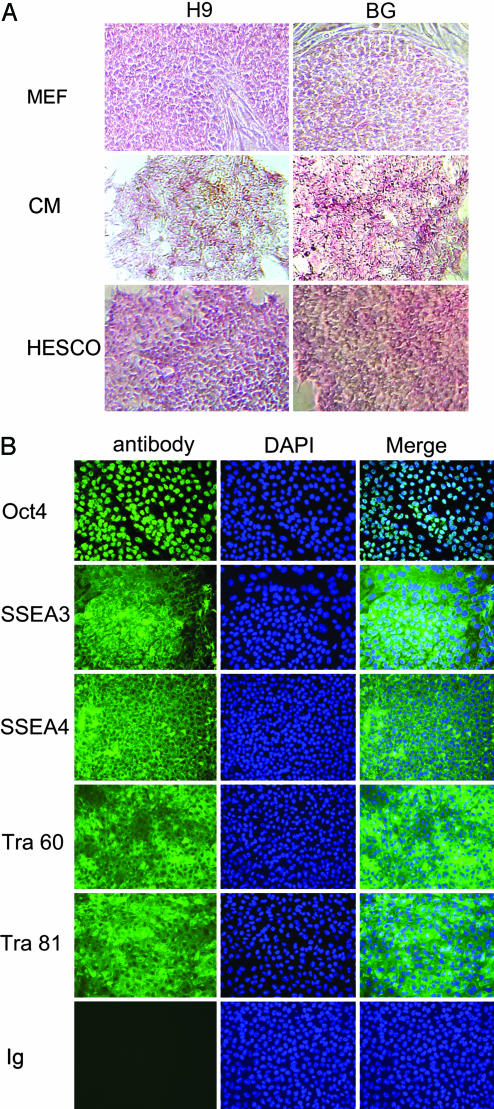
Fig. 2.
Expression of stem cell markers in hESCs cultured in HESCO. (A) Alkaline phosphatase assay of hESCs cultured in the presence of mouse embryonic fibroblast feeder cells (MEF), conditioned medium (CM), or HESCO was performed. (Magnification, ×100.) (B) (Left) Immunofluoresence staining with antibodies to stem cell markers: Oct4, SSEA3, SSEA4, TRA-1-60 (Tra 60), TRA-1-81 (Tra 81), or control mouse IgM (Ig). Mouse IgG and rat IgM controls show results similar to those of mouse IgM control (data not shown). (Center) The nuclei were stained with DAPI. (Right) The overlay of FITC antibody staining and DAPI signals is shown in the column labeled Merge. (Magnification, ×200.)
hESCs Cultured in HESCO Express Stem Cell Markers.
hESCs express stem cell markers that distinguish them from differentiated cells. To confirm that hESCs grown in the HESCO for 2 months are undifferentiated, we measured alkaline phosphatase activities by using an in situ assay (30). Both H9 and BG01 cells have alkaline phosphatase activities comparable with the cells grown in conditioned medium or on feeder cells (Fig. 2A). The undifferentiated state of hESCs was further demonstrated by the expression of the stem cell markers Oct4, stage-specific mouse embryonic antigen (SSEA)3, SSEA4, TRA-1-60, and TRA-1-81 through indirect immunofluoresence assays. In both H9 and BG01 cell lines, >95% of cells cultivated in HESCO stained positive for each of the stem cell markers (Fig. 2B and data not shown). In each case, expression of the stem cell marker revealed that the hESC colonies were not surrounded by differentiated cells (Fig. 2 and data not shown). As negative controls, species-matched IgG and IgM were used to stain hESCs cultured in HESCO, and signal was not detected (Fig. 2B and data not shown). These results indicate that the exogenous factors in the HESCO are sufficient for hESC growth in an undifferentiated stage for more than eight passages.
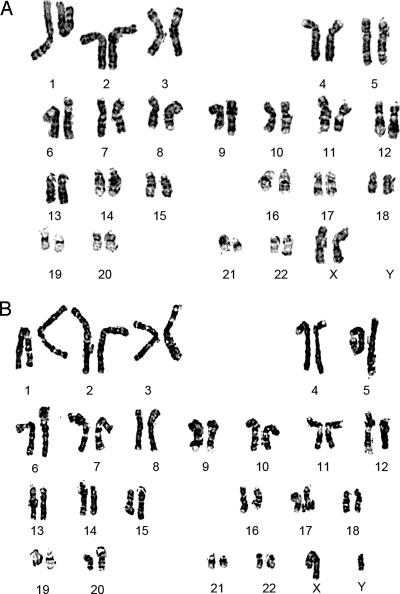
Karyotyping of hESCs Cultured in HESCO.
hESCs cultured in vitro can lose their genetic integrity through passaging (28, 31, 32). For example, BG01 cells cultured in conditioned medium occasionally develop trisomy 12 or 17 (31, 32). To examine the genetic stability of hESCs in HESCO, we karyotyped H9 cells cultured in HESCO for 4, 11, and 23 passages (1–6 months) and BG01 cells cultured for eight passages (2 months). In each case, the karyotype was normal (Fig. 3). No major translocations or other chromosomal changes were observed during this period. Thus, hESCs cultured in HESCO maintain their genomic integrity.
Fig. 3.
The genetic stability of hESCs cultured in HESCO. The karyotypes of H9 (A) and BG01 cells (B) cultured in HESCO for 11 and 8 passages, respectively, were analyzed by using Giemsa staining.
hESCs Cultured in HESCO Are Pluripotent.
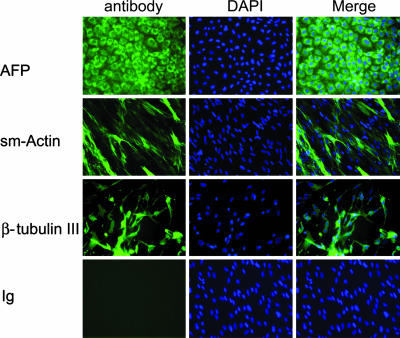
hESCs are pluripotent cells that can differentiate into the three major cell lineages: endodermal, ectodermal, and mesodermal (8, 33). To confirm that hESCs cultured in HESCO still maintain their pluripotency in vitro, we performed embryoid body formation and differentiation assays in H9 and BG01 cells. Three passages of H9 cells (passages 5, 10, 24) and one passage of BG01 cells (passage 9) were tested. After dispersing the cells by enzymatic digestion, hESCs formed embryoid bodies in suspension with high efficiency in both cell lines (data not shown). Subsequently, the embryoid bodies continue to differentiate on gelatin-coated plates for at least 10 days. Expression of endoderm-, mesoderm-, and ectoderm-specific markers in the embryoid body-derived cells were evaluated by using immunofluoresence analysis of α-fetoprotein, smooth muscle actin, and β-tubulin III, respectively. In both hESC lines and in all passages tested, the embryoid body-derived cells contained cells from the three different lineages (Fig. 4and data not shown). Immunofluorescence signal was not evident in the Ig control (Fig. 4 and data not shown). Hence, the HESCO medium is sufficient to maintain the pluripotency of hESCs.
Fig. 4.
Examination of the pluripotency of hESCs cultured in HESCO in vitro. (Left) H9 cells cultured in vitro were induced to form embryoid bodies, and the derived cells were stained with an isoptype-matched Ig control (Ig) and different differentiation markers: endoderm [α-fetoprotein (AFP)], mesoderm smooth muscle (SM) actin, or ectoderm (β-tubulin III). (Center) The nuclei were stained with DAPI. (Right) The overlay of FITC antibody staining and DAPI signals is shown in the column labeled Merge. (Original magnification: ×200.)
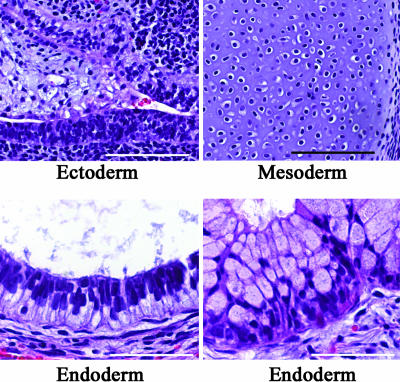
To examine the in vivo pluripotency of hESCs grown in HESCO, we determined their ability to form teratomas. H9 and BG01 hESCs cultured in HESCO for eight passages were injected s.c. into mice. Teratomas were formed, and they contained multiple cell types from each of the major cell lineages, such as neuroepithelium (ectoderm), cartilage (mesoderm), ciliated epithelium (endoderm), and mucus-producing epithelium (endoderm) (Fig. 5 and data not shown). Thus, the cells cultured in HESCO maintain their pluripotency in vivo.
Fig. 5.
In vivo analysis of the pluripotency of hESCs cultured in HESCO. H9 cells were s.c. injected into the severe combined immunodeficient mice. Sections of the resulting teratomas were stained with hematoxylin and eosin. All three germ-layer-derived tissues were observed, including neuroepithelium (ectoderm) (Upper Left), cartilage (mesoderm) (Upper Right), ciliated epithelium (endoderm) (Lower Left), and mucus-producing epithelium (endoderm) (Lower Right). (Scale bars: Upper, 100 μm; Lower, 50 μm.)
Discussion
The identification of all exogenous signals required for hESC growth is important for clinical application and developmental biology studies of hESCs. In this work, we have defined the minimal nutritional requirements for maintaining hESC culture in vitro. The components include Wnt, April/BAFF, bFGF, insulin, transferrin, albumin, cholesterol, and fibronectin.
In the absence of serum and feeders, eight components are required to sustain hESC growth. In contrast, mouse embryonic stem cells (mESCs) survive and propagate well with only leukemia inhibitory factor, bone morphogenetic protein, transferrin, and gelatin (33, 34). Leukemia inhibitory factor is not important for hESC self-renewal, whereas insulin, bFGF, and April/BAFF are not required for mESC survival (33, 35). Thus, hESC and mESC require different exogenous signals to maintain pluripotency and self-renewal. Our results are consistent with published observations in human and mouse development. Insulin is a fetal growth factor for humans (36). Mutations in insulin receptor result in severe growth retardation in newborns (36). High insulin exposure in utero leads to an increase in baby size in diseases, such as maternal diabetes (37), Beckwith–Wiedemann syndrome (38), erythroblastosis fetails (39), and nesidioblastosis (40). In contrast, mice lacking the insulin signal exhibit only a slight impairment of embryonic growth (41, 42). Therefore, insulin signals appear to be more important for hESC than mESC growth.
FGF and Wnt family members are important mammalian developmental and stem cell signals (43, 44). FGF signaling is crucial for hESC maintenance (20). The addition of bFGF at high concentration (100 ng/ml) (20, 45) or bFGF at low concentration (4 ng/ml) along with other cytokines, like Noggin (46) and activin/nodal, can sustain hESC cultures without feeder cells and conditioned medium (47). Wnt3a also can promote hESC proliferation in the absence of a feeder layer or conditioned medium (23). Although Wnt is important for adult stem cell proliferation (48, 49), it is not sufficient to sustain long-term proliferation of hESCs in feeder-free cultures (50).
Albumin is the major protein in serum (51). It functions as a carrier protein and a regulator of steroid, thyroid, and other lipophilic hormones (51). Albumin is also an antioxidant scavenger and can bind fatty acids, ions, drugs, and metabolites. Cholesterol is required for all mammalian cells (52). It is involved in lipid raft assembly, membrane rigidity maintenance, and facilitates post-Golgi sorting (53). In addition to its structural function, cholesterol is also the precursor of steroid hormones, and a component of signaling proteins, such as hedgehog (54). It is possible that antioxidants or growth factors might be able to substitute for albumin and cholesterol.
As this manuscript was under preparation, a recent study (28) described a defined medium (TeSR1) for hESCs that is quite different from HESCO. TeSR1 is a complex medium composed of vitamins, antioxidants, salts, traces minerals, specific lipids, albumin, detergent, GABA, pipecolic acid, TGF, LiCl, and bFGF and uses a combination of collagen, fibronectin, laminin, and vibronectin as supporting matrices (28). In contrast, HESCO includes bFGF, Wnt3a, April/BAFF, albumin, cholesterol, insulin, and transferrin, and it uses fibronectin as the coating material. Both TeSR1 and HESCO share reagents, such as albumin, transferrin, insulin, and bFGF, suggesting that these factors are crucial for hESC self-renewal. It was also reported that optimal culture conditions of TeSR1 require pH (7.2), osmolarity (350 nanoosmoles), and gas atmosphere (10% CO2/5% O2) (28); these special conditions were not necessary for cells grown in HESCO medium. A side-by-side comparison of TeSR1 and HESCO culture conditions and an examination of the effects of their different components and culture environments may further determine the optimal xeno-free culture condition for hESCs.
HESCO is free of non-human, animal-derived components. However, albumin and fibronectin are derived from human sources, and we purchased the recombinant Wnt3a by using mouse sequence. In the future, with our recipe as a template, it should be possible to chemically synthesize or purify all factors from non-animal sources, such as yeast or cell-free systems. This will make the components completely animal-free and prevent contamination. The HESCO medium we formulated will therefore provide a great tool for hESCs to realize their full therapeutic and scientific potential.
Materials and Methods
Cell Culture.
Two hESC lines, H9 (Wicell Research Institute, Madison, WI) and BG01 (BresaGen, Athens, GA), were cultured in DMEM/F12 and supplemented with 20% knockout serum replacement, 1 mM l-gluatamine, 1% nonessential amino acid, and 4 ng/ml human bFGF (all from Invitrogen), and 0.1 mM 2-mercaptoethanol (Sigma). CF-1 mouse embryonic fibroblasts (MEFs) were used as the feeder cells (Chemicon International, Temecula, CA). Conditioned medium was prepared with mouse embryonic fibroblasts as described in refs. 7, 25, and 55. All hESC experiments were performed between passages 25–60 from their initial establishment. Cells were passaged every 4–6 days with 1 mg/ml collagen IV or 0.0025–0.25% trypsin-EDTA (Invitrogen). After PBS washing, the cells were dispersed by scraping. The culture plates were coated with 0.33 mg/ml Matrigel Matrix (BD Biosciences) or 25 μg/ml fibronectin (Invitrogen). HESCO contains 4 ng/ml bFGF (Invitrogen), 160 μg/ml insulin (Invitrogen or Sigma), 88 μg/ml transferrin (Invitrogen or Sigma), 100 ng/ml Wnt3a (R & D Systems), 100 ng/ml April or BAFF (R & D Systems), 2.5 mg/ml albumin (Sigma), and 2.5× cholesterol lipid supplement (Invitrogen).
Immunofluoresence Assay.
Cells were fixed with 4% paraformaldehyde for 15 min or methanol for 3 min at room temperature. After incubation with anti-SSEA3 (Developmental Studies Hybridoma Bank, Iowa, IA), anti-SSEA4 (Developmental Studies Hybridoma Bank), anti-TRA-1-60 (Chemicon), anti-TRA-1-81 (Chemicon), anti-α-fetoprotein (Sigma), anti-smooth muscle actin (Sigma), anti-β-tubulin III (Sigma), control mouse IgG and IgM (Sigma), or control rat IgM (eBioscience, San Diego; DAKO), the cells were washed with PBS and incubated with 200-fold-diluted FITC-conjugated anti-mouse IgG antiserum (all from Jackson ImmunoResearch). The cells were also counterstained with DAPI (Roche, Basel, Switzerland) and examined under a fluorescence microscope.
Alkaline Phosphatase Assay, Karyotyping, and Embryoid Body Formation.
The cells were fixed with 4% paraformaldehyde at room temperature for 15 min and washed with PBS. The alkaline phosphatase assay was performed with an ES cell characterization kit (Chemicon). For karyotyping, hESCs grown in log phase were harvested and karyotyped by using Giemsa stain (Genzyme). Twenty cells were scored in each case. In the embryoid body formation assay, one monoplate of hESCs passaged with 0.025% trypsin was cultured in an uncoated, 10-cm Pertri dish in the presence of DMEM supplemented with 10% FCS (Invitrogen). After 4 days of suspension culture, the embryoid bodies were formed, and the cells were transferred to a plate coated with 0.2% gelatin (Sigma). The cells attached to the plate and were cultured for >10 days. The cells were fixed and processed for immunofluoresence studies.
Teratoma Formation.
hESCs (10 million) were s.c. injected into severe combined immunodeficient Beidge mice (Charles River Laboratories). All animal experiment procedures followed Yale Institutional Animal Care and Use Committee protocols. The teratomas were harvested at least 6 weeks after hESC injection. Teratomas were processed with formalin and sectioned with an Excelsior Processor (Thermo Electron, Pittsburgh, PA), and embedded in paraffin (Blue Ribbon, Surgipath Medical Industries, Richmond, IL). Tissue sections were cut at 5–6 μm and stained with hematoxylin and eosin. Tissues were examined by routine light microscopy on an Axioscope microscope (Zeiss), and digital light microscopic images were taken on Zeiss Axioskop2 Plus microscope, AxoCam HR Camera, and axiovision 5.05.10 imaging software (Zeiss).
Acknowledgments
We thank Michael Smith, Joseph Fasolo (Yale University), and Shao-Yin Chen (Duke University, Durham, NC) for critically reviewing the paper and Xiuqiong Zhou, Cheng Liao, Gordon Terwilliger, Stephen Hartman, and Chris Hart for technical assistance. This work was supported by grants from the National Institutes of Health. J.L. was supported by a training grant from the National Institutes of Health.
Abbreviations
- April
a proliferation-inducing ligand
- BAFF
B cell-activating factor belonging to TNF
- bFGF
basic fibroblast growth factor
- hESC
human embryonic stem cell
- HESCO
hESC Cocktail
- MEF
mouse embryonic fibroblast
- SSEA
stage-specific embryonic antigen.
Footnotes
Conflict of interest statement: M.S. has financial interests with Invitrogen.
References
- 1.Strelchenko N., Verlinsky O., Kukharenko V., Verlinsky Y. Reprod. Biomed. Online. 2004;9:623–629. doi: 10.1016/s1472-6483(10)61772-5. [DOI] [PubMed] [Google Scholar]
- 2.D’Amour K., Gage F. H. Nat. Biotechnol. 2000;18:381–382. doi: 10.1038/74429. [DOI] [PubMed] [Google Scholar]
- 3.Keller G., Snodgrass H. R. Nat. Med. 1999;5:151–152. doi: 10.1038/5512. [DOI] [PubMed] [Google Scholar]
- 4.Trounson A. Reprod. Biomed. Online. 2002;4(Suppl. 1):58–63. doi: 10.1016/s1472-6483(12)60013-3. [DOI] [PubMed] [Google Scholar]
- 5.Odorico J. S., Kaufman D. S., Thomson J. A. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 6.Gertow K., Wolbank S., Rozell B., Sugars R., Andang M., Parish C. L., Imreh M. P., Wendel M., Ahrlund-Richter L. Stem Cells Dev. 2004;13:421–435. doi: 10.1089/scd.2004.13.421. [DOI] [PubMed] [Google Scholar]
- 7.Thomson J. A., Marshall V. S. Curr. Top Dev. Biol. 1998;38:133–165. doi: 10.1016/s0070-2153(08)60246-x. [DOI] [PubMed] [Google Scholar]
- 8.Reubinoff B. E., Pera M. F., Fong C. Y., Trounson A., Bongso A. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 9.Park C. H., Minn Y. K., Lee J. Y., Choi D. H., Chang M. Y., Shim J. W., Ko J. Y., Koh H. C., Kang M. J., Kang J. S., et al. J. Neurochem. 2005;92:1265–1276. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman D. S., Thomson J. A. J. Anat. 2002;200:243–248. doi: 10.1046/j.1469-7580.2002.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assady S., Maor G., Amit M., Itskovitz-Eldor J., Skorecki K. L., Tzukerman M. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 12.Levenberg S., Golub J. S., Amit M., Itskovitz-Eldor J., Langer R. Proc. Natl. Acad. Sci. USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mummery C., Ward D., van den Brink C. E., Bird S. D., Doevendans P. A., Opthof T., Brutel de la Riviere A., Tertoolen L., van der Heyden M., Pera M. J. Anat. 2002;200:233–242. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor C. J., Bolton E. M., Pocock S., Sharples L. D., Pedersen R. A., Bradley J. A. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 15.Wakayama T., Tabar V., Rodriguez I., Perry A. C., Studer L., Mombaerts P. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- 16.Dvash T., Benvenisty N. Best Pract. Res. Clin. Obstet. Gynaecol. 2004;18:929–940. doi: 10.1016/j.bpobgyn.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Martin M. J., Muotri A., Gage F., Varki A. Nat. Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 18.Cobo F., Stacey G. N., Hunt C., Cabrera C., Nieto A., Montes R., Cortes J. L., Catalina P., Barnie A., Concha A. Appl. Microbiol. Biotechnol. 2005;68:456–466. doi: 10.1007/s00253-005-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granerus M., Engstrom W. Cell Prolif. 1996;29:309–314. doi: 10.1111/j.1365-2184.1996.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu C., Rosler E., Jiang J., Lebkowski J. S., Gold J. D., O’Sullivan C., Delavan-Boorsma K., Mok M., Bronstein A., Carpenter M. K. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 21.Beattie G. M., Lopez A. D., Bucay N., Hinton A., Firpo M. T., King C. C., Hayek A. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 22.Amit M., Shariki C., Margulets V., Itskovitz-Eldor J. Biol. Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 23.Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 24.Holden C. Science. 2005;307:1393. doi: 10.1126/science.307.5714.1393b. [DOI] [PubMed] [Google Scholar]
- 25.Xu C., Inokuma M. S., Denham J., Golds K., Kundu P., Gold J. D., Carpenter M. K. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 26.Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 27.Stojkovic P., Lako M., Przyborski S., Stewart R., Armstrong L., Evans J., Zhang X., Stojkovic M. Stem Cells. 2005;23:895–902. doi: 10.1634/stemcells.2004-0326. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig T. E., Levenstein M. E., Jones J. M., Berggren W. T., Mitchen E. R., Frane J. L., Crandall L. J., Daigh C. A., Conard K. R., Piekarczyk M. S., et al. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Powell S., Brunette E., Lebkowski J., Mandalam R. Biotechnol. Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 30.Pease S., Braghetta P., Gearing D., Grail D., Williams R. L. Dev. Biol. 1990;141:344–352. doi: 10.1016/0012-1606(90)90390-5. [DOI] [PubMed] [Google Scholar]
- 31.Brimble S. N., Zeng X., Weiler D. A., Luo Y., Liu Y., Lyons I. G., Freed W. J., Robins A. J., Rao M. S., Schulz T. C. Stem Cells Dev. 2004;13:585–597. doi: 10.1089/scd.2004.13.585. [DOI] [PubMed] [Google Scholar]
- 32.Draper J. S., Smith K., Gokhale P., Moore H. D., Maltby E., Johnson J., Meisner L., Zwaka T. P., Thomson J. A., Andrews P. W. Nat. Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 33.Hyslop L. A., Armstrong L., Stojkovic M., Lako M. Expert Rev. Mol. Med. 2005;7:1–21. doi: 10.1017/S1462399405009804. [DOI] [PubMed] [Google Scholar]
- 34.Ying Q. L., Nichols J., Chambers I., Smith A. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 35.Humphrey R. K., Beattie G. M., Lopez A. D., Bucay N., King C. C., Firpo M. T., Rose-John S., Hayek A. Stem Cells. 2004;22:522–530. doi: 10.1634/stemcells.22-4-522. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi Y., Kadowaki H., Momomura K., Fukushima Y., Orban T., Okai T., Taketani Y., Akanuma Y., Yazaki Y., Kadowaki T. Diabetologia. 1997;40:412–420. doi: 10.1007/s001250050695. [DOI] [PubMed] [Google Scholar]
- 37.Tyrala E. E. Obstet. Gynecol. Clin. N. Am. 1996;23:221–241. doi: 10.1016/s0889-8545(05)70253-9. [DOI] [PubMed] [Google Scholar]
- 38.DeBaun M. R., King A. A., White N. Semin. Perinatol. 2000;24:164–171. doi: 10.1053/sp.2000.6366. [DOI] [PubMed] [Google Scholar]
- 39.Barrett C. T., Oliver T. K., Jr N. Engl. J. Med. 1968;278:1260–1262. doi: 10.1056/NEJM196806062782304. [DOI] [PubMed] [Google Scholar]
- 40.Reinecke-Luthge A., Koschoreck F., Kloppel G. Virchows Arch. 2000;436:1–5. doi: 10.1007/pl00008192. [DOI] [PubMed] [Google Scholar]
- 41.Duvillie B., Cordonnier N., Deltour L., Dandoy-Dron F., Itier J. M., Monthioux E., Jami J., Joshi R. L., Bucchini D. Proc. Natl. Acad. Sci. USA. 1997;94:5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louvi A., Accili D., Efstratiadis A. Dev. Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- 43.Bottcher R. T., Niehrs C. Endocr. Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Wynshaw-Boris A. Curr. Opin. Genet. Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Li L., Menendez P., Cerdan C., Bhatia M. Blood. 2005;105:4598–4603. doi: 10.1182/blood-2004-10-4065. [DOI] [PubMed] [Google Scholar]
- 46.Wang G., Zhang H., Zhao Y., Li J., Cai J., Wang P., Meng S., Feng J., Miao C., Ding M., Li D., Deng H. Biochem. Biophys. Res. Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 47.Vallier L., Alexander M., Pedersen R. A. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 48.Pinto D., Clevers H. Exp. Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R., III, Nusse R. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 50.Dravid G., Ye Z., Hammond H., Chen G., Pyle A., Donovan P., Yu X., Cheng L. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 51.Baker M. E. J. Endocrinol. 2002;175:121–127. doi: 10.1677/joe.0.1750121. [DOI] [PubMed] [Google Scholar]
- 52.Ory D. S. Circ. Res. 2004;95:660–670. doi: 10.1161/01.RES.0000143422.83209.be. [DOI] [PubMed] [Google Scholar]
- 53.Simons K., Ikonen E. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 54.Porter F. D. Curr. Opin. Pediatr. 2003;15:607–613. doi: 10.1097/00008480-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Zeng X., Miura T., Luo Y., Bhattacharya B., Condie B., Chen J., Ginis I., Lyons I., Mejido J., Puri R. K., et al. Stem Cells. 2004;22:292–312. doi: 10.1634/stemcells.22-3-292. [DOI] [PubMed] [Google Scholar]