Abstract
There is a need for an improved test of human ability to assimilate dietary vitamin B12. Assaying and understanding absorption and uptake of B12 is important because defects can lead to hematological and neurological complications. Accelerator mass spectrometry is uniquely suited for assessing absorption and kinetics of carbon-14 (14C)-labeled substances after oral ingestion because it is more sensitive than decay counting and can measure levels of 14C in microliter volumes of biological samples with negligible exposure of subjects to radioactivity. The test we describe employs amounts of B12 in the range of normal dietary intake. The B12 used was quantitatively labeled with 14C at one particular atom of the dimethylbenzimidazole (DMB) moiety by exploiting idiosyncrasies of Salmonella metabolism. To grow aerobically on ethanolamine, Salmonella enterica must be provided with either preformed B12 or two of its precursors, cobinamide and DMB. When provided with 14C-DMB specifically labeled in the C2 position, cells produced 14C-B12 of high specific activity (2.1 GBq/mmol, 58 mCi/mmol) (1 Ci = 37 GBq) and no detectable dilution of label from endogenous DMB synthesis. In a human kinetic study, a physiological dose (1.5 μg, 2.2 kBq/59 nCi) of purified 14C-B12 was administered and showed plasma appearance and clearance curves consistent with the predicted behavior of the pure vitamin. This method opens new avenues for study of B12 assimilation.
Keywords: dimethylbenzimidazole, ethanolamine, metabolic engineering, Salmonella, Schilling test
Vitamin B12 (B12) is a compound of significant nutritional and clinical importance (1). The classical manifestations of B12 deficiency include pernicious anemia, a type of megaloblastic anemia, and neurological dysfunction (2). The Schilling urinary excretion test (3, 4) indirectly measures B12 absorption and has been applied when B12 insufficiency is identified and malabsorption is the suspected cause. The test involves ingestion of a physiological quantity of B12 labeled with gamma-emitting cobalt, followed by administration of a pharmacological parenteral flushing dose of unlabeled B12 to force urinary excretion of radioactivity, which is measured during a 24-h period. The Schilling test is currently the only accepted method for assessing B12 absorption. Despite its utility, the method is semiquantitative and has methodological and practical problems; it is now rarely prescribed, despite the prevalence of B12 malabsorption in older adults (2, 5). We describe a method that has the potential to reinvigorate interest in diagnosis of the underlying causes of vitamin B12 deficiency. The test has several advantages over the Schilling test: It poses a negligible radiation exposure to the subjects and medical workers and can be performed from a capillary-sized blood sample, without the requirement for a flushing dose of B12 or for collection of radioactive urine for an extended period.
The absorption test described uses carbon-14-labeled vitamin B12 (14C-B12) coupled to sensitive detection of the 14C-B12 by accelerator mass spectrometry (AMS). AMS was originally developed for carbon dating in archaeological or earth science samples; however, in the past decade or so, its sensitivity has been exploited for tracing of biological systems (6). In contrast to liquid scintillation counting, which records decay events of a radioisotope, AMS is a direct atom counter that was developed for quantifying long-lived isotopes such as 14C (half-life of 5,370 yr). It is a tandem isotope ratio mass spectrometer that provides the relative abundance of the 14C atom with respect to total carbon (14C/C) down to parts per quadrillion (1 in 1015) (6–8). Thus, it is possible to quantify attomole (10−18 mol) amounts of 14C in a milligram-sized biological sample at high precision (typically <2% imprecision). The remarkable combination of sensitivity and precision of AMS allows quantitation of 14C-B12 from small biological samples and reduces the exposure of subjects to a negligible radiation risk.
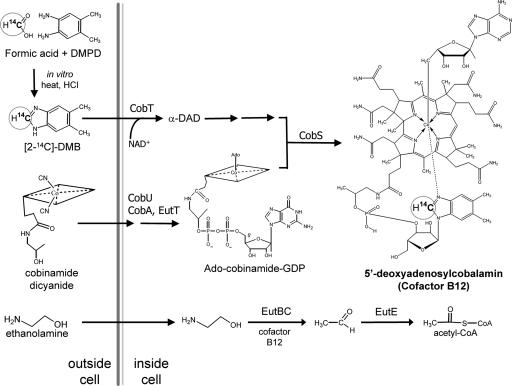
The key to the success of the B12-absorption test we describe is the synthesis of 14C-B12 by an efficient, microscale method that produces 14C-B12 specifically labeled at the carbon 2 position of the dimethylbenzimidazole (DMB) moiety of B12. This B12 is produced by Salmonella enterica, a bacterium that normally produces B12 de novo only under anaerobic conditions and uses it to support growth on ethanolamine. Cells cannot grow aerobically on ethanolamine because they fail to synthesize two B12 precursors, cobinamide and DMB, but they retain the ability to assemble B12 from these precursors supplied exogenously. When isotopically labeled DMB is supplied, B12 is produced with no detectable isotope dilution. The processes leading to the biosynthesis of 14C-B12 by S. enterica are shown in Fig. 1. The 14C-B12 synthesized by this method was used for a quantitative AMS-based assay of human B12 absorption.
Fig. 1.
Synthesis of cofactor B12 from cobinamide and 14C-DMB during growth on ethanolamine. S. enterica enzymes CobTUSC and EutT catalyze the synthesis of adenosylcobalamin (25, 26). Growth on ethanolamine proceeds by means of ethanolamine ammonia lyase EutBC, which requires adenosylcobalamin to catalyze the formation of acetaldehyde from ethanolamine.
Results
Metabolic Engineering of S. enterica for Biosynthesis of 14C-Labeled B12.
The method used to produce labeled B12 relies on two idiosyncrasies of B12 metabolism in S. enterica. Under aerobic conditions on minimal medium with ethanolamine as the sole carbon source, S. enterica produces neither cobinamide (the corrinoid precursor) nor DMB (the lower ligand of B12) but retains the ability to assemble B12 when these precursors are provided exogenously.
The 17-gene ethanolamine (eut) operon (9) of S. enterica is shown in Fig. 2. Induction of eut and the growth conditions pertinent to this work are shown in Table 1. Induction of the eut operon was monitored by assay of β-galactosidase produced by a lacZ gene inserted within a transcribed region of the eut operon that is distal to all genes of the operon. The basal level of eut transcription produces 10 Miller units of β-galactosidase activity from an operon fusion and increases ≈50-fold when the operon is induced by the combination of B12 plus ethanolamine. Surprisingly, the B12 precursor cobinamide can replace B12 as an inducer but does not permit growth on ethanolamine (Table 1). This failure to produce B12 is due to a lack of DMB because B12 production and growth on ethanolamine is restored if DMB is provided in addition to cobinamide. Thus, during aerobic growth on ethanolamine, S. enterica fails to make both cobinamide and DMB but can synthesize B12 if these precursors are provided. The CobA, U, S, T, C, and EutT enzymes, which catalyze conversion of cobinamide plus DMB to B12, are produced at levels sufficient to assemble B12 and permit aerobic cell growth on ethanolamine. These conditions allow efficient conversion of labeled precursors into B12 without label dilution. The growth conditions used appear to avoid feedback repression of B12 synthesis, presumably because B12 is sequestered and bound by the EutBC enzyme.
Fig. 2.
Organization of the 17-gene eut operon. Genes are transcribed from left to right from the primary promoter P1. P2 is a weak constitutive promoter.
Table 1.
Induction of eut and growth on ethanolamine
| Inducer | Mean ± SD | Growth |
|---|---|---|
| 1. None | 10 ± 1 | − |
| 2. EA | 9 ± 3 | − |
| 3. DMB | 14 ± 6 | − |
| 4. CBI | 7 ± 1 | − |
| 5. EA + DMB | 12 ± 3 | − |
| 6. EA + CBI | 447 ± 30 | − |
| 7. EA + CBI + DMB | 313 ± 24 | + |
| 8. EA + B12 | 486 ± 16 | + |
Induction of the eut operon in the presence of ethanalomine and glycerol is compared with growth on ethanalomine as sole carbon source.
Synthesis and Purification of 14C-Labeled DMB and Incorporation into B12.
High specific activity 14C-labeled DMB was synthesized by condensation of 14C formic acid with dimethylphenylenediamine (10) (Fig. 1). The reaction product, [2-14C]-DMB, was purified by HPLC to eliminate unwanted reaction products. When this labeled DMB and unlabeled cobinamide were provided to S. enterica, the cells produced B12 and grew aerobically on ethanolamine.
Purification and Mass Spectral Analysis of 14C-B12.
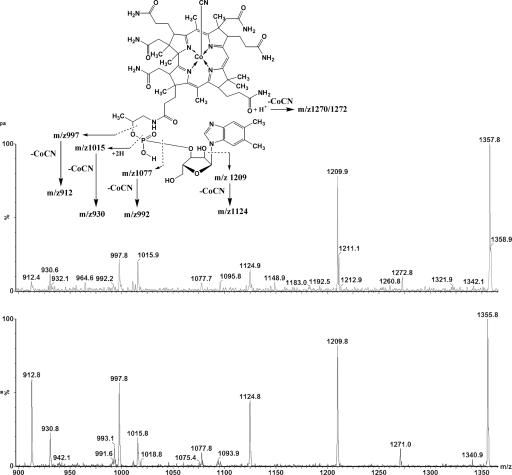
Adenosylcobalamin was extracted from bacteria in the presence of cyanide so that highly stable cyanocobalamin (vitamin B12) would be formed. These extracts were purified by HPLC and analyzed by two methods of mass spectrometry (MS) to establish chemical identity. The 14C radiolabel coeluted precisely with the single chromatographic peak of the vitamin B12 standard (Fig. 3a). The UV/visible absorption spectrum of the purified 14C compound was consistent with that of the B12 standard spectrum (Fig. 3b). The putative 14C-labeled cyanocobalamin (B12) was also analyzed by two forms of MS; the MS/MS product spectra of the vitamin B12 standard, (M+H)+ at m/z = 1,355.8 Thompson (Th), and the 14C-B12, (M+H)+ at m/z = 1,357.8 Th, are shown in Fig. 4. There is a 2 Th mass difference between the molecular ions of the standard and 14C-labeled cobalamins; the fragmentation pattern confirms that this difference is due to a single 14C-label on the 5,6-DMB moiety. The molecular ion (M+H)+ of the unlabeled B12 was compared with the 14C-labeled compound also by high resolution MS. The measured values for both the unlabeled (m/z = 1,355.5741 Th) and 14C-labeled cobalamin (m/z = 1,357.5787 Th) agreed in molecular mass with the predicted values (m/z = 1,355.5752 Th and m/z = 1,357.5782 Th, respectively). Based on the identified decomposition products of the MS/MS experiments, the accurate mass measurement data, and the HPLC data, we conclude that the product of the directed biosynthesis is ([2-14C]5,6-DMB)cyanocobalamin.
Fig. 3.
Comparison of synthesized 14C-B12 with authentic B12. (a) Coelution of the 14C radiolabel with B12. (b) Spectrum of standard vitamin B12 and that of the putative 14C-B12.
Fig. 4.
MS/MS spectra in product mode showing the decomposition products of the molecular ions (M+H)+ of B12 and 14C-B12.
Specific Activity of 14C-B12.
The theoretical specific activity for a compound with one atom of 14C at 100% incorporation is 2.308 GBq/mmol. Mass spectral analysis of the DMB isotopomers synthesized as described above indicated that 91% of the DMB product contained the 14C label; thus, the specific activity was 2.1 GBq/mmol (data not shown). MS analysis of the 14C-B12 indicated that the DMB had been incorporated into the 14C-B12 without detectable dilution by endogenous DMB synthesis. The 14C-B12 therefore had approximately the same specific activity, 2.1 GBq/mmol. The total mass yield of vitamin B12 produced from the incubation was 25 μg (18.6 nmol as cyanocobalamin).
Human B12 Absorption.
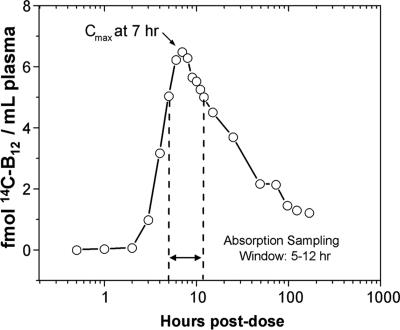
The time course for the appearance and disappearance of 14C in plasma during the 7 days after dosing is presented in Fig. 5. Data are given as femtomoles of 14C-B12 per milliliter of plasma. After 7 h, the circulating 14C-B12 reached a peak, which corresponded to <3% of the administered dose of 14C-B12. The amount of B12 detected at the peak would produce less than one disintegration per min if assayed by scintillation counting, and thus would be unmeasurable by decay counting. There is a 2- to 3-h delay in the appearance of the label in plasma, consistent with the time taken for gastric emptying and facilitated absorption in the ileum. After the maximum level of 14C-B12 was achieved, the concentration of the label decreased at a single rate, with evidence for a small reappearance in plasma 4 days after dosing. A radiochromatogram of cyanidated plasma, taken from the peak sample (Cmax in Fig. 5), revealed that the majority of the 14C comigrated with cyanocobalamin on a reversed-phase HPLC system (data not shown).
Fig. 5.
AMS detection of 14C in human plasma. Units are expressed as femtomolar 14C-B12. Measurements were performed on 30 μl of plasma. The entire sample set consumed <1 ml of whole blood.
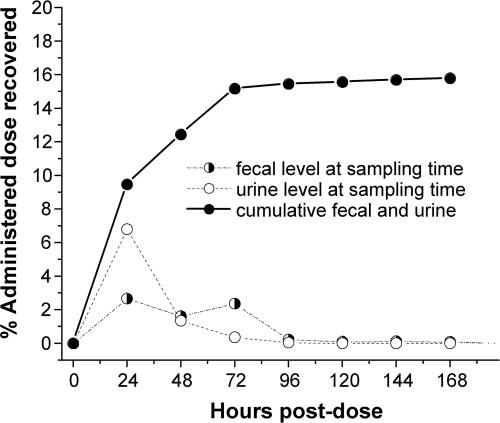
Fig. 6 illustrates recovery of the label in urine and stool specimens 7 days after dosing. The largest single concentration of label appeared in the 24-h urine collection (6.8% of administered dose). The apparent excretion of 6.8% of the B12 dose in the 24-h urine does not agree with previously reported values of 0.10–0.41% urinary B12 excretion for normal subjects given radiocobalt B12 with no flushing dose of B12 (11). However, a radiochromatogram of cyanidated urine indicates that only ≈1.47% of the 14C urinary analytes was 14C-B12 (data not shown). Thus, the total 24-h urine 14C-B12 is ≈0.1% of the total 14C-B12 dose given, in agreement with previous measurements. The chromatographic analysis of the urine showed that the bulk of the label is distributed among several peaks, with mobility distinct from that of B12 or free DMB. These compounds may be breakdown products of B12 that could not be detected by using cobalt-labeled B12. After 7 days, a total of 15.8% of the oral dose was recovered in urine and feces. Ninety-nine percent of the total quantified urinary and fecal output was accounted for 72 h after the dose.
Fig. 6.
Recovery of 14C in urine and fecal specimens and cumulative recovery of 14C. After 7 days, 15.9% of the dose was recovered in the urine and stool.
Discussion
The absorption of B12 in humans is a complex process compared with absorption of other water-soluble vitamins, requiring a specific intestinal transport protein produced in the stomach (intrinsic factor) for active uptake. This active uptake process is quickly saturated, and doses greater than the current U.S. recommended dietary allowance (2.4 μg/day) (12) are predominantly passed unabsorbed into stool. Therefore, sensitive absorption tests are necessary to assess absorption of B12 at normal dietary levels. Recently, a nonradioactive method for assessing vitamin B12 absorption was reported (13). The method seeks to correlate B12 absorption with an increase in holo-transcobalamin by using ELISA. Although this method holds promise, in its current form it lacks the ability to deliver an unequivocal B12 absorption/malabsorption result and cannot convey quantitative or kinetic information. The data described in the present study suggest that a more robust method for assaying B12 absorption and turnover can be developed that relies on use of AMS to detect physiological levels of suitably labeled B12 with high precision. Production of 14C-B12 substrate is critical for this improved method.
B12 biosynthesis starts either from succinyl-CoA and glycine (the Shemin or C4 pathway) or, alternatively, from glutamyl-tRNA (the C5 pathway) (14). These primary metabolites have manifold roles and are not solely committed to B12 biosynthesis; their use as a source of radiolabel for B12 would result in significant label dilution. In the described method, a specific atom of B12 is labeled with 14C by using a committed, late intermediate DMB.
DMB is an ideal molecule for labeling B12 because it appears to have only a single metabolic fate: incorporation into the α-ligand of B12. Fortuitously, it is both stable and inexpensive to radiolabel by a one-step reaction from 14C-formic acid and dimethylphenylenediamine. This precursor is efficiently introduced into B12 by cells of S. enterica because of several metabolic idiosyncrasies of this bacterium. S. enterica has a well characterized de novo biosynthetic pathway for B12 (15) that functions only in the absence of oxygen (16). When grown aerobically on glucose with cobinamide, S. enterica makes B12 by using endogenously synthesized DMB (17), which would dilute the label of any added DMB. However, when ethanolamine is the sole carbon source, aerobic S. enterica cells synthesize neither cobinamide nor DMB, and both must be added to allow B12 production and growth (Fig. 1). Thus, during growth of Salmonella on ethanolamine, 14C-labeled DMB provided with cobinamide allows for efficient and specific labeling of B12.
The use of this labeled B12 and AMS to assay B12 absorption was demonstrated in a single, healthy, normal volunteer. A single 1.5-μg dose of the 14C-B12 was administered orally and detected by AMS as it appeared in the bloodstream. Normally, the release of B12 from intestinal mucosa cells into the portal vein occurs ≈2 h after the oral consumption of the vitamin, and release into systemic circulation takes an additional 1 h (18, 19). Consistent with this finding, the 14C from the labeled B12 appeared in the plasma of the human subject 3 h after the dose. A concentration of 5–6 fmol of 14C-B12 per ml was observed in peak samples (5–12 h) (Fig. 5). This time window would make it possible to assess B12 absorption from a single capillary blood sample. The current Schilling urinary excretion test, which has been the standard method of assessing B12 absorption since its introduction in 1953, is now rarely prescribed because it requires the administration of radiocobalt B12 followed by an intramuscular flushing dose and 24-h total urine collection. By this method, patients with normal B12 absorption excrete 8–40% of the labeled B12 in the urine, compared with patients who have malabsorption, from whom there is little or no recovery of labeled B12 from urine (4). By contrast, the method we describe makes it possible to follow the fate of the vitamin at near ambient levels of radiation exposure by using microliter-sized blood specimens and without a flushing dose. Relatively little of the dose was recovered in either urine or stool; 84% of the administered dose was retained in body tissues after 7 days. This observation is consistent with the very slow body elimination of B12 (0.1% loss per day after initial excretion of the unabsorbed dose) due to efficient enterohepatic recycling. The 6.8% loss in the 24-h urine was unexpected in the absence of a parenteral flushing dose, based on previous experiments carried out using radiocobalt B12. Examination of the chromatographic behavior of 14C-labeled compounds in the urine revealed that the predominant urinary products were not vitamin B12. The accumulation of 14C-B12 degradation products in the urine may be due to acid hydrolysis of some of the 14C-B12 dose in the stomach or bacterial degradation in the gut with consequent release of DMB. Absorption of free DMB and its metabolism, such as through hepatic conjugation to glucuronide, may result in urinary excretion of the product. However, further analysis is necessary to determine the exact nature of the products detected in the urine.
In summary, we describe the biosynthesis of 14C-labeled B12 and human absorption kinetics by using near-ambient levels of radiation that pose little or no risk from exposure. The sensitivity of AMS reduces the needed sample size to only tens of microliters of blood and minimizes exposure to radiation. We believe that combined use of 14C-labeled B12 and AMS detection has the potential to be a powerful clinical diagnostic tool and an improved method for studying the underlying causes of B12-uptake disorders, including the development of a sensitive and quantitative test for B12 absorption in humans.
Methods
Synthesis and Purification of 14C-DMB.
Radiolabeled DMB was synthesized by using a procedure modified from that of Phillips (10). Into a 10-ml boiling flask containing 500 μl of sodium phosphate buffer (pH 7.4, 100 mM) was added 14C-formic acid (1 mCi; 0.0182 mmol, 1.85–2.22 GBq/mmol) (Moravek Biochemicals, Brea, CA). The material was dried under reduced pressure, and the solids were dissolved in 1 ml of 4 M HCl. To initiate the reaction, 15.15 mg (111 μmol) of o-dimethylphenylenediamine was added and the contents taken to a vigorous boil by using a reflux system with the condenser maintained at −10°C by using a recirculating chiller. After 2 h of heating, the reaction solution was neutralized by dropwise addition of concentrated ammonium hydroxide to a pH of ≈7. The reaction product was then loaded onto a solid-phase extraction cartridge (1 g of Bond Elut C18 from Varian), which had been primed with 3 ml of methanol and 3 ml of deionized water. The column was washed with 2 column volumes of deionized water, and the bound 14C-DMB was eluted with 3 ml of methanol.
The solvent was removed under streaming nitrogen, and the product was dissolved in 0.5 ml of absolute ethanol. The product was then purified by multiple injections onto an isocratic reverse-phase HPLC by using an Agilent 1100 chromatograph (Agilent Technologies, Palo Alto, CA) fitted with an Adsorbosphere HS C18 column (150 mm × 4.6 mm; Alltech Associates). The isocratic mobile phase, 34:33:33 water:methanol:acetonitrile, was pumped at a flow rate of 0.80 ml/min; the absorbance of the outflow was monitored at 284 nm. The peaks, which had similar retention and spectral characteristics to a purchased DMB standard (Sigma), were pooled and evaporated to dryness under reduced pressure, dissolved in absolute ethanol, and stored at −70°C. Radioactivity was determined by liquid scintillation counting.
Microorganism Cultivation.
The strain used for reporting β-galactosidase activity was TT10674, genotype eut38::mudA. The strain used for 14C-B12 biosynthesis was S. enterica (serovar Typhimurium) strain TT24733, genotype cbiD24::MudJ. The labeling medium consisted of no carbon E medium (20) supplemented with 40 mM ethanolamine, 250 nM dicyanocobinamide, and 500 nM 14C-DMB synthesized as described above. Approximately 130 ml of labeling medium was added to a sterile, 500-ml conical flask. The medium (130 ml in a 500-ml flask) was inoculated by a 100-fold dilution of a S. enterica culture grown in no carbon E medium supplemented with 20 mM glycerol. Cultures were incubated in the dark for 48 h at 30°C with shaking at 250 rpm.
Extraction and Purification of 14C-Cyanocobalamin from Cells.
Bacterial cells were pelleted by centrifugation for 20 min at 6,000 × g. Supernatants were removed, and cell pellets were washed three times with no carbon E medium (20). The pellets were resuspended in 5 ml of methanol and 500 μl of 50 mg/ml sodium cyanide, vortex mixed, and placed in a 60°C water bath for 12 h with intermittent vortex mixing. The samples were then centrifuged at 20,000 × g for 1 h. The supernatants were removed from the pellet and evaporated to dryness. Dried samples were resuspended in water and filtered (0.22 μm) to remove any insoluble material. A first step in purification of the 14C-B12 was performed by extraction on C18:0 solid-phase extraction cartridges (5-g bond Elut C18 from Varian). The corrinoids were eluted with 50:50 water:methanol, and the solvent was evaporated to dryness. Purification to homogeneity was carried out by HPLC on an Agilent series 1100 HPLC equipped with a diode array detector and fitted with an Agilent Zorbax Eclipse XDB C18 (3.5 μm) column (150 mm × 3.0 mm). Solvent A was 90/10 water/methanol, and solvent B was methanol with initial conditions of 82/18 A/B. At 12 min, a linear gradient was started that reached 25/75 A/B after 16 min. The flow rate was held constant at 0.360 ml/min. Extracts were run in multiple injections, and the peak corresponding to 14C-cyanocobalamin was collected and pooled for each run. The solvent was evaporated to dryness, and samples were resuspended in water for storage at −70°C.
MS.
High-resolution MS: accurate mass measurement.
Exact mass measurement experiments were performed, in positive mode, on a Micromass liquid chromatograph orthogonal acceleration time-of-flight mass spectrometer (Waters-Micromass, Manchester, U.K.). The cone and desolvation gas were set to 50 and 650 liters/h, respectively. Resolution was 8,000, measured at 803-Th mass, based on the definition of full width at half maximum (FWHM). Sample source conditions were as follows: capillary voltage, 3,250 V; sample cone voltage, 30 V; extraction cone voltage, 6 V; source temperature, 100°C; and desolvation temperature, 250°C. Transfer optics settings were as follows: radio frequency (rf) lens, 250 V; rf dc offset-1, 4.0 V; rf dc offset-2, 6.0 V; aperture, 2.0 V; acceleration, 200.0 V; focus, 1.0 V; and steering, −0.3 V. Analyzer settings were as follows: multichannel plate (MCP) detector, 2,430 V; ion energy, 32.0 V; tube lens, 4.0 V; grid-2, 20.0 V; time-of-flight flight tube, 4,599 V; and reflectron, 1,713 V. The pusher cycle time was 50 μs. Data files were acquired in continuum mode, and spectra were stored from m/z = 100 to 1,600 with a 1.1-sec scanning cycle consisting of a 1.0-sec scan and a 0.1-sec interscan time. Typically, 20–30 individual spectra were summarized. TOF calibration: effective length of the flight tube (Lteff) value was set to 1,122.7250 in positive mode by using molecular ions of leucine-enkephalin (L9133 from Sigma) at 556.2771 Th. System calibration was performed by using poly-dl-alanine (P9003 from Sigma), which was also used as an internal standard for accurate mass measurement. To obtain accurate masses, the following procedure was performed: Savitsky–Golay smoothing, by using a ±4-channel window repeated twice, and centering, by using the center value at the 50% height of the peak. Samples were introduced into the mass spectrometer through direct-flow injection by using the Waters Alliance 2795 HPLC system for solvent delivery at the flow rate of 250 μl/min; mobile phase CH3CN/H2O (1/1) was used. masslynx 4.0 sp3 software (Waters-Micromass) was used for instrument control, data acquisition, and data evaluation.
MS/MS experiments.
Positive ion MS/MS experiments were performed in product mode on a Quattro Premier (Waters-Micromass) triple quadrupole mass spectrometer with a configuration of quadrapole-traveling wave collision-cell quadrapole (QtQ) equipped with an atmospheric pressure ionization (API) interface sprayer. The instrument was operated with the following instrumental conditions: source temperature, 120°C; desolvation temperature, 200°C; capillary voltage, 3.1 kV; cone voltage, 50 V; extraction cone, 5 V; and radio frequency lens, 0.5 V. The drying and cone gas was nitrogen. The cone gas flow was set to 50 liters/h, and the desolvation gas was set to flow to 700 liters/h. Quadrupole-1 parameters were as follows: low mass (LM) resolution, 14.0; high mass (HM) resolution, 14.0; ion energy, 0.4–0.6 V; entrance, 7 V; and exit, 16 V. Quadrupole-2 parameters were as follows: LM resolution, 15.5; HM resolution, 15.5; and ion energy, 2.0–2.5 V. Multipliers were set at 550 V. The collision gas was argon (99.9999%) (Airgas, Radnor, PA) with a pressure of 3.6–4.6 × 10−3 mbar (1 bar = 100 kPa) in the collision cell. MS/MS experiments were performed at a collision energy of 50–70 eV (in the case of single charged ions) and 30–40 eV (in the case of double charged ions). MS/MS data were acquired in continuous mode. The scanning speed was in all cases 0.025 sec/decade with a 0.1-sec interscan time. The sampling density was set at 16/Da. Infusion experiments were performed on an integral syringe pump controlled from masslynx, with a flow rate of 20 μl/min, directly connected to the interface. Data acquisition and instrument control was performed by using masslynx 4.0 sp 3.
Subjects and Human Experimental Design.
The subject was a healthy male aged 40 years with a body mass index (BMI) of 27.5. The subject began complete fecal and urine collection 24 h in advance of the 14C-B12 dose and continued complete 24-h collections until day 7. On the day of dose administration, the subject was fitted with an i.v. catheter in a forearm vein. Blood was drawn into 7-ml tubes containing EDTA. A baseline blood sample was drawn (at 7 a.m.), and the 14C-B12 dose consumed that corresponded to 2.2 kBq of radioactivity (1.5 μg) was administered in 50 ml of drinking water in a paper cup. The volunteer was allowed to have water ad libitum thereafter, with a light meal taken 2 h after dosing. Blood samples (5 ml) were drawn at frequent intervals for the first 15 h after dosing and daily thereafter. Other meals were controlled for time and content on the dose-administration day. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Institutional Review Boards at the University of California Davis and Lawrence Livermore National Laboratory. Informed consent was obtained from the subject.
AMS Analysis.
Aliquots of plasma (30 μl), urine (80 μl), and a stool slurry (80 μl) were dried, combusted to CO2, and reduced to filamentous carbon by using procedures described in refs. 21 and 22. No other processing preceded the graphitization step; thus, quantitative recovery was ensured. The 14C measurements were performed at the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Laboratory (23). Measurements were conducted to <3% instrument imprecision, and, in general, signal acquisition was complete to the desired statistical precision in 3–5 min per sample. The radiation exposure of the subject, due to the 14C-B12 dose, was equivalent to or less than exposure due to 16 h of intercontinental plane flight (7, 24).
Acknowledgments
We thank John Thorngate for help with MS and Michael Lamé and Andrew Clifford for technical support and use of laboratory facilities. This work was performed in part under the auspices of the U.S. Department of Energy by the University of California–Lawrence Livermore National Laboratory under Contract W-7405-Eng-48. This work was supported by National Institutes of Health (NIH) Grants DK064302 and 6M34804, NIH/National Center for Research Resources Grant RR 13461, National Institute of Environmental Health Sciences (NIEHS) Superfund Basic Research Program Grant P42 ES04699, NIEHS Center for Environmental Health Sciences Grant P42 ES05707, and NIEHS Center for Children’s Environmental Health and Diseases Prevention Grant NIEHS P01 ES11269.
Abbreviations
- AMS
accelerator MS
- DMB
dimethylbenzimidazole
- Th
Thompson.
Footnotes
Conflict of interest statement: A U.S. patent entitled “Assay for Vitamin B12 Absorption and Method of Making Labeled Vitamin B12” has been filed by P.J.A., S.R.D., J.W.M., R.G., J.R.R., C.C., and B.A.B.
References
- 1.Carmel R. Annu. Rev. Med. 2000;51:357–375. doi: 10.1146/annurev.med.51.1.357. [DOI] [PubMed] [Google Scholar]
- 2.Green R., Kinsella L. J. Neurology. 1995;45:1435–1440. doi: 10.1212/wnl.45.8.1435. [DOI] [PubMed] [Google Scholar]
- 3.Zuckier L. S., Chervu L. R. J. Nucl. Med. 1984;25:1032–1039. [PubMed] [Google Scholar]
- 4.Schilling R. J. Lab. Clin. Med. 1953;42:860–866. [PubMed] [Google Scholar]
- 5.Carmel R. Arch. Intern. Med. 1996;156:1097–1100. [PubMed] [Google Scholar]
- 6.Vogel J. S., Turteltaub K. W., Finkel R., Nelson D. E. Anal. Chem. 1995;67:353A–359A. doi: 10.1021/ac00107a001. [DOI] [PubMed] [Google Scholar]
- 7.Vogel J. S., Turteltaub K. W. Adv. Exp. Med. Biol. 1998;445:397–410. doi: 10.1007/978-1-4899-1959-5_25. [DOI] [PubMed] [Google Scholar]
- 8.Dueker S. R., Lin Y., Buchholz B. A., Schneider P. D., Lame M. W., Segall H. J., Vogel J. S., Clifford A. J. J. Lipid Res. 2000;41:1790–1800. [PubMed] [Google Scholar]
- 9.Kofoid E., Rappleye C., Stojiljkovic I., Roth J. J. Bacteriol. 1999;181:5317–5329. doi: 10.1128/jb.181.17.5317-5329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips M. A. J. Chem. Soc. 1928:2393–2399. [Google Scholar]
- 11.Booth C., Mollin D. Br. J. Haematol. 1956;2:223–236. doi: 10.1111/j.1365-2141.1956.tb06695.x. [DOI] [PubMed] [Google Scholar]
- 12.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: Natl. Acad. Press; 1998. [PubMed] [Google Scholar]
- 13.Bor M. V., Cetin M., Aytac S., Altay C., Nexo E. Clin. Chem. 2005;51:2151–2155. doi: 10.1373/clinchem.2005.055509. [DOI] [PubMed] [Google Scholar]
- 14.Jahn D., Verkamp E., Soll D. Trends Biochem. Sci. 1992;17:215–218. doi: 10.1016/0968-0004(92)90380-r. [DOI] [PubMed] [Google Scholar]
- 15.Roth J. R., Lawrence J. G., Rubenfield M., Kieffer-Higgins S., Church G. M. J. Bacteriol. 1993;175:3303–3316. doi: 10.1128/jb.175.11.3303-3316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson D. I. Mol. Microbiol. 1992;6:1491–1494. doi: 10.1111/j.1365-2958.1992.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson M. G., Escalante-Semerena J. C. J. Biol. Chem. 1992;267:13302–13305. [PubMed] [Google Scholar]
- 18.el Kholty S., Gueant J. L., Bressler L., Djalali M., Boissel P., Gerard P., Nicolas J. P. Gastroenterology. 1991;101:1399–1408. doi: 10.1016/0016-5085(91)90094-2. [DOI] [PubMed] [Google Scholar]
- 19.Doscherholmen A., Hagen P. S. Blood. 1957;12:336–346. [PubMed] [Google Scholar]
- 20.Maloy S. R., Stewart V. J., Taylor R. K. Genetic Analysis of Pathogenic Bacteria: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Lab. Press; 1996. [Google Scholar]
- 21.Vogel J. S. Radiocarbon. 1992;34:344–350. [Google Scholar]
- 22.Ognibene T. J., Bench G., Vogel J. S., Peaslee G. F., Murov S. Anal. Chem. 2003;75:2192–2196. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 23.Ognibene T. J., Bench G., Brown T. A., Peaslee G. F., Vogel J. S. Int. J. Mass Spectrom. 2002;218:255–264. [Google Scholar]
- 24.Vuong L. T., Buchholz B. A., Lame M. W., Dueker S. R. Nutr. Rev. 2004;62:375–388. doi: 10.1111/j.1753-4887.2004.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheppard D. E., Penrod J. T., Bobik T., Kofoid E., Roth J. R. J. Bacteriol. 2004;186:7635–7644. doi: 10.1128/JB.186.22.7635-7644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggio-Hall L. A., Escalante-Semerena J. C. Microbiology. 2003;149:983–990. doi: 10.1099/mic.0.26040-0. [DOI] [PubMed] [Google Scholar]