Abstract
Deuterium spin relaxation was used to examine the motion of enzyme-bound water on subtilisin Carlsberg colyophilized with inorganic salts for activation in different organic solvents. Spectral editing was used to ensure that the relaxation times were associated with relatively mobile deuterons, which were contributed almost entirely by D2O rather than hydrogen–deuteron exchange on the protein. The results indicate that the timescale of motion for residual water molecules on the biocatalyst, (τc)D2O, in hexane decreased from 65 ns (salt-free) to 0.58 ns (98% CsF) as (kcat/KM)app of the biocatalyst preparation increased from 0.092 s−1·M−1 (salt-free) to 1,140 s−1·M−1 (98% CsF). A similar effect was apparent in acetone; the timescale decreased from 24 ns (salt-free) to 2.87 ns (98% KF), with a corresponding increase in (kcat/KM)app of 0.140 s−1·M−1 (salt-free) to 12.8 s−1·M−1 (98% KF). Although a global correlation between water mobility and enzyme activity was not evident, linear correlations between ln[(kcat/KM)app] and (τc)D2O were obtained for salt-activated enzyme preparations in both hexane and acetone. Furthermore, a direct correlation was evident between (kcat/KM)app and the total amount of mobile water per mass of enzyme. These results suggest that increases in enzyme-bound water mobility mediated by the presence of salt act as a molecular lubricant and enhance enzyme flexibility in a manner functionally similar to temperature. Greater flexibility may permit a larger degree of local transition-state mobility, reflected by a more positive entropy of activation, for the salt-activated enzyme compared with the salt-free enzyme. This increased mobility may contribute to the dramatic increases in biocatalyst activity.
Keywords: enzyme activation, organic solvents, salts, subtilisin Carlsberg
In recent years, the application of selective, nonhazardous biocatalysts for chemical synthesis has become an increasingly attractive alternative to traditional chemical methods. This trend is driven in part by the exquisite chemo-, regio-, and enantioselectivities commonly demonstrated by enzymes. The high demand for enantiomerically pure and selectively functionalized molecules, especially within the pharmaceutical industry, continues to spur the expanding interest in biocatalysis. Unfortunately, many compounds of interest to the pharmaceutical and related industries exhibit poor solubility and undergo deleterious side reactions (e.g., hydrolysis) in water; hence, they are not amenable to enzymatic reactions in conventional media.
Nonaqueous biocatalysis, including enzymatic reactions in nearly anhydrous organic solvents, has emerged as an alternative approach to circumvent the limitations of aqueous-based reaction systems. There are drawbacks, however, to performing enzymatic reactions in organic solvents, most notably low biocatalytic activity (1–5). Much effort has been directed toward elucidating the mechanism(s) underlying the low activity exhibited by insoluble enzyme formulations in organic solvents. Poor compatibility between the solvent and enzymatic transition state (6, 7), reduced protein flexibility (8–12), ground-state stabilization of the substrate (13, 14), water stripping (5, 15–17), and partial denaturation of the enzyme (11, 12, 18) are among the factors that have been identified as possible causes of reduced enzyme activity in organic media.
Researchers have attempted to increase enzyme activity in organic media through a variety of techniques [PEGylation (19), ion-pairing (20), solid-state buffers (21)], one of the simplest being the inclusion of excipients during the lyophilization process (22–28). Of the various excipients used, including salts, sugars, and polymers, the most promising results have been obtained with salts. Lyophilized salt-enzyme preparations have shown increases in activity up to 35,000-fold over the lyophilized salt-free preparations (24). Interestingly, Bedell et al. (29) showed conclusively that this activation effect is not attributable to reduced diffusional limitations but is rather an intrinsic property of the biocatalyst. Little has been confirmed about the mechanistic underpinning(s) of salt activation; however, the dramatic activation is believed to involve increased active-site polarity or improved enzyme flexibility, acting alone or in concert. In addition, a high concentration of salt in the prelyophilized enzyme solution may induce preferential hydration of the enzyme (30) and promote the retention of water during the freeze-drying process, thereby helping to preserve enzyme flexibility and increasing catalytic activity.
We have used 2H NMR relaxation methods to examine the motion of enzyme-bound water for a series of enzyme-salt formulations in different organic solvents. These data provide indirect evidence that salt activation is mediated by increased water mobility. Moreover, we show that the presence of salt alters the local mobility of the enzyme-bound water in a manner similar to that of increasing temperature, thereby affording a greater degree of transition-state flexibility for the salt-activated biocatalyst over the salt-free biocatalyst.
Results and Discussion
The inclusion of inorganic salts in lyophilized enzyme preparations can dramatically increase biocatalyst activity in nonaqueous media, by nearly five orders of magnitude in some cases (24, 31). However, a mechanistic explanation for the salt-induced activation has eluded investigators. In particular, the critical importance of water on enzyme structure, function, and dynamics (32–35) suggests that understanding the nature of enzyme-associated water may go a long way toward elucidating the mechanism of salt activation. For this reason, we have investigated the dynamics of enzyme-associated water using a well-studied model enzyme, the bacterial protease subtilisin Carlsberg (SC).
Fig. 1 shows that the total water content of salt-activated SC decreased linearly with the percent NaF content. Furthermore, the molecular dynamics of the water changed dramatically with salt content. The qualitative differences in water mobility between salt-free [(kcat/KM)app = 0.092 s−1·M−1] and salt-activated [98% NaF (wt/wt); (kcat/KM)app = 76.8 s−1·M−1] enzyme samples are readily apparent in the 2H NMR spectra, as evident in Fig. 2. The salt-free sample exhibited a line width at half height of 23,300 Hz, whereas the corresponding value for the salt-activated sample was 55 times narrower (420 Hz). Such dramatic line narrowing is associated with greatly increased motion of the deuterons.
Fig. 1.
Percent water (wt/wt) retained after lyophilization as a function of salt content of the biocatalyst.
Fig. 2.
2H NMR emanating from salt-free (broad) and salt-activated (narrow) samples. Data were obtained with a solid-echo sequence with π/2-pulse widths of 6 μs and a τ value of 100 μs.
Further insights into the role of residual water in salt activation of SC were obtained by considering the relative water content and amount of mobile water for different salt contents. Fig. 3 shows a correlation between biocatalyst activity, as measured by (kcat/KM)app, and the total water [% water (wt/wt)/% enzyme (wt/wt)]. Fig. 3 also shows a similar correlation between (kcat/KM)app and the integrated NMR intensity of relatively mobile deuterons. These results indicate that although the total amount of water is smaller in the more active salt preparations, the amount of water per enzyme and the amount of mobile deuterons (including enzyme-OD, enzyme-ND from hydrogen–deuterium exchange), as determined by the integrated intensities of the Hahn echo spectra, are larger. Deuterium spin counts of salt-activated subtilisin based on the Hahn echo spectra and total water contents measured by Karl–Fischer titration agreed to within 3–5%, indicating that the mobile deuterons were associated primarily with D2O rather than hydrogen–deuteron exchange on the enzyme. That the amount of water present on a per enzyme basis, as well as the mobility of this water, correlates with biocatalyst activity suggests that the salt somehow directs or provides water, including mobile water, to the enzyme.
Fig. 3.
Catalytic efficiency, (kcat/KM)app (•), of salt-activated subtilisin Carlsberg in hexane; % water (wt/wt)/% enzyme (wt/wt) (■); integrated NMR peak area normalized for number of scans and amount of sample in arbitrary units (▴).
The transition state of N-acetyl phenylalanine ethyl ester (APEE) transesterification catalyzed by subtilisin Carlsberg in organic solvents is characterized by a large dipole moment (6), suggesting that greater transition-state stabilization and higher activity would be achieved in more polar organic solvents. However, a consistent correlation between transesterification activity of subtilisins and solvent polarity has not been observed (6, 13). Deviations from the expected trend may arise from several effects, including ground-state stabilization of the substrate (13, 14), the deleterious mechanism of “water stripping” (5, 15–17), and partial denaturation due to increased interaction between the enzyme and solvent (18). The present results, as discussed below, provide evidence for the latter two mechanisms.
Fig. 4 shows enzyme activity and NMR relaxation data for three solvents of differing polarity, as measured by the solvent dielectric constant (hexane, ε = 2.0; tetrahydrofuran, ε = 7.8; acetone, ε = 20.8). The T2 results were derived from NMR data such that signals from solvated D2O molecules present in the bulk solvent due to water stripping were removed via spectral subtraction of NMR data obtained under identical conditions from saturated solvent control samples. Fig. 4 illustrates that the catalytic activity of salt-activated subtilisin [98% NaF (wt/wt)] clearly decreases with increasing solvent polarity, whereas the deuteron (“water”) mobility increases. Assuming that the deuterium NMR data correspond to water associated with the enzyme, this finding is consistent with previous EPR results and molecular dynamic studies on the serine protease α-chymotrypsin (12), indicating that enzyme flexibility increases with increasing solvent dielectric constant.
Fig. 4.
Catalytic efficiency, (kcat/KM)app (•), of salt-activated subtilisin Carlsberg in hexane, THF, and acetone in comparison with T2 (■) of mobile deuterons as a function of dielectric constant of solvent.
Greater enzyme flexibility in more polar organic solvents can be explained, at least in part, by weaker electrostatic interactions with increasing dielectric constant (8, 11, 12). However, the loss of activity evident in Fig. 4 can also be viewed in the context of the water-stripping model. As water is stripped from the enzyme, locations in and on the enzyme previously inaccessible to the solvent may become accessible, thus permitting increased solvent-enzyme interactions. It is possible that these interactions partially denature the enzyme, as exhibited by the decreased activity, with the resulting denatured conformation exhibiting greater flexibility in more polar solvents (10, 18). Thus, any enzyme-bound water associated with the flexible conformation would also appear more flexible, giving rise to the observed trends in transverse relaxation. We conclude that the more hydrophilic solvents may therefore deactivate the enzyme through two mechanisms, water stripping and increased solvent-enzyme interactions.
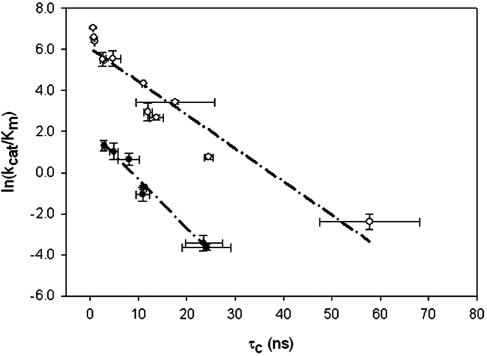
Although a direct trend between water mobility and biocatalyst activity does not appear when comparing activity data from different solvents (e.g., Fig. 4), a compelling trend emerges when examining mobility (as measured by T2-derived correlation times) and activity data from a single solvent. A qualitative inspection of these data suggests a relationship between (kcat/KM)app and the correlation time (τc)D2O of NMR visible deuterons. According to the Stokes–Einstein equation (36), (τc)D2O ∝ 1/T. We therefore plotted ln(kcat/KM)app as a function of (τc)D2O to determine whether a linear or Arrhenius-type dependence was exhibited. Fig. 5 reveals that a linear relationship was indeed obtained. Thus, the physicochemical nature of the salt may alter the local mobility of the enzyme in a manner similar to that of increasing temperature, thereby yielding the implicit free-energy relationships shown in Fig. 5, which reflect “isothermal activation energies” for enzyme activation. The different slopes and y intercepts for the two solvents are likely due to differences in solvent–protein interactions, water stripping, and/or ground state stabilization.
Fig. 5.
Dependence of ln(kcat/KM)app on the correlation times of mobile deuterons, (τc)D2O, for salt-activated subtilisin Carlsberg in hexane (○) and acetone (•). Thelinearity of each plot reflects an Arrhenius-type dependence assuming (τc)D2O ∝ 1/T.
Control experiments on 100% salt preparations in hexane yielded strikingly different deuterium relaxation times than the 98% biocatalysts. The large difference between T2 values of the pure-salt control samples and the 98% enzyme-salt samples (Table 1) indicate that the trend in Fig. 5 is due to the presence of enzyme and is not merely an intrinsic property of deuterons associated with the salt. By using Karl–Fischer titration and counting the exchangeable proton sites on the enzyme, it was estimated that the ratio of D2O to enzyme deuterons (enzyme-ND + enzyme-OD) was ≈10:1, indicating that the majority of observable deuterium relaxation is attributable to D2O rather than deuterated sites on the enzyme. Thus, the large difference in enzyme activity between salt-free [(kcat/KM)app = 0.092 s−1·M−1] and 98% CsF (wt/wt) [(kcat/KM)app = 1,140 s−1·M−1] is accompanied by the presence of a more mobile water population. A similar lubrication phenomenon was observed previously for SC in aqueous solution with and without organic cosolvents wherein faster solvation-layer water dynamics accompanied greater enzyme activity (37). We surmise that enzyme–salt complexes contain a more mobile water (i.e., deuteron) population than salt-free enzymes, which facilitates a more aqueous-like local environment and dramatically increases enzyme activity through increased enzyme flexibility.
Table 1.
Physicochemical and kinetic data for enzyme–salt formulations in hexane 98% (wt/wt)
| Subtilisin formulation | kcat/Km (s−1·M−1) | T2, μs (98% salt) | T2, μs (100% salt) |
|---|---|---|---|
| NaCl | 30.5 | 525 | 351 |
| NaF | 76.8 | 855 | 679 |
| CsCl | 258 | 1,420 | 527 |
| CsF | 1,140 | 4,990 | 200 |
The activation energy of subtilisin-catalyzed transesterification is readily determined from transition-state theory (38), as indicated by Eqs. 1 and 2, where co is the standard-state concentration [generally assumed to be 1.00 M (39)]:
where κ is the preexponential transmission coefficient and the other parameters have their usual definitions. The mobility of the transition state can be inferred from Eq. 2, where increased degrees of freedom and greater entropy may correlate with increased conformational mobility.
Furthermore, ΔΔH* and ΔΔS* can be defined as follows:
These equations form the basis for the effects of temperature on salt-activated biocatalyst activity in a given solvent and clarify whether salt activation is the result of enthalpic and/or entropic effects. Moreover, values of ΔΔS* shed light on how the flexibility of the transition state affects activity.
A series of temperature-dependent experiments were performed on the salt-free preparation and a 98% NaF (wt/wt) preparation in acetone and hexane, and the results are shown as an Eyring plot in Fig. 6. The relative activation enthalpies of the two biocatalyst preparations were nearly identical in both the polar and nonpolar solvent, i.e., (ΔΔH*)hexane ≈ (ΔΔH*)acetone ≈ 0 kJ (Table 2). This result is consistent with the finding in ref. 6, which states that the enzymatic mechanism for transesterification of APEE is highly conserved in a wide range of solvents. Perhaps more interesting is the positive value of ΔΔS* for both solvents; inclusion of salt results in greater degrees of freedom being manifested in the transition state of the salt-activated biocatalyst. This finding supports the hypothesis that salt activation increases biocatalyst activity through increased protein flexibility.
Fig. 6.
Eyring plots for two subtilisin Carslberg preparations: 98% NaF (wt/wt) (•, hexane; ▾, acetone) and salt-free (•, hexane; ▿, acetone).
Table 2.
Transition-state parameters for salt-activated and salt-free biocatalysts
| Solvent | Biocatalyst | ΔH*, kJ·mol−1 | ΔΔH*, kJ·mol−1 | ΔΔS*, J·K−1·mol−1 |
|---|---|---|---|---|
| Hexane | 98% (wt/wt) NaF | 27.6 | 0.0 | 62.2 |
| Salt Free | 27.6 | |||
| Acetone | 98% (wt/wt) NaF | 18.7 | 0.8 | 35.7 |
| Salt Free | 17.9 |
In summary, we have shown that enzyme activity in salt-activated preparations of SC correlates with the transverse relaxation data from enzyme-associated water molecules. A quantitative analysis of the relaxation data, along with temperature-dependent kinetic measurements for various salt-activated preparations, show that the dramatic activation of the biocatalyst preparations is associated with the presence of highly mobile water.
Materials and Methods
Materials.
Subtilisin Carlsberg (from Bacillus licheniformis), APEE, and nonadecane were obtained from Sigma. D2O (99.9%) was obtained from Cambridge Isotope Laboratories (Cambridge, MA). Karl–Fischer titrant and solvent were purchased from GFS Chemicals, Inc. (Powell, OH). All solvents used were of the highest grade commercially available and were stored over 0.3-nm molecular sieves for at least 24 h before use.
Enzyme Preparation.
The enzyme was activated by lyophilization from an aqueous phosphate buffer (KH2PO4). For enzyme samples containing salts, the phosphate buffer concentration (0.25 mg/ml) and enzyme concentration (0.25 mg/ml) remained the same for all samples. The initial salt concentration was chosen to yield the desired salt content (% wt/wt) in the lyophilizate. The pH of all enzyme solutions was adjusted to 7.8 by using a few drops of 0.5 M KOH or 0.1 M H3PO4; in all cases, the amount added did not significantly alter the buffer concentration, ionic strength, or solution volume.
Samples were frozen according to a two-step protocol. First, the Falcon tubes containing samples were vertically immersed in a bath of liquid N2 until boiling subsided. The samples were then placed in a Labconco Freeze Dry 6 freeze dryer (Fisher Scientific) at −49°C and 50 μmHg (1,000 μmHg = 133 Pa) for 44 h. Salt-free enzyme (24.75 mg/ml) solutions in phosphate buffer (0.25 mg/ml, pH 7.8) were frozen and lyophilized in the same manner. The lyophilized samples were immediately assayed for activity and water content. When not in use, the samples were stored at −20°C under N2 in the Falcon tubes. Samples used for 2H NMR were prepared with D2O.
Kinetic Assays.
Catalytic efficiencies of subtilisin Carlsberg formulations [(kcat/KM)app, where app designates that both kinetic parameters of the ping–pong and bi–bi mechanism are apparent values, as per the convention for protease-catalyzed reactions involving competing nucleophiles (40)] were determined in nearly anhydrous tetrahydrofuran, acetone, and hexane. Transesterification of APEE with 1-propanol typically involved adding 5–10 mg of salt-enzyme powder to 5 ml of organic solvent containing 1.5 mM nonadecane (internal standard for gas chromatography), 0.85 M 1-propanol, and varying concentrations of APEE (1–20 mM). Reactions were carried out in 20-ml glass scintillation vials and agitated at 250 rpm in a C-24 Classic Benchtop Incubator Shaker (Neuo Brunswick Scientific) at 30°C.
Product analysis was performed by monitoring the generation of APPE. To this end, 500-μl aliquots were removed from the reaction vials, and each sample was centrifuged in a 1.5-ml Eppendorf tube for 5 min at 12,000 rpm. The resultant supernatant was then analyzed by using a gas chromatograph (model 3800; Varian) equipped with a CP-SIL8, 250-μm capillary column (15 m in length with a 0.25-μm inner diameter), a constant He carrier gas pressure of 15 psi (1.3 ml/min), 250°C injection and detection temperatures, and an isothermal column temperature of 215°C. All measurements were performed in quadruplicate, and initial rates were determined from straight-line fits of average values. In all cases, no measurable amount of hydrolysis product N-Ac-l-Phe-OH was observed. Rate measurements were performed over 60–180 min, during which the rates remained constant (linear plots of concentration vs. time). Kinetic parameters Vmax,app and KM,app were determined by fitting initial rate data to the Michaelis–Menten equation. Values of kcat,app were calculated by dividing Vmax,app by the concentration of active enzyme. The active enzyme concentrations for all preparations used in this study were taken from Ru et al. (41), with the exception of 98% CsF (wt/wt), 98% KHCO3 (wt/wt), 85% NaF (wt/wt), 75% NaF (wt/wt), and 50% NaF (wt/wt), which we determined by active-site titration as described by Wangikar et al. (40). Samples of pure lyophilized salt were determined to be noncatalytic.
Water Content Measurements.
The water content of the lyophilized samples was determined by Karl–Fischer titration. Before addition of the sample, the Karl–Fischer titration apparatus was equilibrated with titrant to neutralize any water present in the chamber. Upon equilibration, a known weight of sample was placed in the sealed chamber and titration commenced. Water content values were measured in triplicate.
2H NMR.
Deuterium relaxation experiments were performed on a home-built spectrometer at 9.39 T (61.4068730 MHz). In a typical experiment, 100 mg of sample (biocatalyst) was packed into a 5-mm (1-ml) NMR tube under N2 to avoid adsorption of ambient H2O. Organic solvent was then added to the NMR tube, and the sample was allowed to equilibrate for at least 1 h. The sample was then placed in an NMR probe, deuterium transverse relaxation times were measured by using the Hahn echo pulse sequence (42), and spectra were obtained by using a solid-echo sequence. Both pulse sequences were performed by using a 900-pulse width of 6 μs. The spectral width was set to 300 kHz, with 8,192 real data points. The number of scans ranged from 5,000–10,000, and the delay between experiments was 1.25 s.
Restriction of the delay time in the Hahn echo sequence to values larger than 100 μs resulted in NMR signals from only relatively mobile nuclei, thereby ensuring that the derived relaxation times are associated with mobile deuteron populations, comprising enzyme-ND, enzyme-OD, and mobile D2O species (42, 43). Signals from solvated D2O molecules in the bulk solvent were removed via spectral subtraction of NMR data obtained under identical conditions from saturated solvent control samples. To this end, the solvents were first equilibrated with lyophilized powders for a period of 1 h, after which the equilibrated solvent was removed by centrifugation and the NMR data collected. The amount of D2O observed in the equilibrated bulk solvent ranged from 5–20% of total D2O signal.
Correlation times for deuterons were calculated according to Eq. 5 (43):
where T2 is the transverse relaxation constant, τ c is a motional correlation time, ωo = 2πν is the angular precession frequency with ν being the NMR frequency of deuterium at 9.39 T, η is the quadrupolar asymmetry parameter, and K is the quadrupole coupling constant. For 2H NMR relaxation measurements of bound water, η = 0.1 and K = 216.4 kHz (44).
Acknowledgments
This work was supported by National Science Foundation Grant BES-0228145 and National Institutes of Health Grant GM66712.
Abbreviations
- SC
subtilisin Carlsberg
- APEE
N-acetyl phenylalanine ethyl ester.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Clark D. S. Philos. Trans. R. Soc. London B. 2004;359:1299–1307. doi: 10.1098/rstb.2004.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klibanov A. M. Nature. 2001;409:241–246. doi: 10.1038/35051719. [DOI] [PubMed] [Google Scholar]
- 3.Klibanov A. M. Trends Biotechnol. 1997;15:97–101. doi: 10.1016/S0167-7799(97)01013-5. [DOI] [PubMed] [Google Scholar]
- 4.Dordick J. S. Biotechnol. Prog. 1992;8:259–267. doi: 10.1021/bp00016a001. [DOI] [PubMed] [Google Scholar]
- 5.Zaks A., Klibanov A. M. J. Biol. Chem. 1988;263:3194–3201. [PubMed] [Google Scholar]
- 6.Michels P. C., Dordick J. S., Clark D.S. J. Am. Chem. Soc. 1997;119:9331–9335. [Google Scholar]
- 7.Xu Z. F., Affleck R., Wangikar P., Suzawa V., Dordick J. S., Clark D. S. Biotechnol. Bioeng. 1994;43:515–520. doi: 10.1002/bit.260430612. [DOI] [PubMed] [Google Scholar]
- 8.Broos J., Visser A., Engbersen J., Verboom W., van Hoek A., Reinhoudt D. N. J. Am. Chem. Soc. 1995;117:12657–12663. [Google Scholar]
- 9.Suzawa V., Khmelnitsky Y. L., Giarto L., Dordick J. S., Clark D. S. J. Am. Chem. Soc. 1995;117:8435–8440. [Google Scholar]
- 10.Burke P. A., Griffin R. G., Klibanov A. M. Biotechnol. Bioeng. 1993;42:87–94. doi: 10.1002/bit.260420112. [DOI] [PubMed] [Google Scholar]
- 11.Affleck R., Xu S. F, Suzawa V., Focht K., Clark D. S., Dordick J. S. Proc. Natl. Acad. Sci. USA. 1992;89:1100–1104. doi: 10.1073/pnas.89.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Affleck R., Haynes C. A., Clark D. S. Proc. Natl. Acad. Sci. USA. 1992;89:5167–5170. doi: 10.1073/pnas.89.11.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J., Clark D. S., Dordick J. S. Biotechnol. Bioeng. 2000;67:112–116. doi: 10.1002/(sici)1097-0290(20000105)67:1<112::aid-bit13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Ryu K., Dordick J. S. Biochemistry. 1992;31:2588–2598. doi: 10.1021/bi00124a020. [DOI] [PubMed] [Google Scholar]
- 15.Gorman L. S., Dordick J. S. Biotechnol. Bioeng. 1992;39:392–397. doi: 10.1002/bit.260390405. [DOI] [PubMed] [Google Scholar]
- 16.Zaks A., Klibanov A. M. J. Biol. Chem. 1988;263:8017–8021. [PubMed] [Google Scholar]
- 17.Zaks A., Klibanov A. M. Proc. Natl. Acad. Sci. USA. 1985;82:3192–3196. doi: 10.1073/pnas.82.10.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke P. A., Griffin R. G., Klibanov A. M. J. Biol. Chem. 1992;267:20057–20064. [PubMed] [Google Scholar]
- 19.Mabrouk P. A. In: Poly(Ethylene Glycol) Chemistry and Biological Applications. Harris J. M., Zalipky S., editors. Washington, DC: Am. Chem. Soc.; 1997. pp. 118–133. [Google Scholar]
- 20.Yang L., Dordick J. S., Garde S. Biophys. J. 2004;87:812–821. doi: 10.1529/biophysj.104.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiros M., Parker M.-C., Turner N. J. J. Org. Chem. 2001;66:5074–5079. doi: 10.1021/jo0101104. [DOI] [PubMed] [Google Scholar]
- 22.Khmelnitsky Y. L., Welch S. H., Clark D. S., Dordick J. S. J. Am. Chem. Soc. 1994;16:2647–2648. [Google Scholar]
- 23.Ru M. T, Wu K. C., Lindsay J. P., Dordick J. S., Reimer J. A., Clark D. S. Biotechnol. Bioeng. 2001;75:187–196. doi: 10.1002/bit.1178. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay J. P., Clark D. S., Dordick J. S. Biotechnol. Bioeng. 2004;85:553–560. doi: 10.1002/bit.20002. [DOI] [PubMed] [Google Scholar]
- 25.Griebenow K., Diaz Laureano Y., Santos A. M., Montanez Clemente I., Rodriguez L., Vidal M., Barletta G. J. Am. Chem. Soc. 1999;121:8157. [Google Scholar]
- 26.Griebenow K., Vidal M., Baez C., Santos A. M., Barletta G. J. Am. Chem. Soc. 2001;123:5380–5381. doi: 10.1021/ja015889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triantafyllou A. O., Wehtje E., Adlercreutz P., Mattiasson B. Biotechnol. Bioeng. 1996;54:67–76. doi: 10.1002/(SICI)1097-0290(19970405)54:1<67::AID-BIT8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Dabulis K., Klibanov A. M. Biotechnol. Bioeng. 1993;41:566–571. doi: 10.1002/bit.260410509. [DOI] [PubMed] [Google Scholar]
- 29.Bedell B. A., Mozhaev V. V., Clark D. S. Biotechnol. Bioeng. 1998;58:654–657. [PubMed] [Google Scholar]
- 30.Timasheff S. N. Ann. Rev. Biophys. Biomol. Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- 31.Ru M. T., Dordick J. S., Reimer J. A., Clark D.S. Biotechnol. Bioeng. 1999;63:233–241. doi: 10.1002/(sici)1097-0290(19990420)63:2<233::aid-bit12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Rupley J. A., Careri G. Adv. Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- 33.Finney J. L, Poole P. L. Comments Mol. Cell. Biophys. 1984;2:129–151. [Google Scholar]
- 34.Bone S., Pethig R. J. Mol. Biol. 1984;181:323–326. doi: 10.1016/0022-2836(85)90096-8. [DOI] [PubMed] [Google Scholar]
- 35.Kuntz I. D., Kauzmann W. Adv. Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- 36.Einstein A. Ann. Phys. 1906;19:289. [Google Scholar]
- 37.Kamal J. K., Xia T., Pal S., Zhao L., Zewail A. H. Chem. Phys. Lett. 2004;387:209–215. [Google Scholar]
- 38.Fersht A. Structure and Mechanism in Protein Science. New York: Freeman; 1999. [Google Scholar]
- 39.Moore J. W., Pearson R. G. Kinetics and Mechanism. New York: John Wiley & Sons; 1981. [Google Scholar]
- 40.Wangikar P. P., Carmichael D., Clark D. S., Dordick J. S. Biotechnol. Bioeng. 1996;50:329–335. doi: 10.1002/(SICI)1097-0290(19960505)50:3<329::AID-BIT11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Ru M. T., Hirokane S. Y., Lo A. S., Dordick J. S., Reimer J. A., Clark D. S. J. Am. Chem. Soc. 2000;122:1565–1571. [Google Scholar]
- 42.Slichter C.P. Principles of Magnetic Resonance. New York: Harper and Row; 1963. [Google Scholar]
- 43.Abragam A. The Principles of Nuclear Magnetism. Oxford: Clarendon; 1961. [Google Scholar]
- 44.Kakalis L. T., Kumosinski T. F. Biophys. Chem. 1992;43:39–49. doi: 10.1016/0301-4622(90)80043-7. [DOI] [PubMed] [Google Scholar]