Abstract
We originally identified senescence marker protein 30 (SMP30) as a distinctive protein whose expression decreases in an androgen-independent manner with aging. Here, we report its sequence homology found in two kinds of bacterial gluconolactonases (GNLs) by using the blast search. Then, through a biochemical study, we identify SMP30 as the lactone-hydrolyzing enzyme GNL of animal species. SMP30 purified from the rat liver had lactonase activity toward various aldonolactones, such as d- and l-glucono-δ-lactone, d- and l-gulono-γ-lactone, and d- and l-galactono-γ-lactone, with a requirement for Zn2+ or Mn2+ as a cofactor. Furthermore, in SMP30 knockout mice, no GNL activity was detectable in the liver. Thus, we conclude that SMP30 is a unique GNL in the liver. The lactonase reaction with l-gulono-γ-lactone is the penultimate step in l-ascorbic acid (AA) biosynthesis, and the essential role of SMP30 in this synthetic process was verified here by a nutritional study using SMP30 knockout mice. These knockout mice (n = 6), fed a vitamin C-deficient diet, did not thrive; i.e., they displayed symptoms of scurvy such as bone fracture and rachitic rosary and then died by 135 days after the start of receiving the deficient diet. The AA levels in their livers and kidneys at the time of death were <1.6% of those in WT control mice. In addition, by using the SMP30 knockout mouse, we demonstrate that the alternative pathway of AA synthesis involving d-glucurono-γ-lactone operates in vivo, although its flux is fairly small.
Keywords: aging, osteogenic disorder, vitamin C
Senescence marker protein 30 (SMP30) is a 34-kDa protein whose tissue levels in the liver, kidney, and lung decrease with aging (1, 2). To examine the physiological function of SMP30, we established SMP30 knockout mice (3) and found that they were viable and fertile, although they were lower in body weight and shorter in life span than WT mice (4). Their livers were also far more susceptible to TNF-α- and Fas-mediated apoptosis than those of WT mice, indicating that SMP30 may act to protect cells from apoptosis (3). The livers of SMP30 knockout mice showed abnormal accumulations of triglycerides, cholesterol, and phospholipids (4). In addition, the lungs of these knockout mice had enlarged alveolar airspaces during their first to sixth month of life (2). However, the molecular mechanism of SMP30 function has remained obscure.
Recently, we reported that SMP30 acts as a hydrolase for diisopropyl phosphorofluoridate (5), a compound resembling chemical warfare nerve agents such as sarine, soman, and tabun. However, a physiological substrate for SMP30 must be present, because this compound is an artificial chemical. Our recent search for amino acid sequences resembling SMP30 was accomplished by using the blast program, which revealed that rat SMP30 is homologous with gluconolactonase (GNL) [EC 3.1.1.17], a lactone-hydrolyzing enzyme, of Nostoc punctiforme and Zymomonas mobilis (6). Therefore, we suspected that SMP30 is a GNL of animal species. In mammalian metabolism, GNL is involved in l-ascorbic acid (AA) biosynthesis, catalyzing the lactonization of l-gulonic acid, the reverse reaction of lactone hydrolysis (7). The product l-gulono-γ-lactone is oxidized to AA (8–10). In this study, to further investigate the identity of SMP30, we have unequivocally proven that it is a GNL. Purified rat liver SMP30 had GNL activity, as did a recombinant rat SMP30 produced in Escherichia coli. Furthermore, SMP30 knockout mice developed symptoms of scurvy when fed a vitamin C-deficient diet, verifying the pivotal role of SMP30 in AA biosynthesis.
Results
Identification of SMP30 as a GNL.
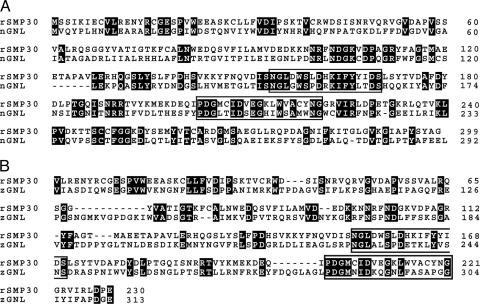
Comparisons of the amino acid sequence of rat SMP30 by means of the blast program revealed that this protein was homologous with two kinds of bacterial GNLs. The total amino acid sequence of rat SMP30 (299 aa) shares 32% homology with that of N. punctiforme GNL (292 aa) (Fig. 1A), and a part of the amino acid sequence of rat SMP30 (222 aa, residues 9–230) shares 26% homology with that of Z. mobilis GNL (247 aa, residues 67–313) (Fig. 1B). Therefore, we speculated that the protein characterized previously as SMP30 in several animals is a GNL. For substantiation, we took this protein from the rat liver and purified it to apparent homogeneity as described in ref. 5. The elution profile of SMP30 obtained with Sephacryl S-200 HR chromatography, which was the final step of the purification process, coincided well with that of GNL activity (Fig. 7A, which is published as supporting information on the PNAS web site), clearly indicating an overlap between them. Conversely, GNL from the rat liver was purified to near homogeneity by a previously reported method (11). The resulting preparation gave a positive band on Western blot analysis by using anti-rat SMP30 antibody (Fig. 7B). Moreover, the main band of a gel processed by SDS/PAGE was subjected to sequence analysis after in-gel digestion with trypsin, and the amino acid sequence of its peptide (YFAGTMAEETAP) proved to be an exact match with an internal sequence (amino acid residues 113–124) of rat SMP30.
Fig. 1.
Alignment of the amino acid sequences of rat SMP30 (rSMP30), Nostoc GNL (nGNL), and Zymomonas GNL (zGNL). (A) rSMP30 [National Center for Biotechnology Information (NCBI) accession no. CAA48786] vs. nGNL (NCBI accession no. ZP_00110023). (B) rSMP30 vs. zGNL (NCBI accession no. CAA47637). Two regions homologous among rat SMP30, nGNL, and zGNL are boxed in A and B.
Expression of Catalytically Active Recombinant SMP30.
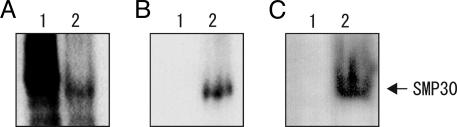
The identity of SMP30 as a GNL was further confirmed by expressing a rat SMP30 cDNA in E. coli. Recombinant SMP30 protein was expressed as a maltose-binding protein (MBP) fusion protein by using a chaperone coexpression system, and a soluble cell lysate was prepared. In this lysate, cells expressing the MBP–SMP30 fusion had clear-cut GNL activity (546 ± 18 nmol/min per mg of protein, mean ± SEM, n = 3) with d-glucono-δ-lactone used as a substrate, whereas the lysate of control cells (expressing a fusion of MBP with the α-fragment of β-gal) had no such activity. We further ascertained that the SMP30 fusion was the entity possessing GNL activity by subjecting this lysate containing the MBP–SMP30 fusion to native gel electrophoresis in triplicate and electroblotting onto a polyvinylidene fluoride membrane. The three parts of the membrane were respectively stained for GNL activity or immunochemically with anti-MBP or anti-SMP30 antibody. The bands stained in these three ways appeared at the same position (Fig. 2, lanes marked “2”). In contrast, a lysate of the cells expressing a fusion protein of MBP with the α-fragment of β-gal as a control did not contain any protein that was stained positive for GNL activity (Fig. 2 A and C, lane 1).
Fig. 2.
Immunoblot analysis after native PAGE and activity staining of recombinant rat SMP30. Samples were electrophoresed on a native polyacrylamide gel, and the proteins on the gel were electroblotted onto a membrane. Detection of specific proteins was carried out with anti-MBP antibody (A), anti-rat SMP30 antibody (B), and activity staining (C). To stain for enzyme activity, d-galactono-γ-lactone was used as substrate. Lane 1, the lysate of E. coli cells expressing a fusion of MBP with β-gal α-fragment (control); lane 2, the lysate of E. coli cells expressing a fusion of MBP with rat SMP30. The arrow shows the position of a fusion of MBP with rat SMP30.
Enzymatic Characterization of SMP30.
Subsequently, we measured GNL activity with a reaction mixture containing 10 mM d-glucono-δ-lactone, 75 μM ZnCl2, and 0.42 μg/ml purified rat SMP30. The SMP30 hydrolyzed d-glucono-δ-lactone with a specific activity of 226 μmol/min per mg at an optimal pH of 6.4 with a linear increase up to an enzyme concentration of 0.42 μg of protein per ml (Fig. 8 A and C, which is published as supporting information on the PNAS web site). However, GNL activity was below the limit of detection in the absence of Zn2+. Because a maximum level of GNL activity was observed at a Zn2+ concentration of 75 μM (Fig. 8B), this amount of Zn2+ was used in all experiments for enzymatic characterization.
Other divalent metal ions were also tested at a concentration of 75 μM in the standard assay mixture. Only Mn2+ ions were effective, giving 27% of the enzyme activity observed with Zn2+ (Fig. 8B), whereas Mg2+, Co2+, Ca2+, and Cd2+ ions gave no activity. However, slight enzyme activity (≪10%) was observed with 3 mM concentrations of these metal ions and a 10-fold higher concentration of SMP30 (4.2 μg/ml) (data not shown).
Next, we studied the kinetics of the GNL reaction. Hyperbolic kinetics were normal (Fig. 9, which is published as supporting information on the PNAS web site), and a Lineweaver–Burk plot of the data yielded an apparent Km value of 9.4 mM and a Vmax value of 345 μmol/min per mg (Fig. 9 Inset). The lactonase activities for a variety of sugar lactones by SMP30 are summarized in Table 2, which is published as supporting information on the PNAS web site. d-glucono-δ-lactone was the best substrate. SMP30 also hydrolyzed l-glucono-δ-lactone, d-gulono-γ-lactone, l-gulono-γ-lactone, d-galactono-γ-lactone, and l-galactono-γ-lactone, indicating that SMP30 has broad substrate specificity for aldonolactones. d-aldonolactones were better substrates than the corresponding enantiomers. SMP30 had no detectable hydrolyzing activity toward d-ribono-γ-lactone, d-mannono-γ-lactone, or d-glucoheptono-γ-lactone.
An Essential Role of SMP30 in AA Biosynthesis.
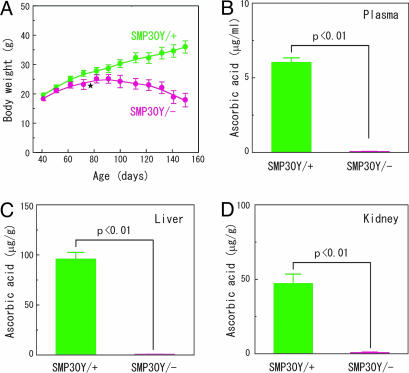
To examine whether these substrates are attacked only by SMP30 in the rat liver, we compared lactonase activity in livers from SMP30Y− and SMP30Y+ mice. Liver extracts from SMP30Y+ mice hydrolyzed d-glucono-δ-lactone, d-gulono-γ-lactone, and d-galactono-γ-lactone, whereas those from SMP30Y− mice had no detectable hydrolyzing activity toward these lactones (Table 1). Clearly, therefore, SMP30 is a unique enzyme that effectively hydrolyzes various aldonolactones in the liver. Because the lactonization of l-gulonic acid to l-gulono-γ-lactone, the reverse reaction of the lactonase reaction, is the penultimate step of AA synthesis, we investigated the role of SMP30 in this metabolic process by using SMP30 knockout mice. Ten days after weaning at the age of 30 days, SMP30Y− and SMP30Y+ mice, six each, were fed a vitamin C-deficient diet. One of the knockout mice started to lose weight after 25 days of consuming this diet (at an age of 65 days), walked with an abnormal gait after 31 days, and died after 37 days. The average body weight of the other five knockout mice started to decrease after 56 days of the vitamin C-deficient diet (at an age of 96 days) (Fig. 3A); their gait became abnormal at that time, and they died by the 106th to 135th days of this dietary deficiency. The AA level in plasma of SMP30Y− mice after 106 days of consuming the deficient diet was <1% of that in SMP30Y+ mice (Fig. 3B). The livers and kidneys of SMP30Y− mice at the time of death contained <1.6% of the AA levels in the SMP30Y+ mice (Fig. 3 C and D).
Table 1.
Absence of GNL activity in livers from SMP30 knockout mice
| Substrate | Specific activity, μmol/min per mg |
|
|---|---|---|
| SMP30Y+ | SMP30Y− | |
| d-glucono-δ-lactone | 9.0 ± 0.6 | ND |
| d-gulono-γ-lactone | 0.5 ± 0.1 | ND |
| d-galactono-γ-lactone | 0.4 ± 0.1 | ND |
The hydrolytic activities for the substrates of SMP30 were determined by the method described in Materials and Methods. The values are the average ± SEM for livers from five animals. ND, no detectable enzyme activity.
Fig. 3.
Body weight changes and AA levels in the plasma, livers, and kidneys of SMP30Y− and SMP30Y+ mice. SMP30Y− and SMP30Y+ mice, six each, were weaned at 30 days of age and fed autoclaved mouse chow (containing ≈55 mg of AA per kg) for 10 days; then, they were fed a vitamin C-deficient diet until all SMP30Y− animals were dead. (A) Body weight changes of SMP30Y− (pink circles) and SMP30Y+ (green circles) mice. One SMP30Y− mouse died after 37 days (indicated by star), and the others died after 106–135 days of consuming the vitamin C-deficient diet. (B–D) Blood was taken from all tested animals (six WT and five knockout mice) after 106 days of this diet, and AA levels in the plasma (B) were measured. All mice were killed after 136 days of receiving the deficient diet, and AA levels in the liver (C) and kidney (D) were measured. Except for blood, all specimens of SMP30Y− mice were taken after death. Values are expressed as mean ± SEM of five or six animals.
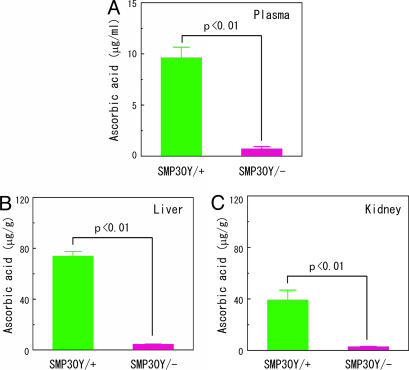
For the previous studies, our SMP30Y− mice were fed autoclaved mouse chow after weaning, and this food contains ≈55 mg/kg of vitamin C. When we assessed the vitamin C status of such mice after 80 days of eating autoclaved chows, their plasma, livers, and kidneys contained only 6–8% of the AA values in the WT mice (Fig. 4). Thus, the knockout mice that were fed autoclaved mouse chow proved to be severely vitamin C-deficient.
Fig. 4.
AA levels in the plasma, livers, and kidneys from SMP30Y− and SMP30Y+ mice that were fed autoclaved mouse chow. SMP30Y− and SMP30Y+ mice, four each, were weaned at 30 days of age and fed autoclaved mouse chow (containing ≈55 mg of AA per kg) for 80 days (age 110 days). AA levels were then measured in their plasma (A), livers (B), and kidneys (C). Values are expressed as mean ± SEM of four animals.
Osteogenic Disorder of SMP30 Knockout Mice.
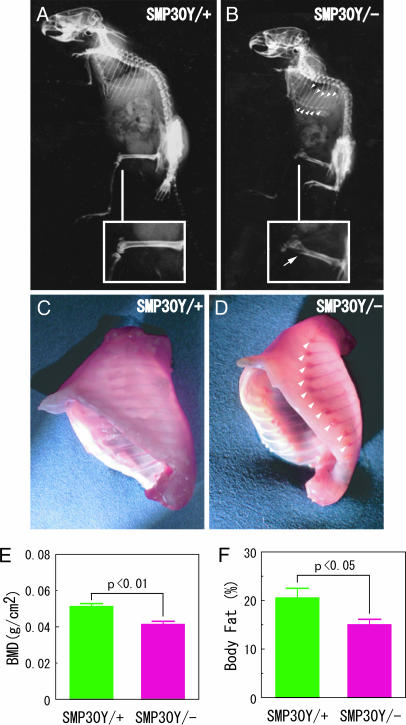
Because thin, brittle bones with a tendency to fracture are known as characteristic manifestations of scurvy, we checked the skeletal structure of SMP30Y− and SMP30Y+ mice by x-ray examination after they had consumed the vitamin C-deficient diet for 59 days (at an age of 99 days) (Fig. 5A and B). Fracture at the distal end of their femurs (Fig. 5B Inset) and rachitic rosaries at the junction of costae and costal cartilages (Fig. 5B) were prominent in a SMP30Y− mouse (Fig. 5D), but not a SMP30Y+ mouse (Fig. 5C). Moreover, subcranial total bone mineral density (BMD) and body fat were significantly decreased in SMP30Y− mice compared with SMP30Y+ mice (Fig. 5 E and F).
Fig. 5.
Osteogenic disorder of SMP30 knockout mice. SMP30Y− and SMP30Y+ mice at an age of 40 days were fed a vitamin C-deficient diet for 59 days (animals’ age was 99 days). (A–D) X-ray images show the skeletal structures of SMP30Y+ (A) and SMP30Y− (B) mice. Insets show enlargements of the femoral region; an arrow points to the distal femur fracture of a SMP30 knockout mouse. A rachitic rosary of the SMP30Y− mouse observed after evisceration (D) is compared with that area in a control mouse (C). Arrowheads in B and D indicate a rachitic rosary at the junction of costae and costal cartilage. (E and F) Subcranial total BMD (E) and body fat percentage (F) of SMP30Y− and SMP30Y+ mice were determined by PIXImus2 densitometry as described in Materials and Methods. Values are expressed as mean ± SEM of five or six animals.
Occurrence of an Alternative Pathway of AA Synthesis.
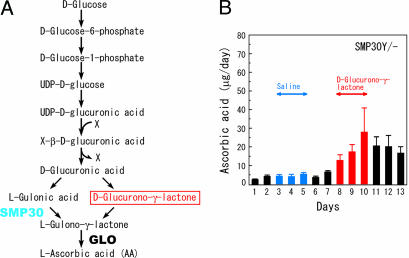
Two pathways were proposed as forming the last part of the AA synthetic pathway (12). Obviously, the main pathway of this process dealt with here includes the steps from d-glucose to l-gulonic acid (detailed on the left side of Fig. 6A). However, for the formation of l-gulono-γ-lactone, the immediate precursor to AA, another pathway branches from d-glucuronic acid (Fig. 6A, right side). To clarify whether the latter pathway exists in the mammalian metabolism, we injected d-glucurono-γ-lactone i.p. into SMP30Y− mice and measured the amount of AA excreted in their urine. We used the lactone for delivery of d-glucuronic acid into liver cells, where AA synthesis occurs, because the lactone would be more easily incorporated into the cells than d-glucuronic acid and would come into equilibrium with this acid by catalysis of uronolactonase (12, 13). As a result, the excretion of AA was appreciably increased by the injection, albeit in a small amount, whereas AA excretion was not affected by the saline injected as a control (Fig. 6B). Thus, the alternative pathway was clearly operational here, regardless of its small flux.
Fig. 6.
Increased excretion of AA in the urine after administration of d-glucurono-γ-lactone. (A) The pathway of AA biosynthesis. The pathway from d-glucose to l-gulonic acid is shared with that of early steps in the uronic acid cycle. X is a conjugating molecule for glucuronidation. GLO, l-gulono-γ-lactone oxidase. (B) SMP30Y− mice were fed autoclaved mouse chow for 60 days after weaning and were housed individually in metabolic cages starting on day 1. Saline was injected i.p. from day 3 to day 5; then, d-glucurono-γ-lactone (0.7 mg/g of body weight) dissolved in saline was injected i.p. from day 8 to day 10. Urine samples were collected, and their AA concentrations were measured. Values are expressed as mean ± SEM of four SMP30Y− mice.
Discussion
This study provides unequivocal evidence that SMP30 is the bona fide GNL in the AA biosynthetic pathway of mammals. In support, (i) SMP30 purified from the rat liver exhibited GNL activity (Fig. 7 and Table 2), (ii) a partial amino acid sequence of GNL purified from rat liver was identical to that of the reported sequence of rat SMP30, and (iii) the GNL was recognized by antibody directed to rat SMP30. Moreover, recombinant rat SMP30 expressed in E. coli showed GNL activity (Fig. 2). Overall, the purified SMP30 had the same substrate specificity toward multiple lactones as previously reported for GNL (7, 11, 13, 14). With respect to the requirement for metal in this process, Zn2+ and Mn2+ were both effective activators, although it was previously reported that Mn2+ was dominantly effective but that Zn2+ had no effect. This discrepancy is possibly due to differing experimental settings (11, 13, 14).
Measurements of GNL activity disclosed that the liver extract of SMP30 knockout mice lacked any such activity under conditions in which the WT extract was markedly active for three known substrates of GNL (Table 1). This outcome indicates that SMP30 is the unique lactone-hydrolyzing enzyme for these substrates in the liver. An essential role of SMP30 in AA biosynthesis in mice was evidenced by a nutritional study using SMP30 knockout mice. AA is produced from the ultimate hexose precursor d-glucose, and, in this pathway, l-gulonic acid, an intermediary metabolite of the uronic acid cycle, is lactonized to form l-gulono-γ-lactone, which in turn is oxidized to AA (Fig. 6A). GNL is known to catalyze the former reaction (8–10). In our experiments, scurvy developed in SMP30 knockout mice that were fed a vitamin C-deficient diet (Figs. 3 and 4). Their symptoms of scurvy included bone fractures, rachitic rosaries, and premature death by 135 days after institution of the vitamin C-deficient diet. In view of this fact, one can understand the phenotypes of SMP30 knockout mice mentioned in the Introduction (abnormally low body weight, shortened life span, susceptibility to hepatic apoptosis, etc., compared with WT mice). For our previous studies (2–4), these mice were fed autoclaved mouse chow, which we now know contains too little vitamin C to maintain normal levels of AA in tissues (Fig. 4). Accumulations of triglycerides, phospholipids, and cholesterol in the livers of these knockout mice may be associated with impaired fatty acid oxidation, probably because of decreased synthesis of carnitine, which plays a role in the transport of long-chain fatty acids into mitochondria. AA is required as a cofactor in two hydroxylation reactions in carnitine biosynthesis (15). In fact, AA-deficient guinea pigs were shown to have abnormalities of lipid metabolism, such as hyperlipidemia and hypercholesterolemia (16). Another clinical feature of young SMP30 knockout mice is enlargement of alveolar airspaces compared with those of WT mice (2). A similar change was reported in ODS (osteogenic disorder Shionogi) rats that survived for a long time on a vitamin C-deficient diet (17). However, never before did we observe scurvy symptoms in the knockout mice that were fed autoclaved mouse chow, despite their smaller body size and shorter life span (3, 4). Assuming that an average weight of mice is 25 g and that the amount of chow taken per day is 4 g (18), SMP30 knockout mice ingest <0.01 mg/g of body weight per day. Based on the observation that 0.02 mg/g of body weight per day cannot sustain normal body function in a scurvy-prone mouse whose l-gulono-γ-lactone oxidase gene was deleted (19), the SMP30 knockout mice seem not to have ingested enough vitamin C. Probably, a small amount of AA may be synthesized through the alternative pathway (Fig. 6A) previously proposed based on an enzymatic study (20) and demonstrated here in SMP30 knockout mice. Furthermore, the absence of GNL may lead to decreased degradation of AA, because GNL can hydrolyze the lactone ring of dehydroascorbic acid, the oxidation product of AA, to 2,3-dioxo-l-gulonic acid, as formerly reported (20).
Because SMP30 is abundant in the kidney and also present, although in lesser amounts, in other organs (21), this protein must have some function other than AA synthesis, which does not occur at all these sites. SMP30 may prevent the production of glycated proteins by hydrolyzing d-glucono-δ-lactone. It is possible that this lactone is formed from d-glucose by glucose dehydrogenase in vivo, and it may be involved in glycation of proteins. In fact, human and rat hemoglobin was shown to be glycated with d-glucono-δ-lactone (22). Glycated proteins, especially advanced glycation end products, are known to cause the deterioration of cellular functions; therefore, SMP30 may protect cells from such an effect.
Materials and Methods
Chemicals.
l-glucono-δ-lactone, d-gulono-γ-lactone, l-gulono-γ-lactone, l-galactono-γ-lactone, d-ribono-γ-lactone, d-glucoheptono-γ-lactone, and d-mannono-γ-lactone were purchased from Sigma-Aldrich. d-glucono-δ-lactone, d-galactono-γ-lactone, and other reagents were purchased from Wako Pure Chemical (Osaka).
Animals.
Male Wister rats, 3–9 months of age, were obtained from the Animal Facility at Tokyo Metropolitan Institute of Gerontology, and their livers were used as a source of purified SMP30. For purification of GNL, 10-week-old male Wister rats purchased from Kiwa Laboratory Animals (Misato-cho, Japan) were used. SMP30 knockout mice were previously generated with the gene-targeting technique (3), and heterozygous female mice (SMP30+/−) were mated with male knockout mice (SMP30Y−) to produce knockout and WT (SMP30Y+) littermates. These littermates were fed autoclaved mouse chow (CRF-1; Charles River Breeding Laboratories) ad libitum with free access to water and used for measurement of GNL activity in the liver when the animals were 6 months old. The autoclaved chow contained ≈55 mg of AA per kg as determined at the time of experimentation.
In a nutritional study, SMP30Y− and SMP30Y+ mice were weaned at 30 days of age and fed autoclaved mouse chow for 10 days, followed by a vitamin C-deficient diet (CL-2; CLEA Japan, Tokyo). Throughout the experiments, animals were maintained on 12-h light/dark cycles in a controlled environment. All experimental procedures using laboratory animals were approved by the Animal Care and Use Committee of Tokyo Metropolitan Institute of Gerontology.
Purification of SMP30 from Rat Liver.
SMP30 was purified from a soluble fraction of rat livers as described in ref. 5. Briefly, liver homogenate was fractionated by ammonium sulfate precipitation followed by a successive series of chromatographies on DEAE-Sephacel, Phenyl Sepharose CL-4B, and Sephacryl S-200 HR columns (all from Amersham Pharmacia Biosciences). The elution of SMP30 was followed by the dot-blot immunoassay described in ref. 5. The purified SMP30 was stored at −70°C until use.
Purification of GNL and Sequence Analysis of Its Peptide.
GNL was purified from rat livers according to the method of Grossman and Axelrod (11), with slight modifications. Briefly, a soluble fraction of the liver homogenate was heated at 50°C for 30 min, and the resulting soluble fraction was fractionated with ammonium sulfate. The fraction with GNL activity was further purified by successive chromatographies on columns of Sephadex G-150 (Amersham Pharmacia Fine Chemicals), Resource Q (Amersham Pharmacia Biosciences), and Bio-Gel HTP (Bio-Rad). After the final preparation was subjected to SDS/PAGE, the main band was excised, and the protein in the gel was digested with trypsin. One of the peptides produced was sequenced through the custom service of APRO Life Science Institute (Naruto, Japan).
GNL and Related Activities.
GNL activity was measured by the change in absorbance of the pH indicator p-nitrophenol caused by free acid formation from the lactone (23). The standard mixture contained 10 mM d-glucono-δ-lactone, 10 mM Pipes (pH 6.4), 0.25 mM p-nitrophenol, 75 μM ZnCl2, and an enzyme in a total volume of 1 ml. The substrate solution was freshly prepared immediately before the assay. The reaction was followed by monitoring a decrease in absorbance at 405 nm, and the acid production rate was determined with a calibration curve obtained by using known amounts of HCl. The rate of spontaneous hydrolysis of the lactone was subtracted from the total rate. To assess whether a divalent metal ion was required for GNL activity, we tested ZnCl2, MnCl2, MgCl2, CoCl2, CaCl2, and CdCl2 in this regard and then determined the hydrolyzing activity for other lactones with the same procedure, except for the substrate.
Lactonase activity was then analyzed in livers removed from mice and homogenized with ice-cold homogenization buffer (10 mM Tris·HCl, pH 8.0/1 mM phenyl methanesulfonyl fluoride) for 30 s at high speed with a Polytron homogenizer. The homogenate was centrifuged at 10,000 × g for 10 min. The protein concentration of the sample was determined by BCA protein assay (Pierce) using BSA as a standard. The lactone-hydrolyzing activity was assayed by using various lactones under the standard conditions described above. In the purification of rat GNL, lactonase activity was measured by recording pH change with a pH meter, essentially as described in ref. 24.
Expression of Recombinant Rat SMP30.
Total RNA was prepared from a rat liver and used to produce a single-strand cDNA with SuperScript II RNase H− reverse transcriptase (Life Technologies, Rockville, MD) following the manufacturer’s protocol. A SMP30 cDNA was amplified by PCR from this cDNA such that an EcoRI site would be produced at the both ends of the product. The PCR was carried out by using PfuUltra DNA polymerase (Stratagene) with a sense primer (5′-GAATTCATGTCTTCCATCAAGATTG-3′) and an antisense primer (5′-GAATTCTTACCCTGCATAGGAATATG-3′). The amplified DNA was digested with EcoRI and inserted into the EcoRI site of the E. coli expression vector pMAL-c2x (New England Biolabs). The constructed plasmid pMAL-c2x-rSMP30 was used to transform E. coli BL21 that had previously been transformed with pGro7 (Takara Bio, Tokyo) for expression of two chaperone proteins: GroEL and GroES. The resulting clone was used to express rat SMP30 as a fusion protein with MBP as follows. It was cultured at 37°C overnight in LB containing 0.2% glucose, 100 μg/ml ampicillin, and 20 μg/ml chloramphenicol. After the cell suspension was diluted 100-fold with the same culture medium, d-arabinose (4 mg/ml) was added for expression of the chaperone proteins and cultured for 1.6 h at 37°C. Then, the expression of MBP–SMP30 fusion protein was induced by adding isopropyl β-d-thiogalactoside to a final concentration of 0.3 mM and culturing for 3 h at 30°C. For control experiments, pMAL-c2x was used instead of pMAL-c2x-rSMP30, and a fusion protein of MBP with the α-fragment of β-gal was induced in the same way as above. The cells were washed with Dulbecco’s phosphate buffered saline, resuspended in 20 mM Tris·HCl buffer (pH 7.4) containing 200 mM NaCl, and stored at −30°C until use.
Activity Staining and Western Blotting for Recombinant MBP–SMP30 Fusion Protein.
Suspensions of the test and control cells were sonicated and centrifuged at 9,000 × g for 30 min at 4°C. Each resulting supernatant (60 μg of protein) was electrophoresed on a 7% polyacrylamide gel by the method of Davis (25), with some modifications. Proteins in the gel were transferred onto a polyvinylidene fluoride membrane with 25 mM Tris/192 mM glycine as a transfer buffer. Subsequent staining for GNL activity involved immersing the membrane successively in 5 mM Tris·HCl buffer (pH 6.8) containing 1 mM DTT and 1 mM MnCl2 (buffer A), then in a mixture of buffer A and 0.04% methyl red in 60% ethanol (9:1), and finally in buffer A containing 80 mM d-galactono-γ-lactone until the red color appeared.
For Western blotting, the supernatant (20 μg of protein) was electrophoresed in duplicate, and proteins were transferred onto a polyvinylidene fluoride membrane in the same way. The membrane was blocked overnight with a mixture of 2% BSA and 7% skim milk and cut into halves. One half was incubated with anti-MBP rabbit antibody (1:3,000 dilution; New England Biolabs), and the other half was incubated with anti-rat SMP30 rabbit antibody (1:3,000 dilution; Cosmo Bio, Tokyo) (3). Then, both half-membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:5,000 dilution; Cappel). MBP and SMP30 were visualized with an ECL (enhanced chemiluminescence) detection kit (Amersham Pharmacia Biosciences).
Measurement of AA.
Plasma was mixed with nine volumes of 20% metaphosphate containing 1% SnCl2, and the mixture was centrifuged at 10,000 × g for 10 min at 4°C. Livers and kidneys were homogenized in 14 volumes of 5.4% metaphosphate, and the homogenate was centrifuged as above. For measurement of AA excreted into urine, a mouse was housed in a metabolic cage, and urine was collected in a bottle with 10% metaphosphate for 24 h. The amount of 10% metaphosphate had been adjusted to keep its concentration >5% after dilution with urine. The volume of the urine–metaphosphate mixture was recorded, and the mixture was centrifuged as above. All samples obtained after centrifugation were kept at −80°C until use. AA in samples was derivatized with dinitrophenylhydrazine and analyzed by HPLC with a Shodex-5SIL-4E column (4.6 × 250 mm; Showa Denko, Tokyo). The mobile phase was hexane/ethylacetate/acetic acid (5:4:1) at a flow rate of 1 ml/min, and the absorbance at 495 nm was recorded (26, 27). AA in mouse chow was determined by the same method after extraction into 15% metaphosphate.
Quantification of BMD and Body Fat Percentage.
Subcranial total BMD and body fat percentage were determined by densitometry with the PIXImus2 imager (General Electric/Lunar, Madison, WI). Field calibration and calibration vs. the quality-control phantom were performed each day before imaging. Each mouse was positioned reproducibly in a prone position on the imaging tray and scanned three times. The coefficients of variance for BMD and body fat percentage were 0.9% and 2.2%, respectively, for in vitro measurements.
Statistical Analysis.
The results are expressed as mean ± SEM. The probability of statistical differences between experimental groups was determined by Student’s t test or ANOVA as appropriate. One and two-way ANOVAs were performed by using kaleidagraph software (Synergy Software, Reading, PA).
Supplementary Material
Acknowledgments
We thank Ms. P. Minick for excellent assistance in the review of English and Mr. H. Hosoi and Ms. Y. Takenaka for participation in the purification of GNL in the training course on basic medicine at Wakayama Medical University. This work was supported by a Grant-in-Aid for Scientific Research (to S.H. and S.K.) and a Grant-in-Aid for Young Scientists (B) (to Y.I.) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and grants from Health Science Research Grants for Comprehensive Research on Aging and Health supported by the Ministry of Health, Labor, and Welfare of Japan (to A.I.), the Smoking Science Foundation (to N.M.), and the Vitamin C Research Committee of Japan (to M.N.).
Abbreviations
- AA
l-ascorbic acid
- BMD
bone mineral density
- GNL
gluconolactonase
- MBP
maltose-binding protein
- SMP30
senescence marker protein 30.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fujita T., Uchida K., Maruyama N. Biochim. Biophys. Acta. 1992;1116:122–128. doi: 10.1016/0304-4165(92)90108-7. [DOI] [PubMed] [Google Scholar]
- 2.Mori T., Ishigami A., Seyama K., Onai R., Kubo S., Shimizu K., Maruyama N., Fukuchi Y. Pathol. Int. 2004;54:167–173. doi: 10.1111/j.1440-1827.2003.01603.x. [DOI] [PubMed] [Google Scholar]
- 3.Ishigami A., Fujita T., Handa S., Shirasawa T., Koseki H., Kitamura T., Enomoto N., Sato N., Shimosawa T., Maruyama N. Am. J. Pathol. 2002;161:1273–1281. doi: 10.1016/s0002-9440(10)64404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishigami A., Kondo Y., Nanba R., Ohsawa T., Handa S., Kubo S., Akita M., Maruyama N. Biochem. Biophys. Res. Commun. 2004;315:575–580. doi: 10.1016/j.bbrc.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y., Ishigami A., Kubo S., Handa S., Gomi K., Hirokawa K., Kajiyama N., Chiba T., Shimokado K., Maruyama N. FEBS Lett. 2004;570:57–62. doi: 10.1016/j.febslet.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Kanagasundaram V., Scopes R. Biochim. Biophys. Acta. 1992;1171:198–200. doi: 10.1016/0167-4781(92)90120-o. [DOI] [PubMed] [Google Scholar]
- 7.Bublitz C., Lehninger A. L. Biochim. Biophys. Acta. 1961;47:288–297. [Google Scholar]
- 8.Burns J. J. In: Metabolic Pathway. Greenberg D. M., editor. Vol. 1. New York: Academic; 1960. pp. 341–356. [Google Scholar]
- 9.Nishikimi M., Yagi K. In: Subcellular Biochemistry–Ascorbic Acid: Biochemistry and Biomedical Cell Biology. Harris J. R., editor. Vol. 25. New York: Plenum; 1996. pp. 17–39. [Google Scholar]
- 10.Nishikimi M., Okamura M., Ohta Y. In: Recent Research Developments in Biophysics and Biochemistry. Part 1. Pandalai S. G., editor. Vol. 3. Kerala, India: Research Signpost; 2003. pp. 531–545. [Google Scholar]
- 11.Grossman S. H., Axelrod B. J. Biol. Chem. 1973;248:4846–4851. [PubMed] [Google Scholar]
- 12.Shimazono N., Mano Y. Ann. N.Y. Acad. Sci. 1961;92:91–104. [Google Scholar]
- 13.Winkelman J., Lehninger A. L. J. Biol. Chem. 1958;233:794–799. [PubMed] [Google Scholar]
- 14.Kawada M., Takiguchi H., Kagawa Y., Suzuki K., Shimazono N. J. Biochem. (Tokyo) 1962;51:405–415. doi: 10.1093/oxfordjournals.jbchem.a127554. [DOI] [PubMed] [Google Scholar]
- 15.Rebouche C. J. Am. J. Clin. Nutr. 1991;54:1147S–1152S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- 16.Ha T. Y., Otsuka M., Arakawa N. J. Nutr. Sci. Vitaminol. (Tokyo) 1990;36:227–234. doi: 10.3177/jnsv.36.227. [DOI] [PubMed] [Google Scholar]
- 17.Kono K., Asai K., Kuzuya F., Harada T. In: Vitamin C and the Scurvy-Prone ODS Rat. Fujita T., Fukase M., Konishi T., editors. Amsterdam: Elsevier; 1990. pp. 147–155. [Google Scholar]
- 18.Bernstein S. E. In: Biology of the Laboratory Mouse. 2nd Ed. Green E. L., editor. New York: Dover; 1966. pp. 337–350. [Google Scholar]
- 19.Maeda N., Hagihara H., Nakata Y., Hiller S., Wilder J., Reddick R. Proc. Natl. Acad. Sci. USA. 2000;97:841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagawa Y., Takiguchi H. J. Biochem. (Tokyo) 1962;51:197–203. doi: 10.1093/oxfordjournals.jbchem.a127521. [DOI] [PubMed] [Google Scholar]
- 21.Fujita T., Shirasawa T., Uchida K., Maruyama N. Biochim. Biophys. Acta. 1992;1132:297–305. doi: 10.1016/0167-4781(92)90164-u. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay R. M., Smith W., Lee W. K., Dominiczak M. H., Baird J. D. Clin. Chim. Acta. 1997;263:239–247. doi: 10.1016/s0009-8981(97)00067-3. [DOI] [PubMed] [Google Scholar]
- 23.Hucho F., Wallenfels K. Biochim. Biophys. Acta. 1972;276:176–179. doi: 10.1016/0005-2744(72)90018-6. [DOI] [PubMed] [Google Scholar]
- 24.Nishikimi M., Koshizaka T., Mochizuki H., Iwata H., Makino S., Hayashi Y., Ozawa T., Yagi K. Biochem. Int. 1988;16:615–621. [PubMed] [Google Scholar]
- 25.Davis B. J. Ann. N.Y. Acad. Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 26.Kishida E., Nishimoto Y., Kojo S. Anal. Chem. 1992;64:1505–1507. [Google Scholar]
- 27.Kodaka K., Inagaki S., Ujie T., Ueno T., Suda H. Vitamin. 1985;59:451–455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.