Abstract
The members of the cytoplasmic 70-kDa heat shock protein family are involved in appropriate folding and trafficking of newly synthesized proteins in the cell. Hsc70, which is expressed constitutively, and Hsp70, the expression of which is stress- and heat shock-induced, are often considered to have similar cellular functions in this regard, but there are suggestions that the intracellular functions of these homologous but not identical proteins may differ. We tested the hypothesis that Hsc70 and Hsp70 would have differential effects on the expression of the epithelial sodium channel (ENaC). In Xenopus oocytes, overexpression of human Hsc70 decreased the functional (defined as amiloride-sensitive whole-oocyte current) and surface expression of murine ENaC (mENaC) in a concentration-dependent fashion. In contrast, coinjection of a moderate amount of Hsp70 cRNA (10 ng) increased the functional and surface expression of mENaC, whereas a higher amount of coinjected Hsp70 cRNA (30 ng) decreased mENaC functional and surface expression. The increase in mENaC functional expression with coinjection of 10 ng of Hsp70 cRNA was antagonized by the additional coinjection of Hsc70 cRNA in a concentration-dependent fashion. These data are consistent with Hsc70 and Hsp70 having differential and antagonistic effects with regard to the intracellular trafficking of mENaC in oocytes, which may have an impact on our understanding and potential treatment of diseases of aberrant ion channel trafficking.
Keywords: chaperone, Xenopus oocyte, cystic fibrosis, ENaC, antagonism
The epithelial sodium channel (ENaC) plays a pivotal role in the regulation of blood volume and blood pressure and may have a vital role in the function of the pulmonary epithelia. The ENaC and the cystic fibrosis transmembrane conductance regulator (CFTR) are colocalized at the apical surface of respiratory epithelia, where CFTR can regulate ENaC activity (1, 2). ENaC functional expression appears increased in cystic fibrosis (CF) compared with non-CF airway epithelia (3), which likely leads to enhanced absorption of water from the airway surface liquid, decreased ciliary propulsion of mucus (4), and a compromised ability to clear bacteria from the CF airway. Thus, control of both CFTR and ENaC trafficking and expression is likely to be critical in airway homeostasis.
Some similarities exist in the intracellular processing of the CFTR and ENaC. Wild-type (WT) CFTR is processed inefficiently; only ≈25% of newly synthesized WT CFTR reaches the plasma membrane (5), although conflicting data have been published recently (6). The most common mutation of the CFTR is the deletion of phenylalanine-508 (ΔF508); protein with this deletion is retained in the endoplasmic reticulum (ER) (7). The majority of WT CFTR and almost all ΔF508 are targeted for intracellular degradation, at least in part by the ubiquitin/proteasome system (5). Such degradation may depend on Hsc70, the constitutively expressed 70-kDa heat shock protein. Hsc70 associates more avidly with ΔF508 than with WT CFTR (8, 9) and is an essential cofactor for ubiquitination (10). Hsc70 associates with nascent CFTR on the ribosome (8), and cessation of this interaction correlates with cessation of CFTR ubiquitination (11). Thus, increased association of ΔF508 and Hsc70 may lead to a higher likelihood of ubiquitination and degradation by the proteasome. Interestingly, a number of pharmacologic agents that improve ΔF508 intracellular trafficking, such as sodium 4-phenylbutyrate (12), decrease the expression of Hsc70 and its association with ΔF508 (9).
Intracellular trafficking of ENaC is, in some ways, similar to that of CFTR. In Xenopus oocytes, only a small fraction of newly synthesized ENaC reaches the plasma membrane (13). Similarly, the degradation of ENaC is also mediated by the proteasome (13–15). However, little is known regarding the role of 70-kDa cytoplasmic molecular chaperones in this process and how agents that modulate chaperone expression may influence this process.
Hsc70 has a number of other intracellular functions, including catalyzing the ATP-dependent uncoating of clathrin-coated pits (16) and promoting lysosomal degradation of intracellular proteins (16, 17). Although most investigators have treated Hsc70 and Hsp70, the stress-inducible 70-kDa heat shock protein, as equivalent and functionally interchangeable, many functions attributed to Hsc70 have not been associated with Hsp70 (16). In Escherichia coli, the Hsp70 homolog DnaK promotes protein folding (16), and overexpression of Hsp70 may improve ΔF508 trafficking in CF epithelial cells (18).
These data suggest that Hsc70 and Hsp70 may affect the intracellular trafficking of ion channels differentially. Decreased Hsc70 expression may decrease the association of an ion channel with Hsc70 and therefore improve its trafficking by decreasing its ubiquitination and degradation. Hsp70 overexpression could decrease the association of an ion channel and Hsc70 by competing with Hsc70 for binding to the ion channel. We therefore tested the specific hypothesis that Hsc70 and Hsp70 overexpression would influence the intracellular trafficking and function of the ENaC differentially. We found that Hsc70 can decrease the functional and surface expression of murine ENaC, whereas Hsp70 has the opposite effect. These data have implications for the treatment of diseases involving aberrant trafficking of ion channels in epithelial cells.
Results
Influence of Hsc70 on the Expression of Murine ENaC (mENaC) in Xenopus Oocytes.
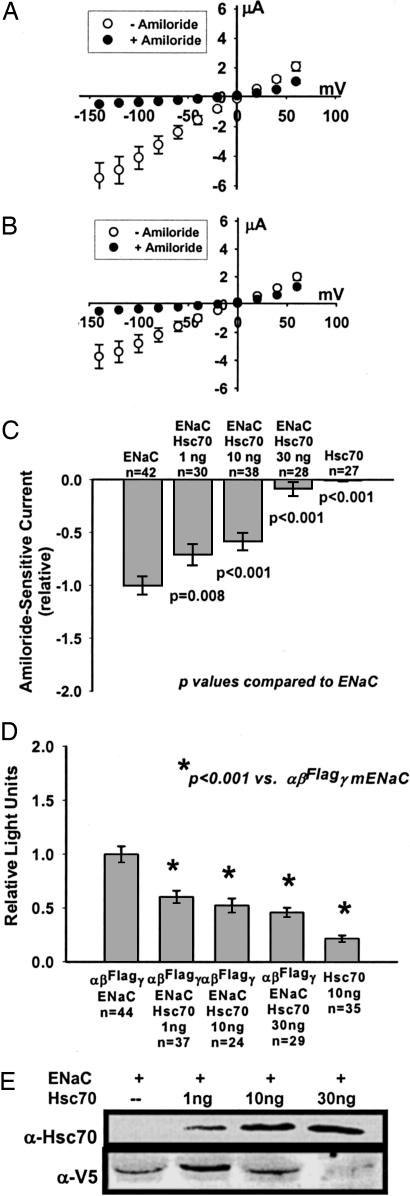
Fig. 1A shows the current/voltage (I/V) relationship by two-electrode voltage clamp (TEV) for oocytes 24 h after injection with mENaC (0.33 ng per subunit) in the absence and presence of 10 μM amiloride. The linear I/V relationship is characteristic of mENaC expressed in oocytes. The reversal potential of ≈0 mV (adjusted for resting membrane potential) suggests that our oocytes are “sodium-loaded” and that sodium feedback inhibition of mENaC (19) is not confounding these observations.
Fig. 1.
Effect of Hsc70 on mENaC expression in Xenopus oocytes. Xenopus laevis oocytes were injected with cRNA for all three subunits of mENaC, α, β, and γ (αβγ-mENaC) (0.33 ng per subunit), either alone (A) or with 10 ng of cRNA for human Hsc70 (B). TEV was performed 24 h after injection as described in Materials and Methods. Shown are the I/V relationships (mean ± SEM) for n = 42 (A) and n = 38 (B) oocytes before (open circles) and after (filled circles) the addition of 10 μM amiloride. (C) Oocytes were injected with cRNA for αβγ-mENaC (0.33 ng per subunit), Hsc70 (10 or 30 ng), or coinjected with cRNA for αβγ-mENaC (0.33 ng per subunit) and the indicated amount of Hsc70 cRNA. TEV was performed 24 h after injection, and data are expressed as the relative amiloride-sensitive current at −100 mV holding potential (mean ± SEM), with P values determined by ANOVA as described in Materials and Methods. The ENaC and ENaC + 10 ng of Hsc70 data in C correspond to the I/V plots of A and B, respectively. (D) Expression of mENaC at the oocyte surface was assessed 24 h after injection with cRNAs for mENaC (0.33 ng per subunit) or Hsc70 (10 ng) alone or injection with mENaC and the indicated amount of Hsc70 cRNAs. In these experiments, the β-mENaC subunit contained an external FLAG epitope (βFLAG). Relative surface expression is expressed as the mean ± SEM, with P values determined by ANOVA. (E) Oocytes were injected with cRNA for αβγ-mENaC (0.33 ng per subunit) where the β subunit contained a C-terminal V5 epitope (β-V5) and the indicated amount of Hsc70 cRNA. Whole-oocyte lysates were prepared 24 h after injection, and expression of Hsc70 and β-V5 was assessed by immunoblot using specific antisera. These data are representative of three or four independent experiments.
Oocytes coinjected with αβγ-mENaC cRNA and Hsc70 cRNA (10 ng) also demonstrated a linear whole-oocyte I/V relationship that was inhibited by amiloride, but the slope of the I/V relationship was decreased (Fig. 1B). The reversal potential of this I/V relationship remained at ≈0 mV, suggesting that sodium loading was not altered by the presence of Hsc70. These data suggest that Hsc70 decreased the mENaC-mediated whole-oocyte conductance.
We next examined the concentration dependence of Hsc70 overexpression on mENaC functional expression (Fig. 1C). Given the linear I/V relationship, we recorded whole-oocyte amiloride-sensitive currents at −100 mV holding potential (corrected for resting membrane potential) for comparison. Coinjection of 1, 10, or 30 ng of human Hsc70 cRNA resulted in a concentration-dependent decrease in mENaC functional expression relative to injection of mENaC alone (Fig. 1C). Oocytes injected with Hsc70 cRNA alone (10 or 30 ng) did not demonstrate amiloride-sensitive currents.
Alterations in mENaC functional expression may reflect changes in the expression of mENaC at the cell surface (N) or in the mENaC open probability (Po) or unitary conductance. To assess whether Hsc70 alters N, and therefore mENaC intracellular trafficking, we determined surface expression (Fig. 1D) by a direct antibody binding and chemiluminescence assay (20, 21), where the β-mENaC subunit contains a FLAG epitope in the extracellular loop (βFLAG). Oocytes were injected with αβFLAGγ-mENaC (0.33 ng per subunit) alone or coinjected with 1, 10, or 30 ng of human Hsc70 cRNA. Control oocytes were injected with 10 ng of Hsc70 cRNA alone and had a background, nonspecific signal of ≈15% of oocytes injected with αβFLAGγ-mENaC. Coinjection of with 1, 10, or 30 ng of Hsc70 cRNA resulted in a concentration-dependent ≈40%, ≈50%, and ≈55% (respectively) decrease in αβFLAGγ-mENaC surface expression compared with oocytes injected with αβFLAGγ-mENaC alone. This Hsc70 dose-dependent inhibition of surface expression parallels the Hsc70 dose-dependent decrease in mENaC functional expression (Fig. 1C) and is consistent with Hsc70 decreasing mENaC functional expression in oocytes by causing a decrease in surface expression.
We prepared immunoblots to assess whole-oocyte expression of Hsc70 and mENaC (Fig. 1E) and used a β-mENaC fusion protein with a C-terminal V5 epitope (β-V5) to increase sensitivity (22). Oocytes were injected with cRNA for αβ-V5γ-mENaC (0.33 ng per subunit) alone or coinjected with increasing amounts of Hsc70 cRNA. Coinjection of increasing amounts of Hsc70 cRNA resulted in a corresponding increase in Hsc70 protein expression in whole-oocyte lysates. Interestingly, overexpression of a low amount of Hsc70 (after injection of 1 ng of cRNA) increased the whole-oocyte content of β-V5-mENaC compared with oocytes injected with αβ-V5γ-mENaC-alone. Higher amounts of coinjected Hsc70 cRNA decreased the whole-oocyte content of β-V5-mENaC significantly compared with oocytes coinjected with 1 ng of Hsc70 cRNA.
Influence of Hsp70 on the Expression of mENaC in Xenopus Oocytes.
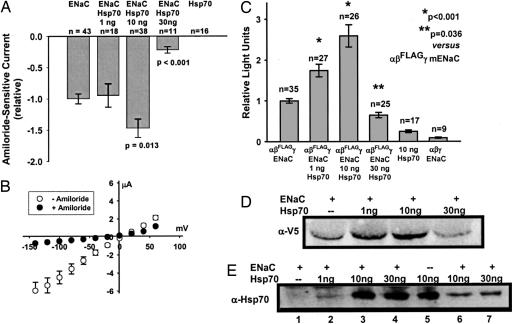
We performed similar experiments to assess the effect of Hsp70 on the expression of mENaC in oocytes (Fig. 2). αβγ-mENaC (0.33 ng per subunit) was injected alone or together with 1, 10, or 30 ng of human Hsp70 cRNA, and whole-cell amiloride-sensitive currents were determined by TEV relative to currents observed for oocytes injected with αβγ-mENaC alone. In contrast to our observations with Hsc70 in Fig. 1, coinjection of 10 ng of Hsp70 cRNA enhanced mENaC functional expression by ≈50%, whereas coinjection of 30 ng of Hsp70 cRNA had the opposite effect and inhibited mENaC functional expression significantly. Oocytes injected with Hsp70 cRNA alone (10 or 30 ng) did not demonstrate an amiloride-sensitive current. Coinjection of 10 ng of Hsp70 cRNA did not alter the mENaC-characteristic linear I/V relationship or the reversal potential of ≈0 mV of sodium-loaded, mENaC-expressing oocytes (Fig. 2B).
Fig. 2.
Effect of Hsp70 on mENaC expression in oocytes. (A) Oocytes were injected with cRNA for αβγ-mENaC (0.33 ng per subunit) or Hsp70 (10 or 30 ng), or coinjected with cRNA for αβγ-mENaC (0.33 ng per subunit) and the indicated amount of Hsp70 cRNA. TEV was performed 24 h after injection, and data are expressed as the relative amiloride-sensitive current at −100 mV holding potential (mean ± SEM), with P values determined by ANOVA. (B) I/V relationship (mean ± SEM) for oocytes (n = 38) coinjected with cRNA for αβγ-mENaC (0.33 ng per subunit) and 10 ng of cRNA for human Hsp70. TEV was performed 24 h after injection before (open circles) and after (filled circles) the addition of 10 μM amiloride. These data correspond to those for ENaC/Hsp70 10 ng in A. (C) Expression of mENaC at the oocyte surface was assessed 24 h after injection with cRNAs for αβFLAGγ-mENaC (0.33 ng per subunit) or Hsp70 (10 ng) alone, or injection with αβFLAGγ-mENaC and the indicated amount of Hsp70 cRNAs. A separate group of control oocytes were injected with cRNA for WT αβγ-mENaC (0.33 ng per subunit, β-mENaC lacking the FLAG epitope). Relative surface expression is expressed as the mean ± SEM, with P values determined by ANOVA. (D) Oocytes were injected with cRNA for αβ-V5γ-mENaC (0.33 ng per subunit) and the indicated amount of Hsp70 cRNA. Whole-oocyte lysates were prepared 24 h after injection, and expression of β-V5 was assessed by immunoblotting. (E) Oocytes were injected with cRNA for αβ-V5γ-mENaC (0.33 ng per subunit, except lane 5) and the indicated amount of Hsp70 cRNA. Whole-oocyte lysates were prepared 24 h after injection, and expression of Hsp70 was assessed by immunoblotting. Lanes 6 and 7 correspond to lanes 3 and 4 but were loaded with one-fourth the amount of sample. D and E are representative of three or four independent experiments.
We next assessed the influence of Hsp70 on the surface expression of mENaC (Fig. 2C). Oocytes were injected with cRNA αβFLAGγ-mENaC alone or coinjected with 1, 10, or 30 ng of Hsp70 cRNA. Coinjection of 1 or 10 ng of Hsp70 resulted in significantly increased surface expression of αβFLAGγ-mENaC compared with oocytes injected with αβFLAGγ-mENaC alone. In contrast, coinjection of 30 ng of Hsp70 cRNA decreased αβFLAGγ-mENaC surface expression significantly. Control oocytes injected with 10 ng of Hsp70 cRNA or with cRNAs encoding non-epitope-tagged (WT) αβγ-mENaC (0.33 ng per subunit) demonstrated nonspecific chemiluminescence of ≤15% of oocytes injected with αβFLAGγ-mENaC. These data suggest that changes in mENaC functional expression caused by overexpression of Hsp70 at least in part reflect changes in the trafficking of mENaC in oocytes.
We also assessed the whole-oocyte expression of β-V5-mENaC in oocytes injected with αβ-V5γ-mENaC either alone or with increasing amounts of Hsp70 cRNA (Fig. 2D). Coinjection of 1 or 10 ng of Hsp70 cRNA increased the whole-oocyte expression of β-V5-mENaC, whereas coinjection of 30 ng of Hsp70 decreased the whole-oocyte expression of β-V5-mENaC. These changes parallel those observed in the surface-expression assay (Fig. 2C). These data are also consistent with the TEV data in which 10 ng of Hsp70 cRNA enhanced and 30 ng of Hsp70 cRNA inhibited mENaC functional expression (Fig. 2A). Fig. 2E demonstrates that injection of increasing amounts of Hsp70 cRNA corresponds to increased Hsp70 protein expression in whole-oocyte lysates.
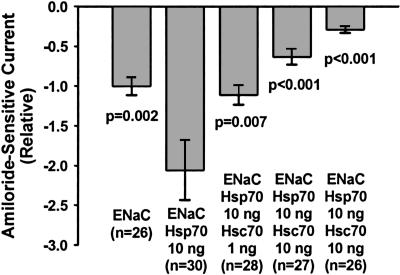
Antagonistic Effects of Hsc70 and Hsp70 on mENaC Functional Expression.
Our model predicts that Hsc70 and Hsp70 should antagonize each other directly with regard to mENaC trafficking and functional expression. To test this hypothesis, oocytes were injected with cRNA for αβγ-mENaC alone (0.33 ng per subunit); coinjected with αβγ-mENaC and an “optimal profunctional expression amount” of Hsp70 cRNA (i.e., 10 ng); or coinjected with αβγ-mENaC, Hsp70, and 1, 10, or 30 ng of Hsc70 cRNA, and mENaC functional expression was then assessed by TEV (Fig. 3). Here, coinjection of 10 ng of Hsp70 cRNA with αβγ-mENaC yielded a 2-fold increase in relative amiloride-sensitive currents compared with oocytes injected with mENaC alone. Oocytes coinjected with αβγ-mENaC and Hsp70 and increasing concentrations of Hsc70 (1, 10, and 30 ng) demonstrated a dose-dependent decrease in relative amiloride-sensitive currents compared with αβγ-mENaC/Hsp70-coinjected oocytes. These data suggest that Hsc70 overexpression can directly antagonize the effect of Hsp70 to increase mENaC functional expression in oocytes and are consistent with the hypothesis that Hsc70 and Hsp70 have differential, nonequivalent, and antagonistic functions with regard to the intracellular trafficking of ion channels such as mENaC.
Fig. 3.
Antagonistic effects of Hsc70 and Hsp70 on mENaC functional expression. Oocytes were injected with cRNAs encoding αβγ-mENaC alone (0.33 ng per subunit), coinjected with αβγ-mENaC (0.33 ng per subunit) and Hsp70 (10 ng) cRNAs, or coinjected with cRNAs for αβγ-mENaC (0.33 ng per subunit) and Hsp70 (10 ng) and the indicated amount of Hsc70 cRNA. TEV was performed 24 h after injection, and relative amiloride-sensitive currents at −100 mV were determined. P values were determined by ANOVA compared with oocytes coinjected with αβγ-mENaC and Hsp70 cRNAs.
Influence of Hsc70 and Hsp70 on mENaC Exocytosis and Endocytosis.
To attempt to delineate the mechanism by which Hsc70 and Hsp70 alter the trafficking of mENaC in oocytes, we sought to determine the influence of these chaperones on the rate of delivery of mENaC to the membrane and on its removal from the membrane. To assess the exocytic rate of delivery, we used a mutant of the amiloride binding site in the γ-mENaC subunit (G542C); such mENaC mutants are blocked irreversibly by treatment with [2-(trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET) (23). The initial rate of recovery of the mENaC-mediated current after MTSET blockade is a direct measure of the rate of mENaC exocytosis. In these experiments, the rate of mENaC exocytosis, as determined by the rate of increase of the inward Na+ current, was −41 ± 6 nA/min (at −100 mV holding potential, n = 17). This rate was not significantly altered by the coinjection of 10 ng of cRNA for either Hsc70 [−54 ± 11 nA/min, n = 18, P = not significant (NS)] or Hsp70 (−45 ± 8 nA/min, n = 22, P = NS).
We also determined the half-life of mENaC at the oocyte membrane in the presence of brefeldin A to block delivery of new channels to the membrane. mENaC was removed from the oocyte membrane with a half-time of 2.6 ± 0.3 h (n = 7). This half-time was again not significantly altered by coinjection of 10 ng of cRNA for either Hsc70 (t½ = 2.3 ± 0.2 h, n = 7, P = NS) or Hsp70 (t½ = 1.7 ± 0.3 h, n = 7, P = NS).
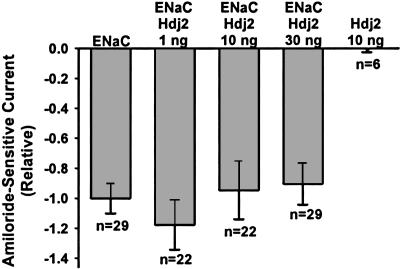
Hdj-2 Overexpression Does Not Alter mENaC Functional Expression.
To ensure that the decreased mENaC functional, surface, and whole-oocyte expression when mENaC was coinjected with 30 ng of cDNA for either Hsc70 or Hsp70 was not a nonspecific effect of competition for oocyte translational machinery, we performed a control experiment with coinjection of Hdj2 cRNA. Hdj2 is a cytosolic 40-kDa heat shock protein that regulates the ATPase activity of Hsc70 and may facilitate CFTR trafficking (8). Oocytes were injected with αβγ-mENaC (0.33 ng per subunit) alone or together with 1, 10, or 30 ng of human Hdj2 cRNA, and whole-cell amiloride-sensitive currents were determined 24 h after injection. Functional expression of mENaC was not altered by coinjection of any amount of Hdj2 cRNA (Fig. 4). These data suggest that the effects on mENaC expression of coinjection of 30 ng of Hsc70 (Fig. 1) or Hsp70 (Fig. 2) cRNA or coinjection of both Hsc70 and Hsp70 cRNA (Fig. 3) were specific and not confounded by competition for translational machinery.
Fig. 4.
Hdj-2 overexpression does not alter mENaC functional expression. Oocytes were injected with cRNAs encoding αβγ-mENaC alone (0.33 ng per subunit) or Hdj2 alone (10 ng), or coinjected with αβγ-mENaC (0.33 ng per subunit) and the indicated amount of Hdj2 cRNA. TEV was performed 24 h after injection, and relative amiloride-sensitive currents at −100 mV were determined. Statistical analysis was performed by ANOVA compared with oocytes injected with αβγ-mENaC cRNA alone. There were no statistically significant differences in amiloride-sensitive currents between oocytes coinjected with mENaC and 1, 10, or 30 ng of Hdj2 compared with oocytes injected with mENaC alone.
Discussion
With increased recognition of the potential role of ENaC in the pathophysiology of CF airway disease, strategies to bring about repair of mutant CFTRs will require attention to the effect of these interventions on the functional expression of ENaC. Based on our observations that sodium 4-phenylbutyrate improves ΔF508 intracellular trafficking (12) and decreases the expression of Hsc70 (9), which is a necessary cofactor for ubiquitination and degradation of a number of cellular proteins (10), and the suggestion of others that overexpression of Hsp70 may promote repair of ΔF508 trafficking (18), we hypothesized that Hsc70 and Hsp70 may have differential effects on the intracellular trafficking of ion channels, such as CFTR and ENaC. Here, we tested this hypothesis, and we observed that, in fact, Hsc70 and Hsp70 can have the opposite effect on mENaC functional and surface expression in oocytes. These data argue against the presumption that Hsc70 and Hsp70 are functionally interchangeable within the cell.
Interestingly, overexpression of large amounts of either Hsc70 or Hsp70 decreased mENaC functional, surface, and whole-oocyte expression markedly. We were able to discern the differential effects only because we examined multiple amounts of injected chaperone cRNA. The decreases in mENaC functional, surface, and whole-oocyte expression at high amounts of injected chaperone cRNA could be a result of the excess of chaperone cRNA competing for translation machinery and precluding the cell from translating the mENaC cRNA. Our data demonstrating a lack of effect of overexpression of Hdj2 on mENaC functional expression (Fig. 4) argue against such an explanation. Furthermore, competition for translational machinery cannot explain that the overexpression of Hsc70 and Hsp70 that results from injection of 10 ng of cRNA has distinct and opposite effects on the functional and surface expression of mENaC.
Our studies assessing the influence of Hsc70 and Hsp70 on the rates of mENaC exocytosis and endocytosis did not yield clear insight into the mechanism by which these chaperones alter mENaC intracellular trafficking. Our assay of exocytosis, or delivery of mENaC to the oocyte membrane, does not discriminate newly synthesized channels from recycling channels. Similarly, channel recycling may complicate our assay of mENaC endocytosis performed in the presence of brefeldin A. These considerations, as well as the relatively small magnitude of the effect of the chaperones and the possibility that these chaperones may influence mENaC trafficking at many locations in the cell, suggest that these assays may not have been sufficiently sensitive to yield these mechanistic insights.
Interestingly, we have also observed that coinjection of 30 ng of Hsp70 cRNA enhances the functional expression of ΔF508 compared with oocytes injected with ΔF508 cRNA alone.‖ These data are consistent with Hsp70 having a higher affinity for ΔF508 (a mutant protein) than for mENaC (a WT protein) and are also consistent with observations that the 70-kDa heat shock proteins associate more avidly with ΔF508 than WT CFTR (9, 24). This may lead to more “free” Hsp70 when Hsp70 is coinjected with the WT protein, mENaC vs. ΔF508. Such free Hsp70 could have secondary effects, such as altering mRNA transcription (25) or altering mRNA turnover (26, 27). An analogous mechanism is proposed for regulation of the unfolded protein response by the ER-resident Hsp70 homolog, BiP. BiP typically sequesters IRE1, ATF6, and PERK, the mediators of the unfolded protein response, but in the presence of excess unfolded protein in the ER, BiP binds preferentially to the unfolded protein and frees these mediators to initiate the unfolded protein response (28).
Low amounts of overexpressed Hsc70 enhanced the whole-oocyte content of β-mENaC while decreasing its functional and surface expression. Although this observation seems counterintuitive, there are potential explanations suggested by the literature. Hsc70 can stabilize peptides and proteins in solution (29) and may act similarly in cells. In cultured cells, ≈20% of the total pool of ENaC channels are expressed at the plasma membrane (30), and channel subunits that do not exit the ER are degraded by proteasomes via an ER-associated degradation pathway (ERAD; refs. 13–15). In cultured cells, ENaC expression is also regulated by ubiquitination at the plasma membrane (14, 15) and clathrin-mediated endocytosis (31), and Hsc70 regulates clathrin-mediated endocytosis by uncoating clathrin-coated pits. It is therefore possible that low levels of Hsc70 overexpression may increase the clathrin-mediated endocytosis of mENaC from the oocyte membrane and increase the size of the intracellular pool, whereas higher levels of overexpression may stimulate either ERAD or proteasomal degradation after removal from the oocyte membrane. Finally, our data suggest correlation, but not absolute congruence, between mENaC functional and surface expression. These data are therefore consistent with the changes in functional expression, with Hsc70 and Hsp70 overexpression being due at least in part to chaperone-induced alterations in the intracellular trafficking and surface expression of mENaC. These minor discrepancies may be the result of batch-to-batch variability in oocytes, which we have tried to account for by normalizing our data, as well as the potentially nonlinear nature of the chemiluminescence surface expression assay compared with TEV. These data do not rule out alterations in mENaC Po or unitary conductance, but subtle changes in Po can be difficult to ascertain because Po can vary widely in ENaC (32).
An alternate potential explanation of this minor discordance arises from recent observations that proteolysis of the ENaC α and γ subunit extracellular domains enhances the Po of ENaC. Such proteolysis can occur within cells by the Golgi resident endopeptidase, furin (33), by the channel-activating proteases (34), or by exogenous proteases, such as trypsin and elastase (35, 36). In this case, chaperone overexpression might modulate the proportion of cleaved/active channels that reach the oocyte membrane as well as the total amount of channel that is trafficked to the membrane. Our experiments, which monitored whole-oocyte and surface expression of the β subunit, are insensitive to this potential mechanism.
In summary, we have demonstrated biochemically and functionally that Hsc70 and Hsp70 have different, nonequivalent, and antagonistic effects on the functional and surface expression and therefore on the intracellular trafficking of mENaC in Xenopus oocytes. These data contradict the assumption that these proteins are functionally interchangeable. Further studies are required to determine the mechanisms underlying these differential effects and whether these effects are also observed in epithelial cells.
Materials and Methods
Except as noted, reagents were purchased from Fisher Chemical, Fair Lawn, NJ.
Xenopus Oocyte Expression.
cDNAs for human Hsc70 (9), human Hsp70 (H. Wong, University of Cincinnati) (37), human Hdj2 (D. Cyr, University of North Carolina, Chapel Hill) (8), and the α, β, and γ subunits of mENaC (38, 39) have been described previously. These proteins were expressed in Xenopus oocytes essentially as described previously (21, 22, 40, 41). cRNAs were prepared by using a cRNA synthesis kit (mmessage mmachine, Ambion). cRNA concentrations were determined spectroscopically. Oocytes were obtained from adult female X. laevis (nasco or Xenopus Express, Plant City, FL) by using protocols approved by the Institutional Animal Care and Use Committees at Children's Hospital of Philadelphia and the University of Pittsburgh. Stage V and VI oocytes were defolliculated enzymatically in 2 mg/ml type IV collagenase and maintained at 18°C in modified Barth's saline [MBS: 88 mM NaCl/1 mM KCl/2.4 mM NaHCO3/0.3 mM Ca(NO3)2/0.41 mM CaCl2/0.82 mM MgSO4/15 mM Hepes, pH 7.6, containing 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulfate, and 100 μg/ml gentamicin sulfate]. Oocytes were injected with cRNAs dissolved in 50 nl of RNase-free water by using a Nanoject II microinjector (Drummond Scientific, Broomall, PA).
Electrophysiological Analyses.
Whole-oocyte current measurements were performed by using the TEV method at room temperature with a DigiData 1320 interface and Axon GeneClamp 500B amplifier (Axon Instruments, Union City, CA) as described previously (39–41). Twenty-four hours after injection, oocytes were placed in a 1-ml chamber containing modified ND96 (96 mM NaCl/1 mM KCl/0.2 mM CaCl2/5.8 mM MgCl2/10 mM Hepes, pH 7.4) and impaled with micropipettes of 0.5- to 5-MΩ resistance filled with 3 M KCl. A series of voltage steps (1 s) from −140 to +60 mV (adjusted for resting membrane potential) in 20-mV increments were performed, and whole-cell currents were recorded 750 ms after initiation of the −100-mV voltage step for data analysis. Data were acquired at 200 Hz, and analyses were performed by using pclamp 8.0 or 8.1 software (Axon Instruments) on 833-MHz Pentium III computers (Dell Computer). mENaC-mediated current, or functional expression, was defined as the difference in whole-oocyte current at −100 mV holding potential (adjusted for resting membrane potential) before and after the addition of 10 μM amiloride·HCl (Sigma) to the bath solution.
Cell-Surface Expression.
Surface expression of mENaC in oocytes was assessed by using a chemiluminescence assay (20, 21, 42). Oocytes were injected with αβγ-mENaC (0.33 ng per subunit), where the β subunit contained a FLAG epitope in the extracellular loop (βFLAG) either alone or coinjected with 1, 10, or 30 ng of either human Hsc70 or human Hsp70 cRNA. For control assays, oocytes were injected with either 10 ng of Hsc70 or Hsp70 cRNA alone or with cRNA encoding non-epitope-tagged WT αβγ-mENaC (0.33 ng per subunit); 18–24 h after injection, the oocytes were fixed in 4% formaldehyde in MBS for 20 min at 4°C. Nonspecific binding was blocked with MBS containing 10 mg/ml BSA (MBS–BSA) at 4°C overnight. The primary antibody was anti-FLAG M2 mouse monoclonal (0.8 μg/ml, Sigma), and the secondary antibody was peroxidase-conjugated AffiniPure F(ab′)2 fragment goat anti-mouse IgG (0.9 μg/ml, Jackson ImmunoResearch). Both were applied in MBS–BSA for 1–2 h at 4°C. Oocytes were washed extensively (12 times over 2 h) at 4°C and transferred to MBS without BSA. Single-oocyte chemiluminescence was revealed with 100 μl of SuperSignal ELISA Femto (Pierce) and quantified for 1 min in a TD-20/20 luminometer (Turner, Torrance, CA).
Immunoblotting.
Oocytes were injected with αβγ-mENaC (0.33 ng per subunit) and increasing amounts (1, 10, and 30 ng) of cRNA for Hsc70 or Hsp70; in these experiments, the β-mENaC subunit had a V5 epitope at its C terminus (43). Twenty-four hours after injection, oocytes (10 per group) were lysed in equal volumes of 0.15 M NaCl/0.01 M Tris·Cl, pH 8.0/0.01 M EDTA/1.0% Nonidet P-40/0.5% sodium deoxycholate/1.0 mM phenylmethanesulfonyl fluoride/0.1 mM Nα-(p-tosyl)lysine chloromethyl ketone/0.1 mM l-1-tosylamido-2-phenylethyl chloromethyl ketone/2 μg/ml aprotinin for 1 h at 4°C and centrifuged at 13,000 × g for 15 min at 4°C. Equal volumes of protein lysates were resolved on SDS/8% polyacrylamide gels and transferred to nitrocellulose, and nonspecific binding was blocked by overnight incubation with PBS and 5% nonfat dry milk at 4°C. Immunodetections were performed by using specific antisera for the V5 epitope (Invitrogen), Hsp70, or Hsc70 (Stressgen). Immunoreactivity was revealed with horseradish peroxidase-conjugated secondary antibodies and the ECL reagent (Amersham Pharmacia).
Rates of mENaC Exocytosis and Endocytosis.
We assessed the rate of mENaC exocytosis as described previously (23, 44). Oocytes were injected with αβγG542C-mENaC either alone or with 10 ng of cRNA for either Hsc70 or Hsp70; 24–36 h after injection, whole-oocyte 100 μM benzamil-sensitive currents were determined by TEV. Whole-oocyte currents were then monitored after a 5-min perfusion of 1 mM MTSET (Toronto Research Chemicals, Downsview, ON, Canada) every minute for 10 min after removal of MTSET from the perfusion buffer and after reapplication of benzamil. The initial rates of reappearance of benzamil-sensitive currents were determined from the linear portion of the current recovery curve (0–2 min).
To assess the rate of mENaC endocytosis, oocytes were injected with cRNAs for αβγ-mENaC alone or together with 10 ng of cRNA for either Hsc70 or Hsp70. Then 24–36 h after injection, the amiloride-sensitive current was determined by TEV before and after 2, 4, and 6 h of incubation with 5 μM brefeldin A. Amiloride-sensitive currents were expressed relative to the initial amiloride-sensitive current, and half-lives for the decline of amiloride-sensitive current were determined.
Statistical Analyses.
Whole-cell amiloride-sensitive current data are expressed relative to those of oocytes injected with mENaC alone. To decrease the influence of batch-to-batch variability in ENaC expression, data were normalized by the mean amiloride-sensitive current within a batch of oocytes before combining data of multiple independent batches for statistical analysis. Cell surface expression data were similarly normalized to the mean αβFLAGγ-mENaC luminescence within a batch before combining data of multiple independent batches for statistical analysis. All data are presented as the mean ± SEM, and P values were determined by a one-way ANOVA. A P value of ≤0.05 was considered significant. All statistical analyses were performed with sigmastat version 2.03.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54354 (to R.C.R.), DK65161 (to T.R.K.), DK66883 (to O.B.K.), and HD043245 (to S.B.G.). R.C.R. is an Established Investigator of the American Heart Association.
Abbreviations
- αβγ-mENaC
α, β, and γ subunits of murine epithelial sodium channel
- βFLAG
β subunit with external FLAG epitope
- β-V5
β subunit with C-terminal V5 epitope
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial sodium channel
- ER
endoplasmic reticulum
- mENaC
murine epithelial sodium channel
- MTSET
[2-(trimethylammonium)ethyl] methanethiosulfonate bromide
- NS
not significant
- TEV
two-electrode voltage clamp.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
‖Goldfarb, S. B. & Rubenstein, R. C. (2003) Am. J. Respir. Crit. Care. Med. 167, 914 (abstr.).
References
- 1.Stutts M. J., Canessa C. M., Olsen J. C., Hamrick M., Cohn J. A., Rossier B. C., Boucher R. C. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 2.Ling B. N., Zuckerman J. B., Lin C., Harte B. J., McNulty K. A., Smith P. R., Gomez L. M., Worrell R. T., Eaton D. C., Kleyman T. R. J. Biol. Chem. 1997;272:594–600. doi: 10.1074/jbc.272.1.594. [DOI] [PubMed] [Google Scholar]
- 3.Knowles M. R., Paradiso A. M., Boucher R. C. Hum. Gene Ther. 1995;6:445–455. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- 4.Matsui H., Grubb B. R., Tarran R., Randell S. H., Gatzy J. T., Davis C. W., Boucher R. C. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 5.Ward C. L., Kopito R. R. J. Biol. Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- 6.Varga K., Jurkuvenaite A., Wakefield J., Hong J. S., Guimbellot J. S., Venglarik C. J., Niraj A., Mazur M., Sorscher E. J., Collawn J. F., et al. J. Biol. Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 8.Meacham G. C., Lu Z., King S., Sorscher E., Tousson A., Cyr D. M. EMBO J. 1999;18:1492–1505. doi: 10.1093/emboj/18.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein R. C., Zeitlin P. L. Am. J. Physiol. 2000;278:C259–C267. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- 10.Bercovich B., Stancovski I., Mayer A., Blumenfeld N., Laszlo A., Schwartz A. L., Ciechanover A. J. Biol. Chem. 1997;272:9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- 11.Fuller W., Cuthbert A. W. J. Biol. Chem. 2000;275:37462–37468. doi: 10.1074/jbc.M006278200. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein R. C., Egan M. E., Zeitlin P. L. J. Clin. Invest. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentijn J. A., Fyfe G. K., Canessa C. M. J. Biol. Chem. 1998;273:30344–30351. doi: 10.1074/jbc.273.46.30344. [DOI] [PubMed] [Google Scholar]
- 14.Staub O., Gautschi I., Ishikawa T., Breitschopf K., Ciechanover A., Schild L., Rotin D. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik B., Schlanger L., Al Khalili O., Bao H. F., Yue G., Price S. R., Mitch W. E., Eaton D. C. J. Biol. Chem. 2001;276:12903–12910. doi: 10.1074/jbc.M010626200. [DOI] [PubMed] [Google Scholar]
- 16.Gething M.-J., Sambrook J. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 17.Chiang H.-L., Terlecky S. R., Plant C. P., Dice J. F. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 18.Choo-Kang L. R., Zeitlin P. L. Am. J. Physiol. 2001;281:L58–L68. doi: 10.1152/ajplung.2001.281.1.L58. [DOI] [PubMed] [Google Scholar]
- 19.Kellenberger S., Gautschi I., Rossier B. C., Schild L. J. Clin. Invest. 1998;101:2741–2750. doi: 10.1172/JCI2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerangue N., Schwappach B., Jan Y. N., Jan L. Y. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 21.Samaha F. F., Rubenstein R. C., Yan W., Ramkumar M., Levy D. I., Ahn Y. J., Sheng S., Kleyman T. R. J. Biol. Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- 22.Yan W., Samaha F. F., Ramkumar M., Kleyman T. R., Rubenstein R. C. J. Biol. Chem. 2004;279:23183–23192. doi: 10.1074/jbc.M402373200. [DOI] [PubMed] [Google Scholar]
- 23.Snyder P. M., Olson D. R., Bucher D. B. J. Biol. Chem. 1999;274:28484–28490. doi: 10.1074/jbc.274.40.28484. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., Janich S., Cohn J. A., Wilson J. M. Proc. Natl. Acad. Sci. USA. 1993;90:9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abravaya K., Myers M. P., Murphy S. P., Morimoto R. I. Genes Dev. 1992;6:1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 26.Wilson G. M., Sutphen K., Bolikal S., Chuang K. Y., Brewer G. J. Biol. Chem. 2001;276:44450–44456. doi: 10.1074/jbc.M108521200. [DOI] [PubMed] [Google Scholar]
- 27.Laroia G., Cuesta R., Brewer G., Schneider R. J. Science. 1999;284:499–502. doi: 10.1126/science.284.5413.499. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman R. J. J. Clin. Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickland E., Qu B. H., Millen L., Thomas P. J. J. Biol. Chem. 1997;272:25421–25424. doi: 10.1074/jbc.272.41.25421. [DOI] [PubMed] [Google Scholar]
- 30.Weisz O. A., Wang J. M., Edinger R. S., Johnson J. P. J. Biol. Chem. 2000;275:39886–39893. doi: 10.1074/jbc.M003822200. [DOI] [PubMed] [Google Scholar]
- 31.Shimkets R. A., Lifton R. P., Canessa C. M. J. Biol. Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 32.Chalfant M. L., Peterson-Yantorno K., O'Brien T. G., Civan M. M. Am. J. Physiol. 1996;271:F861–F870. doi: 10.1152/ajprenal.1996.271.4.F861. [DOI] [PubMed] [Google Scholar]
- 33.Hughey R. P., Bruns J. B., Kinlough C. L., Harkleroad K. L., Tong Q., Carattino M. D., Johnson J. P., Stockand J. D., Kleyman T. R. J. Biol. Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 34.Vuagniaux G., Vallet V., Jaeger N. F., Hummler E., Rossier B. C. J. Gen. Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caldwell R. A., Boucher R. C., Stutts M. J. Am. J. Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell R. A., Boucher R. C., Stutts M. J. Am. J. Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 37.Wong H. R., Menendez I. Y., Ryan M. A., Denenberg A. G., Wispe J. R. Am. J. Physiol. 1998;275:L836–L841. doi: 10.1152/ajplung.1998.275.4.L836. [DOI] [PubMed] [Google Scholar]
- 38.Sheng S., Li J., McNulty K. A., Avery D., Kleyman T. R. J. Biol. Chem. 2000;275:8572–8581. doi: 10.1074/jbc.275.12.8572. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Q., Li J., Dubroff R., Ahn Y. J., Foskett J. K., Engelhardt J., Kleyman T. R. J. Biol. Chem. 2000;275:13266–13274. doi: 10.1074/jbc.275.18.13266. [DOI] [PubMed] [Google Scholar]
- 40.Suaud L., Li J., Jiang Q., Rubenstein R. C., Kleyman T. R. J. Biol. Chem. 2002;277:8928–8933. doi: 10.1074/jbc.M111482200. [DOI] [PubMed] [Google Scholar]
- 41.Suaud L., Carattino M., Kleyman T. R., Rubenstein R. C. J. Biol. Chem. 2002;277:50341–50347. doi: 10.1074/jbc.M209641200. [DOI] [PubMed] [Google Scholar]
- 42.Yoo D., Kim B. Y., Campo C., Nance L., King A., Maouyo D., Welling P. A. J. Biol. Chem. 2003;278:23066–23075. doi: 10.1074/jbc.M212301200. [DOI] [PubMed] [Google Scholar]
- 43.Hughey R. P., Mueller G. M., Bruns J. B., Kinlough C. L., Poland P. A., Harkleroad K. L., Carattino M. D., Kleyman T. R. J. Biol. Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 44.Carattino M. D., Hill W. G., Kleyman T. R. J. Biol. Chem. 2003;278:36202–36213. doi: 10.1074/jbc.M300312200. [DOI] [PubMed] [Google Scholar]