Abstract
Many animals advertise their chemical defense to predators with conspicuous coloration and unpalatability, but little is known about the information in these signal elements. To effectively avoid predation, is it more advantageous to invest in increased conspicuousness or greater noxiousness, or to allocate equally to both signal modalities? Using natural variation among poison frog species measured with spectral reflectance and toxicity assays, we tested the relative importance of warning signal components with predator-learning and avoidance experiments. We demonstrate that closely related species use alternative strategies: increasing either conspicuousness or toxicity affords equivalent avoidance by predators and provides protection to nontoxic mimic species. These equally effective predator avoidance tactics demonstrate different aposematic solutions for two potentially costly signal components, providing a mechanism for natural diversity in warning signals.
Keywords: aposematism, chemical defense, predation
Escaping predation is essential to survival for most animals and has resulted in the evolution of an amazing diversity of predator avoidance tactics. Conspicuous coloration advertises antipredator defense across many taxa, including invertebrates, fish, amphibians, snakes, and birds (1, 2). Such aposematic, or warning, signals are effective when predators associate color pattern with unprofitability and avoid the diagnostic coloration in subsequent encounters. Greater toxicity of brightly colored prey leads to faster avoidance learning by predators (3) and is thought to be proportional to the reduction in attack probability at each encounter (4). Similarly, predators learn faster to associate conspicuous, relative to cryptic, patterns with unpalatability (5–7). No study, however, has empirically evaluated the relative importance of these two components of aposematism, conspicuousness and unpalatability, for avoiding attack by predators. Do species avoid predation by investing in increased conspicuousness or greater noxiousness, or do they allocate equally to both signal modalities? Here, we directly test the relative effectiveness of different combinations of warning signal components using natural variation among poison frog species.
Poison frogs (Dendrobatidae) display some of the most diverse warning signals in nature. Phylogenetic analyses indicate that an incredible variety of color combinations has arisen multiple times from cryptic ancestors in dendrobatid frogs (8, 9). To test the relative benefits of warning signal components, we exploited this natural variation in poison frogs from Ecuadorian Amazonia using three closely related model species that differ in coloration and toxicity, as well as species in a nontoxic clade of putative mimics (10). We tested the efficacy of this putative Batesian mimicry, as well as examined effects of the model’s warning signal for protection afforded to each mimic.
We quantified interspecific variation among aposematic signal components, unpalatability and conspicuousness, using toxicity assays and spectral reflectance. In contrast to the expectation that the most conspicuous species will be the most noxious, we found the most toxic species is only moderately conspicuous, and the most conspicuous species shows only moderate toxicity (11). A diversity of skin alkaloids, which confer noxiousness, exists across poison frogs (12–14). We assessed species’ relative toxicity using an assay of injection of frog skin extract into laboratory mice (3, 12) and found significant interspecific variation. Conspicuousness is a function of a particular viewer’s sensory system (15) and, in aposematism, the most important viewer is the predator. Although accounts of predation on poison frogs are scarce, birds are potential predators (16–18). Accordingly, we evaluated conspicuousness of the three color patterns from a bird’s eye view using an avian visual model that evaluates conspicuousness as a combination of color and brightness contrast (refs. 18 and 19; see Materials and Methods). In increasing order of conspicuousness, the three color patterns examined are “yellow only,” “red only,” and “red + yellow.” Each color pattern is found in a species of noxious Epipedobates and nontoxic Allobates (Fig. 1). The unexpected pattern of variation that we uncovered in aposematic features allowed us to conduct controlled comparisons of the relative importance of conspicuousness and toxicity for warning signal effectiveness (i.e., one pair that differs significantly in conspicuousness but not in toxicity, and another pair that differs significantly in toxicity but not in conspicuousness).
Fig. 1.
Conspicuousness of poison frog species as viewed by a potential avian predator. E. bilinguis (n = 16) and sympatric A. zaparo Y (n = 15) have a mostly red granular dorsum with yellow blotches in axilla and groin regions (red + yellow); E. parvulus (n = 16) and A. zaparo no Y (n = 12) have a red dorsum but lack the yellow regions (red only); and E. hahneli (n = 10) and A. femoralis (n = 11) have a dark brownish dorsum with the yellow blotches in the axilla and groin (yellow only). The y axis is color contrast (ΔS = spectral discrimination), and the x axis is brightness contrast (ΔL = long wavelength sensitivity cone contrast) as computed using frog color radiances in an avian visual model (18, 19). Conspicuousness is based on dorsal internal contrast comparing head, back, axilla, and groin areas to side body accounting for the relative body area for each color patch. Ellipses show 95% confidence intervals for each species; the ellipse of each mimic (gray) overlaps with each respective model species (black). Phylogeny of Dendrobatidae is adapted from ref. 8.
We took advantage of this measured variation in conspicuousness and toxicity to examine the comparative saliency of warning signal components to predators and to test the effectiveness of mimetic convergence. We conducted predator learning and avoidance experiments using live frogs and naïve chicken predators. Predators were exposed to one of three learning stimuli in a series of learning trials: (i) high conspicuousness, moderate toxicity (Epipedobates bilinguis); (ii) moderate conspicuousness, high toxicity (Epipedobates parvulus); or (iii) moderate conspicuousness, moderate toxicity (Epipedobates hahneli). The degree to which predators avoid the aposematic individuals was assessed with pre- and post-learning choice trials. We then investigated whether convergence on the toxic Epipedobates conspicuous coloration by nontoxic Allobates is effective for escaping predation: are these true Batesian mimics? This research experimentally tests the relative importance of the two components of aposematism for avoiding attack by predators, providing insight into different strategies of relative investment (11) and yielding testable predictions for the evolution of warning signal diversity.
Results
Variation in Unpalatability.
Relative unpalatability of poison frog species was assessed by using a toxicity assay, because a quantitative assay for oral noxiousness does not exist. Species’ relative toxicity was measured by using a standard protocol of s.c. injection of frog skin extract into laboratory mice (3, 12). Time to recovery from injection of E. parvulus skin extract was significantly greater than that of either E. bilinguis or E. hahneli skin extract (see Table 1, which is published as supporting information on the PNAS web site; n = five mice per treatment; Kruskal–Wallis test; Zparvulus-bilinguis = 2.507, two-tailed P = 0.012; Zparvulus-hahneli = 2.507, P = 0.012). The recovery times from injection of the less toxic species skin extracts were not significantly different from one another (Zbilinguis-hahneli = −1.571, P = 0.116). Injection of Allobates zaparo Y, A. zaparo no Y, and Allobates femoralis skin extract caused no adverse reaction (no difference among reactions from A. zaparo and A. femoralis skin extracts and saline control injections; ANOVA, P = 0.535). These results demonstrate variation in chemical defense among Epipedobates species and confirm the absence of alkaloids in Allobates (3, 14, 20), suggesting an adaptive function for color pattern convergence (Fig. 1).
Variation in Conspicuousness.
Darst and Cummings (3) demonstrated color pattern convergence by the two color morphs of A. zaparo (Y and no Y) to geographically localized models (E. bilinguis in the north and E. parvulus in the south). By converging on a toxic model’s color pattern, the mimic is ultimately adopting the model’s degree of visual salience (conspicuousness). We evaluated conspicuousness of the three color patterns (red only, yellow only, and red + yellow) from a bird’s eye view using an avian visual model that evaluated conspicuousness as a combination of color and brightness contrast (18, 19). We calculated conspicuousness as the dorsal internal contrast comparing head, back, axilla, and groin areas to side body accounting for the relative body area for each color patch (Fig. 1). Hence, both color and brightness contrast (ΔS and ΔL, Fig. 1) are weighted functions of the relative body area for each color patch, producing a measure of whole body conspicuousness that is more appropriate than single patch comparisons (21). Total conspicuousness was evaluated as vector distance in a perceptual space (i.e., Euclidean distance; see Table 1). We found that conspicuousness varies across species, and that each nontoxic Allobates has converged on the conspicuousness of a toxic sympatric Epipedobates species (Fig. 1; Kruskal–Wallis test; Zparvulus-zaparo no Y = −1.319, P = 0.187; Zbilinguis-zaparo Y = −0.184, P = 0.854; Zhahneli-femoralis = 0, P = 1.00). E. bilinguis, with both red and yellow color elements, is the most conspicuous of the toxic frogs, followed by E. parvulus and E. hahneli, each with single color elements, which do not differ significantly from one another in conspicuousness (Fig. 1; Zbilinguis-parvulus = −4.336, P < 0.001; Zbilinguis-hahneli = −4.005, P < 0.001; Zparvulus-hahneli = −1.606, P = 0.108).
We found that the most toxic species, E. parvulus (red only), is not the most conspicuous, whereas the most conspicuous species, E. bilinguis (red + yellow), shows only moderate toxicity. E. hahneli (yellow only), displays moderate levels of both signal components (Fig. 1). This unexpected pattern of variation allows for controlled comparisons of the relative importance of conspicuousness and toxicity for warning signal effectiveness (i.e., E. bilinguis and E. hahneli, which differ significantly in conspicuousness but not in toxicity; and E. parvulus and E. hahneli, which differ significantly in toxicity but not in conspicuousness). Interestingly, the color patterns of all three brightly colored toxic species are mimicked by a nontoxic Allobates, suggesting Batesian mimicry (Fig. 1).
Effectiveness of Aposematic Signal Components for Avoiding Predation.
We examined the relative contributions of conspicuous coloration and unpalatability to escaping predation with two measures: speed of avoidance learning and degree of avoidance after learning. Predator learning experiments were conducted by using live frogs and naïve chicken predators in which predators were exposed to one of three learning stimuli in a series of learning trials: (i) high conspicuousness, moderate toxicity (E. bilinguis); (ii) moderate conspicuousness, high toxicity (E. parvulus); or (iii) moderate conspicuousness, moderate toxicity (E. hahneli). We found that speed of learning was mediated by toxicity. Predators learned most quickly on the most toxic frog (Fig. 2; n = six chicks per treatment; E. parvulus mean learning slope, 40.33 ± 8.11; E. bilinguis, 18.04 ± 7.4; E. hahneli, 16.60 ± 2.36; Zparvulus-bilinguis = 1.992, P = 0.046; Zparvulus-hahneli = 2.005, P = 0.045). Toxic frogs were rejected with no harm to the predator, suggesting that greater toxicity confers protection through increased unpalatability. Our results also showed that increased conspicuousness had no effect on learning speed: predators learned at similar rates on highly and moderately conspicuous frogs of similar toxicity (Zbilinguis-hahneli = 0.7488, P = 0.810).
Fig. 2.
Predators learn not to attack toxic conspicuous poison frogs over a series of learning trials. Learning proceeded fastest with the most toxic frog (E. parvulus evoked full learning by trial 4.33 ± 0.95 (SE); E. bilinguis, 6.33 ± 0.99; E. hahneli, 6.55 ± 0.56). A learning trial (x axis) consisted of presenting chicks with one of the brightly colored toxic frogs under a glass dome for 1 min or until chicks pecked the dome; the dome was then removed, and latency to peck the stimulus (sampling event) was recorded up until 120 seconds (y axis). Data are mean ± standard deviation (n = six chicks per treatment).
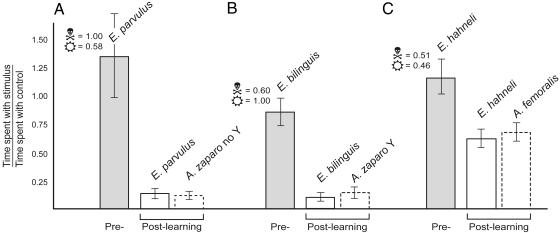
Although the first measure, speed of avoidance learning, is important for protection from predation, the ultimate determination of advantage is the second measure, the degree to which predators avoid aposematic individuals (2). A classic and enduring argument for the advantage of conspicuousness is that bright coloration makes predators less likely to confuse toxic prey with palatable prey, which are typically cryptic (22–24). This argument is particularly applicable when predators do not show innate aversion to bright colored prey, which was the case with our naïve chick predators (pre-learning time spent by chicks in each frog’s quadrant; E. bilinguis 34.17 ± 3.0, Colostethus 40.83 ± 4.9, Zbilinguis-Colostethus = 1.959, P = 0.375; E. parvulus 49.17 ± 6.2, Colostethus 54.16 ± 8.9, Zparvulus-Colostethus = 1.959, P = 1.77; E. hahneli 36.67 ± 7.5, Colostethus 34.17 ± 8.9, Zhahneli-Colostethus = 1.959, P = 0.582). We tested the discriminability hypothesis with the pair of Epipedobates that vary significantly in conspicuous coloration but not in toxicity (E. bilinguis and E. hahneli). The degree to which predators avoid the aposematic individuals was assessed with post-learning choice trials. Having learned to associate conspicuous coloration with unpalatability, educated predators were given both a cryptic nontoxic dendrobatid (Colostethus awa) as a control and the conspicuous toxic frog with which the predator had been trained. As predicted (22–24), greater conspicuousness of E. bilinguis resulted in significantly greater avoidance by educated predators (Fig. 3; time spent with stimulus frog/time spent with control: E. bilinguis as stimulus, 0.12 ± 0.03; E. hahneli, 0.44 ± 0.34; Zbilinguis-hahneli = 2.732, P = 0.006). High toxicity with moderate conspicuousness proved to be an equally effective combination. E. parvulus, the most toxic species, garnered the same degree of avoidance as the more conspicuous E. bilinguis (Fig. 3; E. parvulus, 0.13 ± 0.03; Zparvulus-bilinguis = −0.161, P = 0.872; Zparvulus-hahneli = −2.566, P = 0.010). Hence, high toxicity with moderate conspicuousness and moderate toxicity with high conspicuousness are equally successful signal component combinations for achieving effective predator avoidance.
Fig. 3.
Educated predators avoid the toxic conspicuous Epipedobates species and their respective Allobates mimics. The y axis represents the relative time spent by predators with the brightly colored frog (stimulus) in pre- and post-learning choice trials (x axis) (data are mean ± SE; n = six chicks per treatment); significance was measured comparing pre- to post-learning avoidance (in all cases: Z = 2.802, two-tailed P < 0.005). Skull-and-crossbones icons represent relative toxicity; sun icons represent relative conspicuousness. (A–C) Chicks spent significantly less post- than pre-learning time with toxic frogs, which is conferred to each respective nontoxic mimic (bars outlined by dashes). (C) The degree of avoidance received by E. hahneli and A. femoralis was significantly less than the degree of avoidance provided to more conspicuous E. bilinguis or more toxic E. parvulus and their mimics (Zhahneli-parvulus = 2.566, two-tailed P = 0.010; Zhahneli-bilinguis = 2.7312, two-tailed P = 0.006).
Batesian Mimicry.
Having demonstrated convergence on toxic frogs’ conspicuous coloration by nontoxic Allobates, we tested whether this mimicry is effective for escaping predation: is convergence on conspicuousness functional Batesian mimicry? We found that the mimics successfully deceive predators. Chick predators trained with each model avoided the respective mimic as well, an empirical confirmation of Batesian mimicry by not one but two closely related species in a distantly related clade. Mimics of either the more toxic or more conspicuous model received the high degree of avoidance afforded to their respective model (Fig. 3; A. zaparo no Y, 0.08 ± 0.01, Zparvulus-zaparo no Y = 1.046, P = 0.295; A. zaparo Y as stimulus, 0.15 ± 0.03, Zbilinguis-zaparo Y = −0.646, P = 0.518). Accordingly, the mimic of the moderately conspicuous and moderately toxic frog received the same moderate degree of avoidance afforded to its model, significantly less than that conferred to A. zaparo Y and no Y (Fig. 3; A. femoralis, 0.46 ± 0.06; Zfemoralis-zaparo Y = −2.802, P < 0.005; Zfemoralis-zaparo no Y = −2.807, P < 0.005).
Discussion
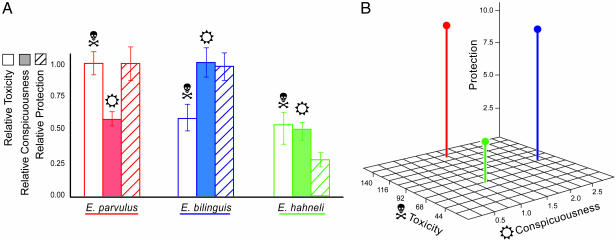
Our results uncover different aposematic solutions to effectively avoid predation that take advantage of the relative benefits of toxicity and conspicuousness. Predators learn more quickly to avoid highly versus moderately toxic prey, whereas an increase in greater conspicuousness does not increase the speed of learning. However, enhancing the complexity of the prey environment with both conspicuous and cryptic prey, the advantage of increased conspicuousness becomes apparent. The benefit of increasing conspicuousness, independent of toxicity, is a significant gain in protection from predation, suggesting that conspicuous coloration helps predators distinguish toxic from palatable prey. We find that poison frog species use different combinations to achieve the same effect; equal protection is achieved with a combination of moderate toxicity and high conspicuousness as with high toxicity and moderate conspicuousness. Our findings reveal equally effective aposematic strategies, providing a mechanism for natural diversity in warning signals (Fig. 4).
Fig. 4.
Conspicuous toxic poison frogs achieve equal protection from predation with different combinations of warning signal components. Skull-and-crossbones icons represent toxicity; sun icons represent conspicuousness. Protection from predation is measured as the ratio of pre- to post-learning time spent with the stimulus frog. (A) E. parvulus achieves equal protection from predation with high toxicity and moderate conspicuousness as E. bilinguis achieves with moderate toxicity and high conspicuousness. Relative toxicity, conspicuousness, and protection are scaled to a maximum of 1.00 (data are mean ± SE; n = six chicks per treatment). (B) The comparative benefits of warning signal components, conspicuousness, and toxicity support alternative strategies for an effective and efficient warning signal. Measured (nonrelative) data for toxicity, conspicuousness, and protection from predation are shown.
Aposematism succeeds when predators associate conspicuousness with unprofitability, and in dendrobatid frogs, multiple origins of conspicuousness are correlated with multiple acquisitions of toxicity (8). During origins of aposematism (evolutionary transitions from cryptic to aposematic signals), a positive correlation between conspicuousness and the strength of defense is predicted (2) and has been reported (25). Our empirical data suggest that after this correlation is achieved, degree of conspicuousness and level of defense may become dissociated and adjusted independently. We find that conspicuousness and unpalatability are decoupled: E. bilinguis and E. hahneli differ significantly in conspicuousness but not in toxicity, whereas E. parvulus and E. hahneli differ significantly in toxicity but not in conspicuousness (Fig. 4). Our results suggest the hypothesis of a tradeoff between the two components of aposematism for effectively and efficiently escaping predation. Theoretical work has anticipated cross-compensation between potentially costly unprofitability and bright coloration, predicting that optimal investment in secondary defense will diminish when more cost-effective conspicuousness evolves as primary defense (11, 26). There will, however, be constraints in how signal components can be adjusted, particularly in cases of Müllerian mimicry and limited genetic variability. Theoretical predictions and our results support a dynamic, complex relationship between signal components that should be further investigated.
The relative costs of increased conspicuousness versus high toxicity remain unknown, although growing empirical evidence indicates that chemical defenses are costly in a variety of circumstances (2). Additionally, complete dissociation of conspicuousness and toxicity in Batesian mimics suggests that if warning coloration can be exploited without investment in noxiousness, then Batesian mimicry is the preferred strategy. The noxious alkaloids in the skin of poison frogs are sequestered from a specialized diet of leaf-litter arthropods (13, 20). An animal that accumulates toxic metabolites not only has to ingest toxic prey (27) but also is restricted to a specialized diet (28). If the cost of either diet specialization or sequestration becomes too great (for example, with change in prey resources), shedding the expense of high toxicity in favor of increased conspicuousness may be a more efficient predator avoidance tactic. This depends, however, on the relative costs of conspicuousness due to acquiring or producing conspicuous pigmentation or simply the cost of increased detectability to predators, which is unknown in poison frogs. Moderate levels of toxicity and conspicuousness may be favored when costs associated with high levels of signal components are disadvantageous and moderate protection is sufficient. Such a selective advantage may occur when a surplus of palatable nontoxic prey is available, and predators, therefore, only rarely resort to moderately toxic prey (29). Thus, the fitness benefits of moderate toxicity and moderate conspicuousness may depend upon the availability of alternative, nontoxic prey, which generates predictions that are testable in the field.
Warning coloration would initially be favored only after the acquisition of chemical defense, suggesting that conspicuous mutants arise from defended cryptic species (30, 31). New aposematic forms, therefore, will be selected against because of their conspicuousness and rarity (32). Interestingly, in poison frogs, the benefits of signaling may be conferred by individual selection; we found that 79.24% ± 1.78 of frogs sampled survived the attack, i.e., were tasted by the chick and promptly rejected with no harm to the frog (n = 62 sampled frogs). Hence, individuals with novel combinations of the two signal components are able to survive and reproduce, providing greater evolutionary lability in aposematic signals.
Our results demonstrate alternative strategies for combining toxicity and conspicuousness, suggesting that decoupling warning signal components enables effective and efficient predator avoidance and provides a mechanism for the generation and maintenance of diversity in aposematism. We hypothesize a tradeoff between conspicuous coloration and unpalatability in achieving protection from predation (11) and suggest a role for other ecological factors, such as availability of alternative prey. Further information on the relative costs of signal components will improve our understanding of forces that generate variation in aposematism. Our results provide insight into different aposematic solutions of relative investment and yield testable predictions for the evolution of warning signal diversity.
Materials and Methods
Collection.
Dendrobatid frogs were collected in the Amazonian lowland rainforest and Western Andean slopes of Ecuador, January–May 2003, 2004, and 2005. The five collection sites were Estación Científica Yasuní, Francisco de Orellana Province (E. hahneli and A. femoralis); Estación Biológica Jatun Sacha, Napo Province (E. bilinguis and A. zaparo Y); Río Santiago, ≈1 km east of Santiago, Morona-Santiago Province (E. parvulus and A. zaparo no Y); and Río Toachi, ≈2 km north of La Unión del Toachi, Pichincha Province (C. awa). Taxonomy follows (http://research.amnh.org/herpetology/amphibia/index.php).
Unpalatability.
Five frogs from each A. femoralis, A. zaparo Y, A. zaparo no Y, E. bilinguis, E. hahneli, and E. parvulus were killed, skinned, and deposited following ref. 20. Toxicity assay methods follow (3). Methanol extracts from individual frog skins were evaporated to dryness and redissolved in sterile saline (≈1 ml of saline per skin extract). Resultant alkaloid fractions were s.c. injected into seven treatments of five mice each (3, 12): single-skin extracts of (i) A. femoralis, (ii) A. zaparo Y, (iii) A. zaparo no Y, (iv) E. bilinguis, (v) E. hahneli, (vi) E. parvulus, or (vii) saline-control injection. Each mouse was injected with extract of one frog skin or saline control (n = 35 mice, International Care and Use Committee no. 03110501). Sleeping behavior was used as a baseline for all toxicity assays. Mice were awakened with injection, and time to complete recovery (return to sleep) was recorded. Mouse recovery time after injection was used to estimate degree of toxicity. Skin extracts were only marginally “toxic,” given that only one mouse died in our assay (death was from injection of an E. parvulus skin extract; because this mouse did not yield a time to recovery, its data were removed from the analysis). Thus, we use the term “toxicity” to refer to relative irritant effect of frog skin alkaloids and as a proxy for unpalatability. We used a Kruskal–Wallis nonparametric test for all comparisons among recovery times, and ANOVA was used to compare recovery times among groups.
Conspicuousness.
Eighty poison frogs were collected and transported to Museo de Zoología, Pontificia Universidad Católica del Ecuador for reflectance measurements (Fig. 1). Spectral reflectances were measured by using an Ocean Optics (Dunedin, FL) PS2000 spectrometer, full spectrum light source (DT-1000), Spectralon white standard, and reflectance probe (R400-7) at 2-mm distance from eight body regions: head, dorsum, left and right axillas, groins, and flanks (side body), with two measurements per region. Twenty samples of leaf litter found near or upon where frogs were first sighted were collected. Spectral reflectances of leaf-litter background were measured by using the same protocol as above. Habitat spectral irradiance measurements were collected at 0900 h on 9 different days with the PS2000 and cosine collector connected to a 400-μm fiber optic. Frog and background radiance estimates were computed as the product of spectral reflectances and average habitat irradiance spectrum for all locations.
To evaluate the conspicuousness of the different color patterns, we used a passerine tetrachromatic visual model following ref. 19 that includes both a chromatic (color) and achromatic (brightness) channel, as in ref. 18. The avian vision model was used to describe color (ΔS) and brightness (ΔL) discrimination, where vision is limited by photoreceptor noise. The model begins with photoreceptor photon capture (cone quantum catch), Qc, which represents a certain level of excitation for cone class, c, while viewing target, t, stimuli under specific irradiance measurements: Qc = Σλ = 300700 Ii(λ)Rt(λ)Ac(λ). Cone quantum catch of target radiances, Qc, is evaluated as the summed product of illuminating irradiance Ii(λ); target reflectance, Rt(λ); and the absorptance spectrum (including ocular or screening pigments where appropriate), Ac(λ), for a given photoreceptor cone class c. These photon capture responses are then adjusted for the adapting background light through a process known as the von Kries transformation, where qc = kc Qc, and kc = 1/Σλ = 300700 Ib(λ)Ac(λ) where Ib(λ) is the irradiance of the adapting visual background.
The next stage in this visual model assumes that photoreceptor adaptation follows the Weber–Fechner laws (19, 33), where the signal of each cone channel is proportional to the logarithm of the background adjusted quantum catch: fc = ln (qc) Color differences between frog body color reflectances were evaluated as the receptor (cone class) channel differences normalized by noise in each receptor channel [e.g., Δfc = ln(qL(red back)) –ln(qL(side body))]. Noise in each receptor channel, ωc, is assumed to be independent of quantal fluctuations and was set by the relative number of receptor types within a typical avian receptive field (ωU = 1.0; ωS = 0.857; ωM = 0.520; ωL = 0.515; where U, UV sensitive; and S, short-wave sensitive; cone proportions are from ref. 34).
The spectral distance, ΔS, or the distance separating two spectra in perceptual space is defined as
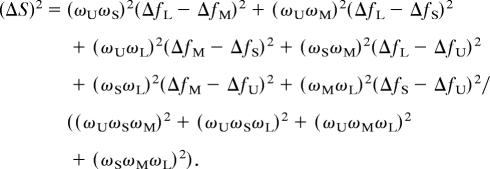 |
Brightness contrast, or the achromatic processing channel, of the avian visual system is considered to be a function of the double cone class that represents the absorption spectra of long-wavelength sensitivity (LWS) cone photoreceptors (18). Brightness for the potential bird predators in this system was therefore calculated for LWS cones (L = fL), and brightness contrast estimates, ΔL, were evaluated as the absolute difference between two color elements: ΔL = (L1 –L2)/ωL).
We evaluated frog conspicuousness as internal contrast viewed dorsally (e.g., by an avian predator) in terms of spectral (ΔS) and brightness (ΔL) contrast by comparing head, back, axilla, and groin areas to flanks (side body) accounting for the relative body area of each color patch. We used photographs of model frogs viewed from above to estimate the percent body area of each color patch in adobe photoshop (Adobe Systems, San Jose, CA), with head and dorsal regions accounting for 88% and remaining areas 12%. Hence, each ΔS and ΔL is a weighted function of the relative body area for each color patch, producing a measure of whole body conspicuousness that is more appropriate than single patch comparisons (21). Conspicuousness viewed from above was evaluated as the Euclidean distance of color and brightness contrast, E = , producing vector distance in a perceptual space. Confidence ellipses (95%) were calculated for each species (Fig. 1; E. parvulus, n = 24; A. zaparo no Y, n = 11; E. bilinguis, n = 19; A. zaparo Y, n = 17; E. hahneli, n = 10; and A. femoralis, n = 12). We used a Kruskal–Wallis nonparametric test for all comparisons among Euclidean distances.
Predator Learning Experiments.
Predator learning experiments generally followed methods as described in ref. 3. Although few data exist, birds may be potential poison frog predators (16–18). Thus, in Quito, Ecuador, we conducted a series of learning experiments using ≈1-mo-old domestic chickens (Gallus gallus domesticus) as naïve model predators (35) and wild-caught dendrobatids (toxic species, E. bilinguis, E. hahneli, and E. parvulus; nontoxic species, A. femoralis, A. zaparo Y, and A. zaparo no Y). Birds were tested individually in a 1-m2 dirt-floor test arena of four 50-cm2 quadrants outside, under natural lighting conditions. Chickens were fed chicken mash and cracked corn twice daily and water ad libitum. We assessed Allobates palatability by presenting nine naïve chickens an Allobates (three A. femoralis, three A. zaparo Y, and three A. zaparo no Y). Naïve chickens readily ate all Allobates and control frogs (Colostethus awa). We assessed the effects of conspicuousness on innate predator behavior (baseline) with pre-learning choice experiments in which the brightly colored learning stimulus species was paired with a cryptic control frog (C. awa). Chicks were presented with both the brightly colored frog and control frog, each under a glass dome, for 2 min; time spent in each dome’s test-arena quadrant was recorded.
We had three experimental groups (six chicks each), differing in learning stimulus species (E. bilinguis, E. hahneli, or E. parvulus), in eight learning trials (Institutional Care and Use Committee no. 04071901). A learning trial consisted of presenting a chick with a learning stimulus under a glass dome for 1 min or until the chick pecked the dome. The dome was then removed, and latency to peck the stimulus was recorded up to 2 min or until first peck (sampling event) (Fig. 2). A typical sampling event involved the chick grabbing the frog in its beak and spitting the frog out. Only one chick fully ingested a poison frog (E. bilinguis). This animal died 3 days later, and its data were therefore removed from the experiment. We defined learning rate as the slope (latency to peck per no. of trials) until full learning (no subsequent sampling in further trials). Learning slopes were compared by using a Kruskal–Wallis test. Control frogs were presented to chicks after trials nos. 2 and 6 to ensure chicks were still motivated to eat frogs.
After training was complete, degree of avoidance was assessed in two choice experiments, one that paired the control frog with the toxic learning-stimulus model (the same choice as in the pre-learning trial), and the second that paired the control with the appropriate Allobates mimic of the learning stimulus. Chicks were presented with both the brightly colored frog and control frog, each under a glass dome, for 2 min; time spent in each dome’s test-arena quadrant was recorded. Placement of frogs within the test arena was randomized across trials. We assessed degree of learned avoidance by comparing time spent by the predator with the learning stimulus to the time spent with the control frog in post-learning choice trials (Fig. 3). Protection from predation was measured as the ratio of pre-learning (baseline) to post-learning time spent with the stimulus frog (Fig. 4). All comparisons were made by using a Kruskal–Wallis test.
Supplementary Material
Acknowledgments
This work would not have been possible without the generous guidance and support of Luis A. Coloma and Pontificia Universidad Católica del Ecuador. We also thank G. Onore for the use of his back yard to house chickens and conduct predator experiments; M. Domjan for advice regarding predator learning protocols; E. Tapia, S. Ron, J. C. Santos, S. Padilla, M. Bustamante, P. Menéndez-Guerrero, and D. Paucar for assistance in the field; and L. Coloma, J. Daly, M. Ryan, M. Speed, W. C. Funk, G. Pauly, one anonymous reviewer, and the Cannatella and Cummings laboratories for helpful comments on the manuscript. The Ecuadorian Ministerio Ambiente provided research and collection permits 004-IC-FAU-DNBAP/MA (to C.R.D.) and 016-IC-FAU-DNBAP/MA (to Luis A. Coloma). This work was supported by University of Texas graduate fellowships, the Philanthropic Educational Organization, and National Science Foundation Grant 0078150.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Edmunds M. Defence in Animals: A Survey of Anti-Predator Defences. London: Longman; 1974. [Google Scholar]
- 2.Ruxton G. D., Sherratt T. N., Speed M. P. Avoiding Attack: The Evolutionary Ecology of Crypsis, Aposematism, and Mimicry. Oxford, U.K.: Oxford Univ. Press; 2004. [Google Scholar]
- 3.Darst C. R., Cummings M. E. Nature. 2006;440:208–211. doi: 10.1038/nature04297. [DOI] [PubMed] [Google Scholar]
- 4.Turner J. R. G., Kearney E. P., Exton L. W. Biol. J. Linn. Soc. 1984;23:247–268. [Google Scholar]
- 5.Gittleman J. L., Harvey P. H. Nature. 1980;286:149–150. [Google Scholar]
- 6.Roper T. J., Redston S. Anim. Behav. 1987;35:739–747. [Google Scholar]
- 7.Lindström L., Alatalo R. V., Mappes J., Riipi M., Vertainen L. Nature. 1999;397:249–251. [Google Scholar]
- 8.Santos J. C., Coloma L. A., Cannatella D. C. Proc. Natl. Acad. Sci. USA. 2003;100:12792–12797. doi: 10.1073/pnas.2133521100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vences M. J., Kosuch J., Boistel R., Haddad C. F., LaMarca E., Lötters S., Veith M. Org. Div. Evol. 2003;3:215–226. [Google Scholar]
- 10.Bates H. W. Trans. Linn. Soc. London. 1862;23:495–566. [Google Scholar]
- 11.Leimar O., Enquist M., Sillén-Tullberg B. Am. Nat. 1986;128:469–490. [Google Scholar]
- 12.Daly J. W., Myers C. W. Science. 1967;156:970–973. doi: 10.1126/science.156.3777.970. [DOI] [PubMed] [Google Scholar]
- 13.Daly J. W. J. Med. Chem. 2003;46:445–452. doi: 10.1021/jm0204845. [DOI] [PubMed] [Google Scholar]
- 14.Daly J. W., Myers C. W., Whittaker N. Toxicon. 1987;25:1023–1095. doi: 10.1016/0041-0101(87)90265-0. [DOI] [PubMed] [Google Scholar]
- 15.Endler J. A. Biol. J. Linn. Soc. 1990;41:315–352. [Google Scholar]
- 16.Master T. L. Herp. Rev. 1998;29:164–165. [Google Scholar]
- 17.Summers K. Herp. Rev. 1999;30:91. [Google Scholar]
- 18.Siddiqi A., Cronin T. W., Loew E. R., Vorobyev M., Summers K. J. Exp. Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. [DOI] [PubMed] [Google Scholar]
- 19.Vorobyev M., Osorio D., Bennett A. T. D., Marshall N. J., Cuthill C. J. Comp. Physiol. A. 1998;183:621–633. doi: 10.1007/s003590050286. [DOI] [PubMed] [Google Scholar]
- 20.Darst C. R., Menéndez-Guerrero P. A., Coloma L. A., Cannatella D. C. Am. Nat. 2005;165:56–69. doi: 10.1086/426599. [DOI] [PubMed] [Google Scholar]
- 21.Endler J. A., Mielke P. W. Biol. J. Linn. Soc; 2005. pp. 405–431. [Google Scholar]
- 22.Wallace A. R. Proc. Entomol. Soc. London March; 1867. pp. Ixxx–Ixxxi. [Google Scholar]
- 23.Fisher R. A. The Genetical Theory of Natural Selection. Oxford, U.K.: Oxford Univ. Press; 1930. [Google Scholar]
- 24.Sherratt T. N., Beatty C. D. Am. Nat. 2003;162:377–389. doi: 10.1086/378047. [DOI] [PubMed] [Google Scholar]
- 25.Summers K., Clough M. E. Proc. Natl. Acad. Sci. USA. 2001;98:6227–6232. doi: 10.1073/pnas.101134898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speed M. P., Ruxton G. D. Evolution (Lawrence, Kans.) 2005;59:2499–2508. [PubMed] [Google Scholar]
- 27.Duffey S. S. Annu. Rev. Entomol. 1980;25:447–477. [Google Scholar]
- 28.Mebs D. Toxicon. 2001;39:87–96. doi: 10.1016/s0041-0101(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 29.Sherratt T. N., Speed M. P., Ruxton G. D. J. Theor. Biol. 2004;228:217–226. doi: 10.1016/j.jtbi.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Poulton E. B. The Colours of Animals: Their Meaning and Use, Especially Considered in the Case of Insects. Trench, Trübner, London: Kegan Paul; 1890. [Google Scholar]
- 31.Cott H. B. Adaptive Coloration in Animals. London: Methuen; 1940. [Google Scholar]
- 32.Speed M. P., Ruxton G. D. Proc. R. Soc. London Ser. B; 2005. pp. 431–438. [Google Scholar]
- 33.Chiao C.-C., Vorobyev M., Cronin T., Osorio D. Vision Res. 2000;40:3257–3271. doi: 10.1016/s0042-6989(00)00156-5. [DOI] [PubMed] [Google Scholar]
- 34.Hart N. S., Partridge J. C., Cuthill I. C. J. Exp. Biol. 1998;201:1433–1446. doi: 10.1242/jeb.201.9.1433. [DOI] [PubMed] [Google Scholar]
- 35.Osorio D., Vorobyev M., Jones C. D. J. Exp. Biol. 1999;202:2951–2959. doi: 10.1242/jeb.202.21.2951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.