Abstract
Administration of daclizumab, a humanized mAb directed against the IL-2Rα chain, strongly reduces brain inflammation in multiple sclerosis patients. Here we show that daclizumab treatment leads to only a mild functional blockade of CD4+ T cells, the major candidate in multiple sclerosis pathogenesis. Instead, daclizumab therapy was associated with a gradual decline in circulating CD4+ and CD8+ T cells and significant expansion of CD56bright natural killer (NK) cells in vivo, and this effect correlated highly with the treatment response. In vitro studies showed that NK cells inhibited T cell survival in activated peripheral blood mononuclear cell cultures by a contact-dependent mechanism. Positive correlations between expansion of CD56bright NK cells and contraction of CD4+ and CD8+ T cell numbers in individual patients in vivo provides supporting evidence for NK cell-mediated negative immunoregulation of activated T cells during daclizumab therapy. Our data support the existence of an immunoregulatory pathway wherein activated CD56bright NK cells inhibit T cell survival. This immunoregulation has potential importance for the treatment of autoimmune diseases and transplant rejection and toward modification of tumor immunity.
Keywords: CD25, IL-2, immunoregulatory natural killer cells
Multiple sclerosis (MS) is an inflammatory/demyelinating disease of the CNS that is one of the leading causes of neurological disability in young adults (1). It is believed that MS is a T cell-mediated autoimmune disease, and therefore the search for new therapies focuses on agents that affect lymphocyte function. Daclizumab (Zenapax), a humanized mAb that blocks the IL-2 binding site on the IL-2Rα chain, CD25 (i.e., Tac epitope), is among these novel agents (2). The IL-2R complex is comprised of three subunits: IL-2Rα (CD25), IL-2Rβ (CD122), and IL-2Rγ (CD132). CD122 and CD132 have intracellular signaling motifs and together form the intermediate-affinity (Kdis ≈ 0.1–1 nM) IL-2R. CD25 binds IL-2 with low (Kdis ≈ 10 nM) affinity, but when it associates with CD122/CD132 it stabilizes the complex to form the high-affinity (Kdis ≈ 10 pM) receptor (3). CD25 is present at low levels in resting human T cells (with the exception of T regulatory cells) but is significantly up-regulated on activated T cells, enabling them to receive a high-affinity IL-2 signal (4). Therefore, it is believed that the blockade of CD25 will result in selective functional inhibition of activated T cells (5). Although it has been demonstrated that daclizumab (or the original murine anti-Tac mAb) inhibits early IL-2R signal transduction events (6, 7) and blocks T cell activation and expansion in vitro (8), a comprehensive characterization of its in vivo effects is still lacking.
We recently concluded a phase II, open-label, baseline-versus-treatment crossover trial of daclizumab in 10 MS patients with incomplete therapeutic response to IFN-β. Daclizumab showed a profound inhibitory effect on brain inflammatory activity (78% reduction) and subsequent stabilization of disability progression (9). Both the inhibition of brain inflammation by daclizumab and reappearance of inflammation after cessation of the therapy developed gradually over a period of 2–3 months, consistent with the hypothesis that daclizumab induced gradual and prolonged immunomodulatory changes in vivo. Based on these results, we initiated a second trial to test whether the inhibition of brain inflammation is maintained during long-term daclizumab monotherapy. Here we present in vivo observations complemented by in vitro experiments from a total of 22 MS patients from both phase II trials of daclizumab in MS that suggest a mechanism of action of daclizumab via a regulatory circuit between innate and adaptive immune responses that involves the action of immunoregulatory CD56bright natural killer (NK) cells on T cells.
Results
Daclizumab Therapy Has only Marginal Effects on Functional T Cell Responses in Vivo.
To assess T cell functions in daclizumab-treated patients, the average proliferation from two baseline samples (months −2 and 0) was compared with the average of three treatment samples (months 1.5, 3.5, and 5.5) for each patient. We saw no inhibition of T cell proliferation to polyclonal (plate-bound anti-CD3/CD28, IL-2, and IL-15) stimuli when daclizumab was not present in culture media (data not shown). Only modest (≈20%) but significant inhibition of CD4+ (but not CD8+) T cell proliferation was observed when daclizumab was added to culture medium at 10 μg/ml [the peak concentration achieved in vivo at 1 mg/kg every 4 weeks i.v. dosing (10)] (Fig. 4, which is published as supporting information on the PNAS web site). The antiproliferative effect of daclizumab was further analyzed by titrating the TCR stimulus and IL-2 (Fig. 4B and data not shown). In these experiments the inhibition of CD4+ T cell proliferation by daclizumab was most pronounced at lower levels of TCR stimulation, and the inhibitory effect could be overcome by high amounts of IL-2. No significant inhibition of cytokine production (IL-2, IL-4, IL-6, IL-8, and IFN-γ; ELISA and intracellular cytokine staining) from these cultures was observed during daclizumab therapy (data not shown). The lack of a direct functional inhibition of adaptive immune responses by daclizumab was supported by in vivo observations that delayed-type hypersensitivity responses (i.e., skin test results) against the recall antigens tetanus, candida, and mumps at the end of the daclizumab dosing were comparable to those observed at baseline.
Thus, contrary to published in vitro data (6, 7), daclizumab therapy had only marginal effects on adaptive immune responses in vivo.
Daclizumab Therapy Leads to Modest Declines in Circulating CD4+ and CD8+ T Cells and to a Robust Expansion of CD3−/CD56bright NK Cells.
To consider possible cell-depleting properties of daclizumab, changes in lymphocyte subpopulations were monitored by flow cytometry during the daclizumab trial. By comparing the average of two baseline samples with the average of two therapy samples for each patient (Table 1), small but highly statistically significant decreases were observed in CD4+ and CD8+ T cell counts (6–12%) as well as a significant expansion of CD4−/CD8αdim cells (≈35%).
Table 1.
Changes in cellular subpopulations during the daclizumab trial
| Markers examined by flow cytometry | Baseline IFN-β, mean | IFN-β therapy + dacliz., mean | % change | P value |
|---|---|---|---|---|
| Lymphocytes subpopulations (n = 22) | ||||
| All lymphocytes | ||||
| % | 24.39 | 23.81 | −2.39 | NS |
| Absolute no. | 1,748 | 1,633 | −6.56 | NS |
| CD4+ T cells | ||||
| % | 51.6 | 48.68 | −5.67 | <0.001 |
| Absolute no. | 917 | 815 | −11.20 | 0.009 |
| CD8+ T cells | ||||
| % | 18.68 | 17.19 | −7.97 | <0.001 |
| Absolute no. | 333 | 295 | −11.53 | 0.015 |
| CD8αdim lymphocytes | ||||
| % | 7.49 | 10.52 | +40.55 | <0.001 |
| Absolute no. | 124 | 168 | +35.62 | <0.001 |
| Expression of IL-2R chains, % (n = 22) | ||||
| CD25 (Tac)+ CD4+ T cells | 40.28 | 0.58 | −98.56 | <0.001 |
| CD25+ (7G7) CD4+ T cells | 58.39 | 39.49 | −32.38 | 0.002 |
| CD25 (Tac)+ CD4− T cells | 9.65 | 0.55 | −94.33 | <0.001 |
| CD25+ (7G7) CD4− T cells | 25.84 | 20.64 | −20.14 | NS |
| CD122bright CD4− T cells | 19.66 | 28.52 | +45.10 | <0.001 |
| NK cells and γ/δ-T cells (n = 12) | ||||
| CD56dim NK cells, % | 24.07 | 24.32 | +1.00 | NS |
| CD56bright NK cells % | 3.30 | 8.78 | +166.10 | <0.001 |
| Absolute no. | 23 | 68 | +197.11 | <0.001 |
| CD56+/CD3+ T cells, % | 2.61 | 2.80 | +7.30 | NS |
| γ/δ-T cells, % | 2.96 | 3.09 | +4.65 | NS |
Absolute numbers are per microliter of whole blood. NS, not significant; Dacliz., daclizumab.
The Tac epitope of CD25, the molecular target of daclizumab, was blocked throughout the duration of daclizumab therapy (>95% inhibition at trough levels; Table 1). However, the CD25 epitope detected by a mAb (7G7) that binds outside of the Tac epitope (11, 12) persisted on cell surfaces and was selectively down-modulated (≈32%) on CD4+ T cells by daclizumab. Additionally, daclizumab therapy led to a significant expansion (≈45%) of CD122bright/CD4− lymphocytes.
Because the CD4−/CD8αdim cells and CD4−/CD122bright cells expanded during daclizumab treatment could represent the same cellular population, i.e., NK cells, the immunology protocol was modified during the second phase II trial (n = 12) to prospectively examine NK cell markers. Almost all CD4−/CD8αdim lymphocytes were CD122bright CD3−/CD56+ NK cells. Furthermore, daclizumab therapy led to a selective expansion of CD56bright NK cells (≈200%). CD56dim NK cells, CD56+ T cells, and γ/δ-T cells were not expanded (Table 1).
Daclizumab Therapy Leads to Activation and Expansion of CD56bright NK Cells Through an IL-2-Dependent Mechanism.
The next logical question was how does daclizumab therapy lead to highly selective expansion of CD56bright NK cells?
Comparing the expression of effector molecules on NK cells by flow cytometry from samples before and during daclizumab therapy, we observed significant modulation of expression patterns on CD56bright but not CD56dim NK cells or T cells (Fig. 5A, which is published as supporting information on the PNAS web site). CD56bright cells consistently expressed the highest levels of IL-2Rβ chain (CD122) among all lymphocytes, and this expression was further enhanced during daclizumab therapy (Δ = +30.3%, P = 0.004). We observed surface expression of CD25 only in a subgroup of CD56bright NK cells, and the expression levels did not change significantly by daclizumab therapy (Fig. 5 A and B). Compared with CD56dim cells, CD56bright NK cells expressed the IL-7Rα chain and had consistently higher expression of all adhesion molecules and chemokine receptors studied (CD44, CXCR3, and CCR7). Both IL-7Rα chain (Δ = +22.9%, P = 0.027) and CD44 (Δ = +53.8%, P = 0.001) were further up-regulated by daclizumab treatment. Neither population expressed IL-4Rα and IL-15Rα chains (data not shown). Most NK cell effector molecules examined (CD2, CD94/NKG2A, NKG2D, NKp46, KIR2DL4, and TRAIL) were expressed in higher levels on CD56bright as compared with CD56dim NK cells, and NKG2A (Δ = +82.3%, P = 0.001), NKG2D (Δ = +28.7%, P = 0.009), NKp46 (Δ = +44.7%, P = 0.027), and KIR2DL4 (Δ = +30.5%, P = 0.002) were further up-regulated on these cells during daclizumab therapy (Fig. 5A). CD16 and perforin (intracellularly) had higher expression on CD56dim as compared with CD56bright NK cells. CD16 was down-modulated in daclizumab therapy samples on both CD56dim (Δ = −31.8%, P = 0.032) and CD56bright (Δ = −51.4%, P = 0.02) NK cells, whereas no significant changes in perforin expression were observed.
Because the detected changes in expression patterns on CD56bright NK cells during daclizumab therapy affected molecules that are known to be regulated by IL-2 (i.e., IL-2Rβ, IL-7Rα, and KIR2DL4) (13) one plausible explanation for the expansion of these cells during daclizumab treatment was their heightened ability to receive IL-2/IL-15 signals by means of the intermediate-affinity IL-2R. Although it was previously reported that CD56bright NK cells proliferate to picomolar doses of IL-2 (14) and IL-15 (15), it was the role of CD25, not CD122, that was traditionally emphasized in this phenomenon (16). We were able to address the role of CD25 versus CD122 in the proliferation of NK cells during the daclizumab trial: the prospectively collected 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assays were reanalyzed, and gating on CD4−/CD8αdim lymphocytes (i.e., NK cells) revealed that the proliferation of these cells to IL-2 (Δ = +223.6%, P = 0.026) and IL-15 (Δ = +177.9%, P = 0.021) was significantly increased in therapy as compared with baseline samples (Fig. 5C). Because the CD25 Tac epitope was blocked by daclizumab and only CD122 was up-regulated, and because NK proliferation was increased to both IL-2 and IL-15 during daclizumab therapy, it is likely that the activation signal was delivered via intermediate-affinity IL-2/IL-15R.
The Expansion of CD56bright NK Cells on Daclizumab Therapy Correlates with Decreased Brain Inflammation.
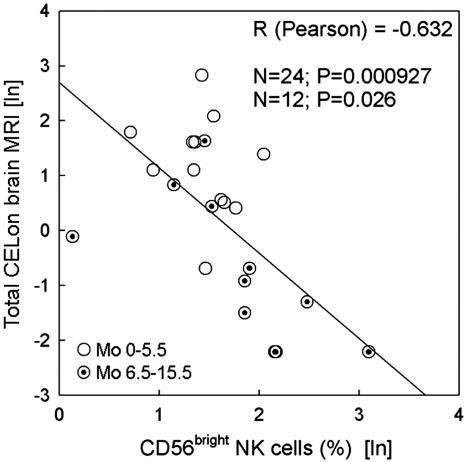
The relationship between the daclizumab-induced expansion of CD56bright NK cells and its therapeutic effect was examined by correlating the inhibition of contrast-enhancing lesions on brain MRI (an established measure of brain inflammation) with the percentage of CD56bright NK cells. Using either nonparametric Spearman or parametric Pearson correlations (after logarithmic transformation of the data to achieve normality and linearity assumption of parametric correlation) strong (RSpearman = −0.745, RPearson = −0.632) and highly significant (PSpearman < 0.0001; PPearson < 0.000927) correlations were observed between expansion of CD56bright NK cells by daclizumab therapy and inhibition of contrast-enhancing lesions (Fig. 1; only Pearson correlation data are presented). We observed that both the expansion of CD56bright NK cells and inhibition of contrast-enhancing lesions developed gradually and continued during daclizumab dosing. Therefore, to increase the relevant sample size, we included two therapy time points for 12 patients on whom we have prospective NK cell data: short-term (average of three time points per patient during months 0–5.5; IFN-β plus daclizumab therapy, open circles) and long-term (average of five time points per patient during months 6.5–15.5; daclizumab monotherapy, target circles). The correlations remained significant (PSpearman < 0.0001; PPearson < 0.026) when only long-term data points were evaluated. There was a trend that did not reach significance (P = 0.079) when only short-term data points were considered.
Fig. 1.
Correlation between expansion of CD56bright NK cells and inhibition of brain inflammatory activity during the daclizumab trial. Percentages of CD56bright NK cells were averaged from two combination therapy samples (months 0–5.5; IFN-β plus daclizumab, open circles) and two monotherapy samples (months 6.5–15.5; daclizumab, target circles) for each patient (n = 12) and correlated with the average number of contrast-enhancing lesions on brain MRI during 5.5 combination therapy months and 12 monotherapy months.
NK Cells Expanded During Daclizumab Therapy Limit the Survival of Activated T Cells in a Contact-Dependent Manner.
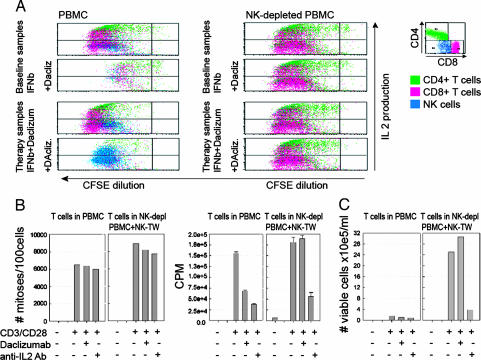
The correlation between expansion of CD56bright NK cells and its effect on the primary outcome measure suggested that these cells may be directly responsible for the therapeutic effect of daclizumab in MS. Although daclizumab had only marginal effects on the T cell effector functions, it seemed possible that it could affect longer-term activities of activated T cells, including survival. Therefore, a previously described experimental paradigm (17) was used in which CFSE-labeled peripheral blood mononuclear cells (PBMC) stimulated in vitro by anti-CD3/CD28 were washed after 72 h, transferred into IL-7 plus IL-15-enriched medium, and followed for survival. Addition of daclizumab led to diminished T cell survival in PBMC cultures, irrespective of whether these cultures originated from PBMC collected before (baseline) or during (therapy samples) daclizumab dosing [Fig. 2 A Left; compare proportion of red and green dots (T cells) and blue dots (NK cells)]. Inclusion of daclizumab gave rise to expansion/survival of NK cells in therapy samples (Fig. 2A, increased proportion of blue dots).
Fig. 2.
Inhibitory effect of daclizumab on T cell survival in in vitro cultures appears to be mediated by NK cells and requires NK–T cell contact. (A) PBMC or NK-depleted PBMC (by CD56 microbeads) from the same samples were stained with CFSE, polyclonally activated (plate-bound CD3/CD28) for 72 h in the presence/absence of daclizumab, washed and reseeded in T cell media enriched for IL-7 and IL-15 (with or without daclizumab), and followed for long-term survival. Corresponding flow cytometry profiles of equivalent proportions of cultures (CFSE proliferation together with intracellular cytokine staining for IL-2) at day 8 after stimulation are depicted from a representative patient from samples before and during daclizumab therapy. (B and C) Similar experimental design to A except that the NK cells that were depleted from PBMC were added into TW cultures at a 1:10 NK:T cell ratio so that T cells and NK cells were not in contact. (B Left) Rate of T cell proliferation by CFSE dilution at day 6 (no. of mitoses per 100 gated cells). (B Right) Thymidine incorporation from the same cultures at same time point (cpm). (C) T cell survival at day 18 of culture was assessed by two independent investigators counting the live cells in culture by light microscopy using trypan blue exclusion and by calculating the final number of T cells based on the proportions of CD3+ T cell in the cultures analyzed by flow cytometry. For each of the three panels, T cells in the PBMC are compared with T cells in NK-depleted PBMC, and depleted NK cells were placed in TW. Each plot represents representative experiment from two to five patients.
The elimination of NK cells from these PBMC cultures completely restored T cell survival and function regardless of the presence of daclizumab (Fig. 2A Right).
The mechanism of action of NK cells on T cell survival was investigated by transwell (TW) experiments comparing T cell survival in PBMC versus NK-depleted PBMC with NK added to TW and separated from the T cells by a semipermeable membrane (Fig. 2 B and C). Early after stimulation (day 6) the rate of T cell proliferation (CFSE dilution: no. of mitoses per 100 surviving T cells) was only minimally inhibited by daclizumab (Fig. 2B Left). However, the thymidine incorporation (cpm), which reflects the total number of proliferating cells in the PBMC culture, was already decreased at day 6 by >50% by daclizumab and >70% by IL-2-blocking Ab (Fig. 2B Right). Because this discrepancy between CFSE dilution (which focuses on the proportion of surviving cells) and thymidine incorporation (which reflects the absolute number of cells that were placed in the culture and are still proliferating) indicated lack of survival of T cells in PBMC versus NK-depleted PBMC, cultures were followed in long-term survival assays (Fig. 2C). When NK cells were not in contact with T cells, daclizumab had absolutely no effect on long-term survival of activated T cells, whereas very few T cells survived in PBMC cultures where T cells were in contact with NK cells. In contrast to IL-2 blockade, daclizumab affected T cell survival only when NK cells were in contact with T cells.
IL-2-Activated NK Cells Can Kill Activated Autologous T Cells.
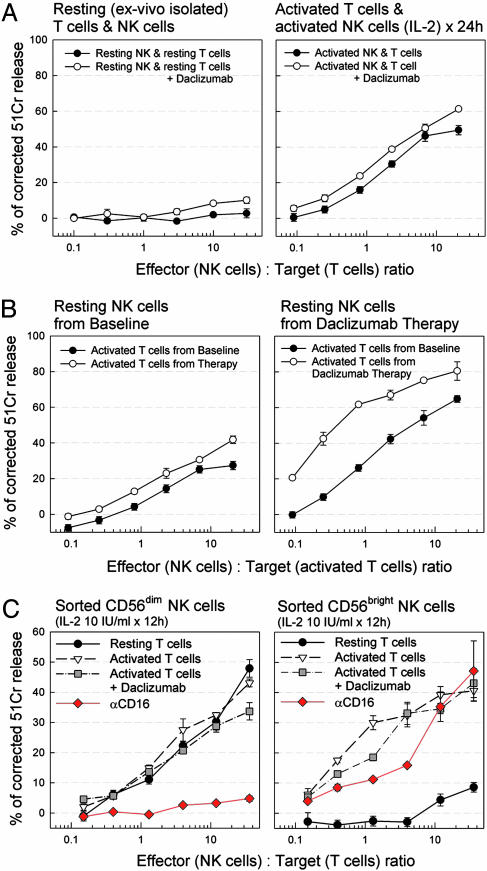
Because of contact dependence, we considered cytotoxicity a possible explanation for the NK-mediated inhibition of T cell survival. Chromium-release cytotoxicity assays were performed with negatively selected NK cells as effectors and autologous resting or polyclonally activated T cells as targets (Fig. 3). Anti-CD3/CD28 activation of T cells was used initially but was replaced by PMA/ionomycin in subsequent experiments to exclude the possibility of Ab-dependent cellular cytotoxicity.
Fig. 3.
NK cells are cytotoxic toward activated autologous T cells. (A) NK-mediated cytotoxicity against autologous T cells from a healthy donor. Both T cells and NK cells were purified by negative magnetic bead selection to >95% purity and tested in a 4-h chromium-release cytotoxicity assay immediately (Left) or after the T cells were polyclonal activated for 24 h (Right), and NK cells were cultured with 10 units/ml IL-2. Open circles indicate the effect of daclizumab (10 μg/ml) in the culture and cytotoxicity assay. (B) NK-mediated cytotoxicity against autologous activated T cells from a daclizumab-treated MS patient. T cells were purified by negative selection and activated (by PMA/ionomycin) from frozen samples of a MS patient from pretreatment baseline period and during daclizumab therapy and stained overnight with chromium. The next morning the NK cells were purified from the same frozen samples by negative selection and used fresh as effector cells. Left compares cytotoxicity of NK cells isolated from baseline samples, and Right shows the activity of NK populations from therapy samples against the same targets. (C) Cytotoxicity of purified CD56dim and CD56bright NK cells from MS patients undergoing daclizumab therapy on autologous T cells. T cells were left unstimulated or were stimulated with PMA/ionomycin for 12 h. Daclizumab (10 μg/ml) was added to the assays as indicated. Inhibition of NK subset cytotoxicity by anti-CD16 Ab (1 μg/ml) is depicted in red. Each plot corresponds to a representative experiment from two to five subjects.
Freshly isolated NK cells from healthy donors did not exert significant cytotoxicity against resting T cells (Fig. 3A Left). In contrast, after overnight culture with IL-2 (10 units/ml) NK cells readily killed activated autologous T cells, irrespective of the presence or absence of daclizumab (Fig. 3A Right).
Freshly isolated NK cells from MS patients during daclizumab therapy showed cytotoxicity toward activated autologous T cells (PMA/ionomycin) without need for an IL-2 activation step. When comparing NK cells isolated from the same patient before (baseline) and during daclizumab therapy, greater NK cytotoxicity was found in the therapy samples (Fig. 3B). We observed a mild degree of cytotoxicity toward autologous activated T cells by resting NK cells even when isolated from some healthy donors and MS patients before daclizumab therapy (i.e., at baseline) (Fig. 3B and data not shown).
Because only CD56bright NK cells were expanded during daclizumab therapy and low cytotoxicity potential has been attributed to these cells previously (14), CD56bright and CD56dim NK cells were sorted (>99% purity) from patients under daclizumab therapy by using flow cytometry and were tested for their cytotoxicity against autologous T cells (Fig. 3C). Both populations were comparably cytotoxic toward activated T cells. Addition of daclizumab did not enhance this cytotoxicity further. Whereas CD56bright NK cells had virtually no cytotoxicity toward resting T cells, CD56dim NK cells killed resting T cells equally well as activated T cells. This cytotoxicity of CD56dim NK cells was completely abrogated by anti-CD16 Ab, whereas the same Ab had only a mild inhibitory effect on the cytotoxicity of CD56bright NK cells (Fig. 3C, red).
To assess whether NK-mediated killing of activated T cells may occur in vivo, the relationship between the expansion of CD56bright NK cells and decline in CD4+ and CD8+ T cells was analyzed for individual patients. A significant correlation was found between the expansions of CD56bright NK cells and contractions of CD4+ (RSpearman = −0.458; P = 0.0277) and CD8+ (RSpearman = −0.623; P = 0.00154) T cells in daclizumab-treated MS patients (Fig. 6, which is published as supporting information on the PNAS web site).
Discussion
We initiated a clinical trial of daclizumab in MS based on the hypothesis that in MS patients lymphocytes are chronically activated, rendering them functionally dependent on high-affinity IL-2R signaling. However, despite extensive collected data, we observed virtually no signs of inhibition of adaptive immune responses in daclizumab-treated patients. This apparent discrepancy from in vitro studies (6, 7) can be explained by the redundancy in cytokine systems in vivo. In contrast to predictions from in vitro experiments, immune responses in IL-2-deficient mice are not suppressed. These animals show normal lymphocyte development and mount normal cytotoxic T and B cell responses against viruses but have reduced NK cell activity (18). By 5 weeks they develop uncontrolled proliferation of T cells resulting in autoimmunity (19). Therefore, although IL-2 is not required for the lymphocyte activation in vivo, it controls excessive lymphoproliferation. However, IL-2 deficiency is not equivalent to daclizumab therapy, because daclizumab blocks only high-affinity IL-2 signaling while permitting signaling through the intermediate-affinity receptor (4). Indeed, daclizumab and IL-2-blocking Ab had divergent effects on T cell survival in NK-depleted PBMC cultures (Fig. 2 B and C), and daclizumab-treated patients had increased, not decreased, NK cell numbers (Table 1) and functional activities (Fig. 3B).
Daclizumab inhibits CD4+ T cell proliferation in vitro, particularly under conditions of low-potency stimuli and at low IL-2 concentrations (Fig. 4) that preferentially occur during stimulation by autoantigens. However, this inhibition is dose-dependent and is fully reversible when daclizumab fails to saturate CD25-binding sites, which occurs in patients 6–8 weeks after cessation of daclizumab dosing (data not shown). Therefore, the sole inhibition of T cell function by daclizumab does not appear to explain the gradually developing and lasting therapeutic effect observed during a daclizumab trial in MS (9). In contrast, daclizumab therapy resulted in a gradual expansion of CD56bright NK cells that correlated strongly with the decrease in brain inflammatory activity (Fig. 1). Expanded CD56bright NK cells limited survival of activated T cells in vitro in a contact-dependent manner, and NK cells isolated from treated patients were directly cytotoxic to autologous activated T cells. Finally, we observed a gradual decline in CD4+ and CD8+ T cells in daclizumab-treated patients (Table 1), and the correlation between expansion of CD56bright NK cells and decline in T cell numbers (Fig. 6) indirectly supports the hypothesis of NK cell-mediated immunoregulation of activated T cells in vivo.
CD56bright NK cells have been labeled “immunoregulatory” based on their ability to secrete cytokines (20) and home to lymph nodes (21) and tissues (22) and their expansion in humans during states characterized by increased immune tolerance (23, 24) such as pregnancy. Animal studies demonstrated a regulatory role of NK cells in autoimmunity in general (25–28), and in particular in experimental autoimmune encephalomyelitis (EAE) (25, 27), the animal model of MS. In an informative study Zhang et al. (25) depleted NK cells before immunization of susceptible mice with encephalitogenic myelin oligodendrocyte glycoprotein35–55 with the hypothesis that NK cells may contribute to CNS destruction. Contrary to their expectation, depletion of NK cells resulted in earlier onset of EAE, strikingly worse symptomatology, and occurrence of relapses in this normally nonrelapsing EAE model (25). In a rat EAE Smeltz et al. (27) showed that NK cells inhibit autoimmune T cell responses (proliferation to myelin basic protein and IFN-γ production) (27, 28) and suggested that NK cells may kill activated autoreactive T cells (27). Such killing of activated T cells by syngeneic IL-2-activated NK cells was confirmed by Rabinovich et al. (29) in mice. Our observation that NK cells isolated from daclizumab-treated MS patients, but also activated NK cells from healthy donors, are capable of killing activated T cells (Fig. 3 A and B) suggests that NK cell cytotoxicity against activated T cells may represent one physiological mechanism for the termination of adaptive immune responses that is enhanced in vivo by daclizumab administration.
Is immunoregulation via CD56bright NK cells solely responsible for the profound therapeutic effect of daclizumab in MS? Our study did not examine other regulatory populations, such as CD4+/CD25+ T regulatory cells (30, 31). Because IL-2 is necessary for the generation and maintenance of CD4+/CD25+ T regulatory cells in animals (32), daclizumab could theoretically exert negative influences on these cells. However, our data suggest that it is unlikely that it does: daclizumab therapy leads to inhibition rather than activation of autoimmune diseases (9, 10, 33). Additionally, we did not observe any changes in effector T cell function between baseline and daclizumab therapy, which would be expected if daclizumab inhibited T regulatory cell function. Physiological immunoregulation encompasses multiple cellular populations and different mechanisms (34) that jointly balance effective clearance of pathogen with limiting potential damage to the host. Our data suggest that such regulatory circuits in humans include CD56bright NK cells and that these cells control the adaptive immune responses by the negative immunoregulation of activated lymphocytes.
Materials and Methods
Study Design and Subjects.
The MS patient data are from an open-label, baseline (4 months; IFN-β therapy) versus treatment (5.5 months; IFN-β plus daclizumab i.v. infusions at 1 mg/kg twice every 2 weeks and then five times every 4 weeks) crossover trial in 10 MS patients with incomplete response to IFN-β therapy. The clinical/MRI results of this trial are reported elsewhere (9). Additionally, we included immunological data on 12 MS patients from an ongoing second phase of the daclizumab trial. This new trial has an identical trial design for the first 5.5 months (IFN-β plus daclizumab combination therapy) but has an added extension during which IFN-β is withdrawn and patients continue on daclizumab monotherapy (1 mg/kg every 4 weeks) for an additional 12 months (months 6.5–15.5). Although the monotherapy extension phase is ongoing, all patients completed the IFN-β/daclizumab combination therapy to give us total of 22 patients with an entirely identical trial design. Both trials were approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board.
Immunological Assays.
Blood sample collection.
Blood samples were collected between 0830 hours and 1130 hours and were processed within 2 h. Lymphocytapheresis were collected during baseline, at month 5.5 of IFN-β plus daclizumab therapy, and at month 13.5 of daclizumab monotherapy and were cryopreserved.
Prospective flow cytometry analysis of the cell surface markers.
Surface markers were evaluated from fresh cells prospectively by three- to four-color flow cytometry after RBC lysis as described (35). Specific sources of used mAb are outlined in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Analysis of the functional immune status.
Functional analyses included skin testing to tetanus, candida, and mumps, all performed at baseline and at month 5.5 of daclizumab therapy, and prospective analysis of T cell proliferation by CFSE-based flow cytometry proliferation assay as described (36) bimonthly during baseline and treatment. Briefly, PBMC were isolated by density gradients, diluted to 1 × 107 PBMC per ml in PBS, and stained with CFSE (Molecular Probes; 1 μM). After washing, PBMC were resuspended in serum-free X-vivo 15 medium (BioWhittaker, Walkersville, MD) and seeded in 24-well plates (2 × 106 PBMC per well) with the following stimuli: plate-bound anti-CD3 (20 ng/ml; preincubated at 37°C for 2 h), plate-bound CD3/CD28 (CD28 at 10 μg/ml), IL-2 (50 units/ml), IL-15 (20 ng/ml) (all PeproTech), and unstimulated sample. After 72 h of incubation (37°C and 5% CO2) 1 ml of supernatant from each culture was collected and stored (−20°C) for ELISA (Cyto-Sets from BioSource International). Ninety-six hours after stimulation cells were stained with anti-CD3, anti-CD4, anti-CD8, or anti-CD56 Ab and analyzed by flow cytometry. We calculated the total number of mitoses per 100 gated cells using the following formula: no. of mitoses = Σ (Xn × 100 − Xn × 100/2n) where X is the percentage of cells that underwent n divisions.
Additional in vitro experiments from the frozen samples.
PBMC from baseline (IFN-β) and therapy (IFN-β plus daclizumab) apharesis were thawed and processed simultaneously. Cells were stained with CSFE and seeded unstimulated or with polyclonal stimuli [plate-bound CD3 (20 ng/ml)/CD28 (10 μg/ml) with or without 10 units/ml IL-2] in 24-well plates with daclizumab (10 μg/ml) or equimolar concentration of control anti-CD25 Ab M-A251 (BD Pharmingen), which does not block the IL-2 binding site. After 72 h of incubation one-half of each culture was washed and reseeded into fresh medium supplemented with IL-7 plus IL-15 (10 ng/ml each), with or without daclizumab or M-A251, to study T cell survival (17). Days 8–10 after stimulation one-half of the cultures were stimulated for 5 h by PMA (20 ng/ml)/ionomycin (1 μM) in the presence of brefeldin A (GolgiPlug, BD Pharmingen) at 1 μg/ml per 106 PBMC and followed by intracellular cytokine staining (IL-2, IL-4, IL-6, IL-10, IFN-γ, and perforin; all from BD Pharmingen). Remaining cells were followed for long-term survival until days 14–18 after stimulation by counting viable cells. The same experimental design was performed simultaneously on NK-depleted PBMC: before CFSE staining, NK cells were depleted by CD56 MACS microbeads (Miltenyi Biotec, Auburn, CA). Where indicated, separated NK cells were plated into TWs (3-μm pore size, Polycarbonated Membrane, Corning Costar) at a 1:10 NK:T cell ratio. IL-2 blockade was achieved by anti-IL-2 mAb (10 μg/ml; clone 5334, R & D Systems). Flow cytometry confirmed >95% purity of all separated populations.
Cytotoxicity assays.
Cytotoxicity of NK cells against T cells was determined by 51Cr release. Lymphocytes were isolated from fresh or cryopreserved apharesis samples by negative selection (T cell isolation kit II MACS, Miltenyi Biotec) and used fresh or polyclonally activated. At determined time points cells were harvested, and 5 × 106 cells were labeled overnight with 200 μCi (1 Ci = 37 GBq) of Na251Cr2O7 (MP Biomedicals). Freshly purified NK cells (negative selection via NK cell isolation kit II, MACS, Miltenyi Biotec) from frozen or freshly acquired apharesis were mixed with labeled targets (8 × 103 per well) at the indicated effector-to-target ratio in 96-well plates in triplicate. Alternatively, NK cells were cultured overnight with IL-2 (10 units/ml). When indicated CD56bright and CD56dim NK subsets were sorted by flow cytometry to >99% purity. After a 4-h effector-to-target incubation (37°C and 5% CO2), supernatants were harvested and counted in a gamma counter. Lysis was calculated from the supernatant 51Cr with the spontaneous release subtracted. To test for Ab-dependent cellular cytotoxicity, CD16 blockade was performed by using mAb anti-CD16 (1 μg/ml; BD Pharmingen).
Statistical Analysis.
To evaluate immunological changes from baseline to treatment, two baseline samples and two to three treatment samples were averaged for each individual and compared by the signed-rank test or, if permitted, by a paired t test. Correlations between MRI measures and immunological indices were assessed by nonparametric Spearman correlation or by Pearson correlation. When Pearson correlation was used, values were logarithmically transformed (zero values were replaced by half the smallest non-zero value in the data set because ln 0 does not exist) to acheive normality and linearity of distribution required by parametric test. All statistics were performed by using sigmastat 2.03 (Jandel Scientific) with a preset limit of statistical significance of P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. E. O. Long (National Institute of Allergy and Infectious Diseases, National Institutes of Health) for helpful advice and Dr. P. A. Muraro (National Institute of Neurological Disorders and Stroke, National Institutes of Health) for his critical review of the manuscript. The research was supported by the Intramural Research program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Abbreviations
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cell
- NK
natural killer
- TW
transwell
- CFSE
5-(and 6)-carboxyfluorescein diacetate succinimidyl ester.
Footnotes
Conflict of interest statement: B.B., T.A.W., H.M., and R.M. are coinventors on National Institutes of Health-owned patents related to the use of daclizumab in multiple sclerosis and as such are receiving royalty payments.
References
- 1.Bielekova B., Martin R. Curr. Treat. Options Neurol. 1999;1:201–219. doi: 10.1007/s11940-999-0004-x. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann T. A., O’Shea J. Curr. Opin. Immunol. 1998;10:507–512. doi: 10.1016/s0952-7915(98)80215-x. [DOI] [PubMed] [Google Scholar]
- 3.Roessler E., Grant A., Ju G., Tsudo M., Sugamura K., Waldmann T. A. Proc. Natl. Acad. Sci. USA. 1994;91:3344–3347. doi: 10.1073/pnas.91.8.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldmann T., Tagaya Y., Bamford R. Int. Rev. Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann T. A. J. Clin. Immunol. 2002;22:51–56. doi: 10.1023/a:1014416616687. [DOI] [PubMed] [Google Scholar]
- 6.Goebel J., Stevens E., Forrest K., Roszman T. L. Transplant Immunol. 2000;8:153–159. doi: 10.1016/s0966-3274(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 7.Tkaczuk J., Milford E., Yu C., Baksh S., Carpenter C., Burakoff S., McKay D. Transplant. Proc.; 2001. pp. 212–213. [DOI] [PubMed] [Google Scholar]
- 8.Queen C., Schneider W. P., Selick H. E., Payne P. W., Landolfi N. F., Duncan J. F., Avdalovic N. M., Levitt M., Junghans R. P., Waldmann T. A. Proc. Natl. Acad. Sci. USA. 1989;86:10029–10033. doi: 10.1073/pnas.86.24.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielekova B., Richert N., Howard T., Blevins G., Markovic-Plese S., McCartin J., Wurfel J., Ohayon J., Waldmann T. A., McFarland, et al. Proc. Natl. Acad. Sci. USA. 2004;101:8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nussenblatt R. B., Thompson D. J., Li Z., Peterson J. S., Robinson R. R., Shames R. S., Nagarajan S., Tang M. T., Mailman M., Velez G., et al. J. Autoimmun. 2003;21:283–293. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 11.Vincenti F., Nashan B., Light S. Transplant. Proc. 1998;30:2155–2158. doi: 10.1016/s0041-1345(98)00571-5. [DOI] [PubMed] [Google Scholar]
- 12.Lehky T. J., Levin M. C., Kubota R., Bamford R. N., Flerlage A. N., Soldan S. S., Leist T. P., Xavier A., White J. D., Brown M., et al. Ann. Neurol. 1998;44:942–947. doi: 10.1002/ana.410440613. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi-Maki A., Yusa S., Catina T. L., Campbell K. S. J. Immunol. 2003;171:3415–3425. doi: 10.4049/jimmunol.171.7.3415. [DOI] [PubMed] [Google Scholar]
- 14.Cooper M. A., Fehniger T. A., Caligiuri M. A. Trends Immunol. 2001;22:633–640. [Google Scholar]
- 15.Carson W. E., Giri J. G., Lindemann M. J., Linett M. L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M. A. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. J. Exp. Med. 1990;171:1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gett A. V., Sallusto F., Lanzavecchia A., Geginat J. Nat. Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 18.Kundig T. M., Schorle H., Bachmann M. F., Hengartner H., Zinkernagel R. M., Horak I. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 19.Sadlack B., Lohler J., Schorle H., Klebb G., Haber H., Sickel E., Noelle R. J., Horak I. Eur. J. Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 20.Trotta R., Parihar R., Yu J., Becknell B., Allard J., II, Wen J., Ding W., Mao H., Tridandapani S., Carson W. E., Caligiuri M. A. Blood. 2005;105:3011–3018. doi: 10.1182/blood-2004-10-4072. [DOI] [PubMed] [Google Scholar]
- 21.Fehniger T. A., Cooper M. A., Nuovo G. J., Cella M., Facchetti F., Colonna M., Caligiuri M. A. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 22.Dalbeth N., Callan M. F. Arthritis Rheum. 2002;46:1763–1772. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 23.Cooper M. A., Fehniger T. A., Turner S. C., Chen K. S., Ghaheri B. A., Ghayur T., Carson W. E., Caligiuri M. A. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa K., Saito S., Morii T., Hamada K., Ako H., Narita N., Ichijo M., Kurahayashi M., Sugamura K. Int. Immunol. 1991;3:743–750. doi: 10.1093/intimm/3.8.743. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B., Yamamura T., Kondo T., Fujiwara M., Tabira T. J. Exp. Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French A. R., Yokoyama W. M. Arthritis Res. Ther. 2004;6:8–14. doi: 10.1186/ar1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeltz R. B., Wolf N. A., Swanborg R. H. J. Immunol. 1999;163:1390–1397. [PubMed] [Google Scholar]
- 28.Wolf N. A., Swanborg R. H. J. Neuroimmunol. 2001;119:81–87. doi: 10.1016/s0165-5728(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovich B. A., Li J., Shannon J., Hurren R., Chalupny J., Cosman D., Miller R. G. J. Immunol. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 30.Shevach E. M., McHugh R. S., Thornton A. M., Piccirillo C., Natarajan K., Margulies D. H. Adv. Exp. Med. Biol. 2001;490:21–32. doi: 10.1007/978-1-4615-1243-1_3. [DOI] [PubMed] [Google Scholar]
- 31.Viglietta V., Baecher-Allan C., Weiner H. L., Hafler D. A. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setoguchi R., Hori S., Takahashi T., Sakaguchi S. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nussenblatt R. B., Fortin E., Schiffman R., Rizzo L., Smith J., Van Veldhuisen P., Sran P., Yaffe A., Goldman C. K., Waldmann T. A., et al. Proc. Natl. Acad. Sci. USA. 1999;96:7462–7466. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncarolo M. G., Levings M. K. Curr. Opin. Immunol. 2000;12:676–683. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 35.Muraro P. A., Leist T., Bielekova B., McFarland H. F. J. Neuroimmunol. 2000;111:186–194. doi: 10.1016/s0165-5728(00)00362-3. [DOI] [PubMed] [Google Scholar]
- 36.Lyons A. B., Parish C. R. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.