Abstract
Recombinant human erythropoietin (rhEPO) is receiving increasing attention as a potential therapy for prevention of injury and restoration of function in nonhematopoietic tissues. However, the minimum effective dose required to mimic and augment these normal paracrine functions of erythropoietin (EPO) in some organs (e.g., the brain) is higher than for treatment of anemia. Notably, a dose-dependent risk of adverse effects has been associated with rhEPO administration, especially in high-risk groups, including polycythemia–hyperviscosity syndrome, hypertension, and vascular thrombosis. Of note, several clinical trials employing relatively high dosages of rhEPO in oncology patients were recently halted after an increase in mortality and morbidity, primarily because of thrombotic events. We recently identified a heteromeric EPO receptor complex that mediates tissue protection and is distinct from the homodimeric receptor responsible for the support of erythropoiesis. Moreover, we developed receptor-selective ligands that provide tools to assess which receptor isoform mediates which biological consequence of rhEPO therapy. Here, we demonstrate that rhEPO administration in the rat increases systemic blood pressure, reduces regional renal blood flow, and increases platelet counts and procoagulant activities. In contrast, carbamylated rhEPO, a heteromeric receptor-specific ligand that is fully tissue protective, increases renal blood flow, promotes sodium excretion, reduces injury-induced elevation in procoagulant activity, and does not effect platelet production. These preclinical findings suggest that nonerythropoietic tissue-protective ligands, which appear to elicit fewer adverse effects, may be especially useful in clinical settings for tissue protection.
Keywords: β common receptor, cytokine, tissue protection, thrombosis, hypertension
Therapy for anemia using recombinant human erythropoietin (rhEPO) has been remarkably safe, yet complications of rhEPO therapy can be life threatening. Although insufficient information exists to accurately predict the association between rhEPO dosing and adverse effects (AEs), results of clinical trials suggest that higher doses of rhEPO are more likely to be associated with AEs. Even though peak hematopoietic effects of rhEPO are reached at a dose of 300 units/kg of body weight (bw) administered intravenously (i.v.) (1), larger doses have been used recently in an effort to reduce the frequency of administration (2) or in an attempt to treat refractory anemia (3). Notably, several clinical trials evaluating the use of rhEPO in anemic patients with breast (4, 5) or head and neck (6) malignancies have raised concerns because of lower survival within the rhEPO treatment arms. Similarly, rhEPO therapy also has been identified as a strong risk factor for venous thromboembolism in cancer patients undergoing chemotherapy (7).
The high-dose-related AEs have recently assumed a new significance because multiple investigators have shown that many nonhematopoietic tissues [e.g., the kidney (8), among others (9)] express the erythropoietin receptor (EPOR) and are protected from injury in the setting of metabolic stress by rhEPO (10). In some tissues (e.g., the central nervous system), cytoprotection requires higher doses than those required to treat anemia (reviewed in ref. 11), whereas others like the heart (12) or skin (13) may not. The anticipated therapeutic use of rhEPO for tissue protection, therefore, may be associated with unacceptable AEs in certain patient groups.
The biology of the homodimeric receptor complex (EPOR)2 through which erythropoietin (EPO) maintains erythrocyte production has been intensively studied (14). In addition to triggering erythrocyte survival and maturation, EPO mediates other effects within the hematopoietic system. Recent interest has focused on vascular endothelial cells of the hematopoietic lineage, in which rhEPO administration has been observed to increase the levels of circulating biomarkers of endothelial cell injury, including thrombomodulin, von Willibrand factor, and tissue plasminogen activator (15), and to enhance production of reticulated, hyperactive platelets (16–18), E-selectin and P-selectin (16), which together induce a procoagulant state. Not surprisingly, assessments carried out in vitro (19) or in vivo (17, 20) have shown a clear propensity for rhEPO-induced thrombosis that has also been observed in clinical trials (4–7, 21). Further, rhEPO’s procoagulant activity can be amplified in the setting of acute and subacute injury (22–24). rhEPO also affects other organs, e.g., the kidney (25), where it modulates regional blood flow (BF) and affects both water and electrolyte excretion (25), which could be problematic in certain clinical situations. rhEPO also increases systemic blood pressure (BP) particularly in individuals with renal failure (26, 27) but also in normal individuals, especially after submaximal exercise. Extreme exercise predisposes the vasculature to thrombosis, as has been observed in athletes engaging in “blood doping” with rhEPO (28).
We recently showed that tissue protection does not depend on (EPOR)2 but, rather, utilizes an alternative EPOR complex, which includes the β common receptor (βc or CD131) and is widely expressed in numerous organs, including the brain, heart, and kidney (29). Further, tissue-protective and nonerythropoietic molecules have been developed that signal via EPOR–βc and not (EPOR)2 (30). For example, carbamylated rhEPO (CEPO) has no affinity for (EPOR)2 and is, therefore, nonerythropoietic and yet maintains full tissue-protective activity (30). Thus, biological activities possessed by rhEPO, but not by CEPO, functionally define the effects of (EPOR)2. These discoveries provide the conceptual framework and tools with which to assess the relative contributions of each receptor isoform to the AEs of rhEPO. In the present studies, we used rhEPO and CEPO to engage differentially the homodimer (EPOR)2 and EPOR–βc. We demonstrate that (EPOR)2 specifically mediates a number of potentially significant AEs in several model systems, whereas EPOR–βc does not.
Results
Comparative Bone Marrow Effects of rhEPO or CEPO.
CEPO was added to standard colony-forming cultures of human bone marrow CD34+ cells to assess its erythropoietic and thrombopoietic activity. We found that CEPO failed to stimulate CD34+ cell proliferation as hematopoietic colonies even in the presence of thrombopoietin (TPO) (Fig. 1A). To determine whether CEPO acted as a competitive inhibitor or synergistically with EPO, an EPO dose–response analysis was performed in the presence of a high (100 ng/ml) concentration of CEPO. CEPO neither enhanced nor reduced the colony-forming capacity of EPO (Fig. 1B). Similar in vitro results were observed by using unselected BDF1 murine bone marrow cells (data not shown). CEPO and rhEPO exhibited a similar constellation of activities in vivo. For example, rhEPO (10 μg/kg of bw, i.v.) rapidly increased circulating reticulocytes, whereas CEPO did not (ref. 30 and data not shown). Only rhEPO significantly increased circulating platelet levels in vivo in the rat (Fig. 1C).
Fig. 1.
Unlike rhEPO, CEPO does not stimulate hematopoietic colony growth. (A) Effects of compounds on human bone marrow CD34+ cells capable of differentiating into erythrocytes, leukocytes, or megakaryocytes in the presence of thrombopoietin (TPO) in vitro. A representative experiment (of a total of three) is shown with either 0.5–50 ng/ml CEPO or 0.05–50 ng/ml rhEPO in the presence or absence of 10 ng/ml human TPO. (B) The number of erythroid colonies (±SEM) formed when 500 human marrow CD34+ cells were cultured, in duplicate, with 0.05–100 ng/ml CEPO, 0.05–50 ng/ml rhEPO, or the same concentrations of EPO plus 100 ng/ml CEPO. None of the CEPO plus rhEPO results differed significantly from those seen with the same concentration of EPO alone. (C) Unlike rhEPO, CEPO (30 μg/kg s.c.) administered for 2 weeks twice weekly does not stimulate in vivo platelet production (±SEM) in the rat. (∗∗, P < 0.01 vs. saline or CEPO.)
Differential Effects on Vascular Endothelium.
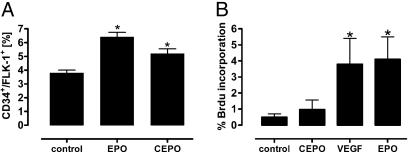
Subcutaneous (s.c.) administration of either CEPO or rhEPO to mice for 2 weeks (10 μg/kg of bw twice weekly) stimulated an increase in CD34+/Flk-1 positive endothelial progenitor cells (EPCs) in the bone marrow (Fig. 2A). In contrast, human umbilical vein endothelial cells (HUVECs) responded differently to the two compounds in vitro, with rhEPO significantly stimulating mitogenesis similar to that of VEGF, whereas CEPO was inactive (Fig. 2B).
Fig. 2.
Although rhEPO and CEPO stimulate accumulation of EPCs within the bone marrow, rhEPO alone is mitogenic for HUVECs in vitro. (A) In vivo accumulation of EPCs in the mouse is similar for both compounds. (∗, P < 0.05 vs. control). (B) CEPO is not mitogenic for HUVECs. (Error bars, ± SD.) (∗, P < 0.05 vs. control or CEPO.)
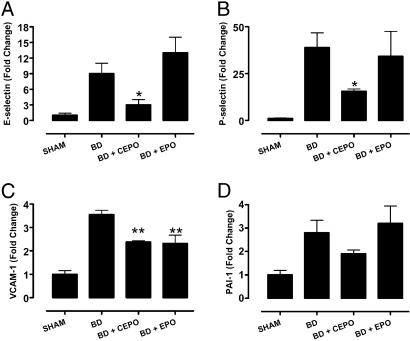
By using a rat model of brain death (BD), which primes the vasculature to release procoagulants and up-regulates expression of cell adhesion molecules, we analyzed the mRNA levels of the proinflammatory molecules E- and P-selectin, plasminogen activator inhibitor (PAI), and vascular cell adhesion molecule 1 (VCAM-1) in the kidney, a tissue known to respond favorably to the tissue protective effects of rhEPO. After 4 h of BD, inflammatory markers were induced 3- to 40-fold relative to sham-treated animals as determined by real-time RT-PCR (Fig. 3). Only CEPO pretreatment significantly attenuated the BD-induced production of E-selectin and P-selectin mRNA (Fig. 3 A and B). In contrast, both EPO and CEPO decreased VCAM-1 expression in the kidney (Fig. 3C). Although CEPO also reduced PAI-1 in contrast to EPO, this effect was not at the level of significance (Fig. 3D).
Fig. 3.
CEPO, but not rhEPO, reduce the expression of proinflammatory factors. Compounds were given 30 min before induction of BD and kidney mRNA quantitated after 4 h of beating heart BD. The relative amounts of E-selectin (A), P-selectin (B), VCAM-1 (C), and PAI-1 (D) were determined by real-time PCR. All error bars are ±SEM. (∗, P < 0.05; ∗∗, P < 0.01 vs. untreated BD).
Divergent Hemodynamic Responses to CEPO or rhEPO.
Arterial pressure and peripheral vascular resistance frequently rise during rhEPO therapy in patients with chronic renal disease (31). Although the increased BP often parallels the rise in hematocrit, studies in both iron-deficient rodents (32) and humans (33) have conclusively demonstrated that these two events are not causally linked and that rhEPO acts directly on the vasculature. To determine whether the pressor action of rhEPO is mediated through the hematopoietic (EPOR)2 or the tissue-protective EPOR–βc receptor, we treated rats with rhEPO or CEPO while monitoring systolic BP plethysmographically via tail cuff. Chronic administration of rhEPO at either low (1.2 μg/kg of bw twice weekly) or high (40 μg/kg of bw three times weekly) doses was associated with significant and comparable increases in systolic BP of 12 ± 3% (low dose) and 5 ± 2% (high dose) of baseline (118 ± 6.1 mmHg; 1 mmHg = 133 Pa), respectively (P < 0.05 vs. saline and P < 0.01 vs. CEPO). In contrast, CEPO administration was associated with a small, nonsignificant decrease in BP compared with control animals. Diastolic pressure increased to a similar extent (data not shown).
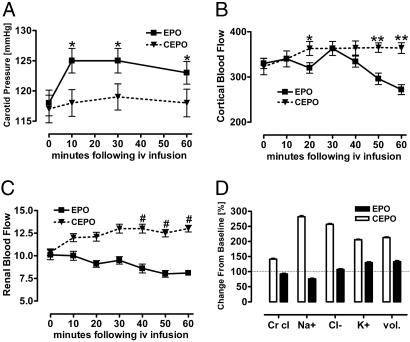
We performed additional hemodynamic studies in normal adult male rats to determine whether acute dosing of rhEPO or CEPO may have similar divergent effects on regional differences in tissue BF. Before anesthesia, awake systolic BPs (tail cuff) were indistinguishable, as were baseline data obtained 1 h after instrumentation and just before CEPO or rhEPO administration (Table 1). After infusion of 50 μg/kg of bw rhEPO, the carotid BP increased within 10 min and remained elevated throughout the 60-min observation period (Fig. 4A). Renal cortical BF initially rose but then fell progressively, whereas total renal BF decreased progressively (Fig. 4 B and C). Although the creatinine (Cr) clearance remained stable, the urinary excretion of Na+ fell, consistent with salt retention (Fig. 4D). The hematocrit was stable, and serum Cr and blood urea nitrogen (BUN) rose slightly (data not shown). In contrast, after CEPO infusion the carotid BP did not change significantly (Fig. 4A), whereas cortical BF and total renal BF significantly increased by ≈25% and 12%, respectively. Cr clearance rose, as did urinary Na+ and Cl− excretion and urine volume (Fig. 4D), consistent with solute diuresis. Similar to the rhEPO group, the hematocrit remained stable with slight rises in serum Cr and BUN (data not shown).
Table 1.
Baseline renal parameters of anesthetized rats undergoing renal studies
| Experimental group | Carotid BP, mmHg | Renal BF, ml/min per kidney | Cortical BF, arbitrary units | Cr clearance, ml/min | Na+ excretion, μeq per 60 min | Cl− excretion, μeq per 60 min | K+ excretion, μeq per 60 min | Volume, ml per 60 min |
|---|---|---|---|---|---|---|---|---|
| CEPO | 117 ± 2.3 | 10.4 ± 0.4 | 323 ± 18 | 2.8 ± 0.07 | 11.8 ± 0.5 | 38.6 ± 1.3 | 58.5 ± 1.7 | 0.3 ± 0.01 |
| EPO | 118 ± 2.1 | 10.1 ± 0.5 | 330 ± 11 | 2.9 ± 0.08 | 13.8 ± 0.4 | 39 ± 1.2 | 52.8 ± 1.7 | 0.3 ± 0.01 |
Values are means ± SEM. n = 6 for CEPO and EPO.
Fig. 4.
Divergent effects rhEPO and CEPO on systemic and renal hemodynamics. Acute changes in carotid artery BP (A) (∗, P < 0.05 vs. CEPO), renal cortical BF (B) (∗, P < 0.05; ∗∗, P < 0.002 vs. EPO), total renal BF (C) (#, P < 0.0001 vs. EPO), and urinary excretion (D) (P < 0.0001 CEPO vs. EPO) are shown.
Discussion
Data from the widespread clinical use of rhEPO for anemia has shown that rhEPO dose predicts mortality in certain patient groups (34, 35). For example, a retrospective study of >94,000 dialysis patients showed a dose-dependent increase in mortality, with a >2-fold increase in mortality among anemic patients receiving intensive dosing of rhEPO (35). Further, a randomized, prospective trial of 1,265 hemodialysis patients with clinical heart disease targeting hematocrit at 42% or 30% unexpectedly found a 7% increased mortality within the more intensively treated group, which led to early termination of the trial (36). Although assignment of true cause and effect of rhEPO therapy on mortality cannot be assessed in these studies, myocardial infarction, vascular access thrombosis, and other thrombotic complications were noted (36), all of which are potential AEs of rhEPO administration.
Preclinical data suggest that tissue protection using rhEPO may require doses higher than those used for treatment of anemia. For example, a successful proof of concept human trial evaluating rhEPO in middle cerebral artery infarction used a total of 100,000 units of rhEPO administered in three equal daily doses of ≈450–500 units/kg of bw (37). Although it is currently unknown whether lower doses would also be effective, preclinical data obtained in rodents have shown that a minimum dose of ≈500 units/kg of bw is required for the treatment of central nervous system lesions (38).
Many of the AEs of rhEPO arise through effects on the broadly defined hematopoietic system, especially the vascular endothelium. One dramatic example of rhEPO AEs occurs within the population of highly conditioned athletes engaged in EPO “doping,” as, for example, long-distance bicyclists who have experienced sudden death secondary to pulmonary embolism (28). Additionally, patients predisposed to thrombotic complications, e.g., those with malignancy (4–6) or undergoing chemotherapy (7), also frequently exhibit rhEPO-associated AEs, which are not prevented by antithrombosis therapy (39). Although increased AEs associated with the more dose-intense EPO regiment might have several possible explanations related to red cell mass per se, AEs do occur without any change in the hematocrit, and these AEs could arise from a direct effect of rhEPO on the endothelium.
Our previous work showed that although CEPO does not bind monomeric or dimeric EPOR, this compound possesses equivalent or enhanced cytoprotective potency when compared with rhEPO in diverse preclinical models (30). Subsequent work has identified an additional receptor comprised of the EPOR and the βc receptor (CD131) subunit, the latter also used for granulocyte/macrophage colony-stimulating factor (GM-CSF), IL-3, and IL-5 signaling (29). CEPO is therefore a useful ligand to distinguish between effects mediated by the hematopoietic (EPOR)2 and cytoprotective EPOR–βc receptors.
The data described here, obtained after doses reasonably anticipated for future study of tissue protection in humans, suggest that stimulation of human multipotent hematopoietic colonies and rat platelets, as well as E- and P-selectin (and possibly PAI-1) up-regulation are mediated by the (EPOR)2 receptor. In contrast, VCAM-1, a ligand expressed by the endothelium (and monocytes) that is activated by proinflammatory cytokines for recruitment of leukocytes into damaged tissues (40), was similarly reduced in the kidneys of the BD rat model pretreated with either rhEPO or CEPO. VCAM-1 expression also has been reported to decrease in dialysis patients receiving rhEPO (41). The observation that CEPO elicits the same effect implies that VCAM-1 suppression is mediated through the activity of the tissue protective EPOR-βc receptor complex. Moreover, modulation of inflammation via VCAM-1 suppression may partly explain the striking reduction of inflammatory infiltrates observed after EPO dosing in preclinical models of stroke (42) and spinal cord injury (38).
Our results also demonstrate that rhEPO and CEPO act differently on the vascular endothelium. Whereas both compounds stimulate EPC production, only rhEPO appears to be mitogenic for differentiated HUVECs. The observed differential effect of rhEPO and CEPO on endothelial cells is also consistent with the view that these cells predominantly express (EPOR)2. However, because rhEPO also has been shown to be antiapoptotic for endothelial cells (43), it is of interest to assess whether this cell expresses the tissue-protective heteromeric receptor as well. Further study is clearly needed in this area.
Both rhEPO and CEPO enhanced EPC numbers within the bone marrow of mice treated for 2 weeks, and therefore both compounds may stimulate the formation of blood vessels. Because blood vessel formation is a key step in both normal development as well as the growth of tumors, this differential vasculogenic/angiogenic response may have important clinical implications (reviewed in refs. 44 and 45). The angiogenic potency of rhEPO may differ depending on the organ or tissue involved. For example, rhEPO has been shown to promote angiogenesis within uterine tissue (46) and ovarian and uterine tumors (47) and to be equipotent angiogenically with VEGF in myocardial tissue (48). Recent observations suggest, however, that rhEPO administration also can be associated with diminished capillary formation within tumors by means of a direct suppression of hypoxia inducible factor-1α (49).
Collectively, the results of the BP experiments performed on normal rats show that both chronic and acute rhEPO administration increases systemic BP (13, 32, 50, 51), whereas, in contrast, chronic treatment with CEPO mediates a slight decrease in systolic pressure. The increased BP induced by acute administration of rhEPO has been reported to occur by means of contraction of vascular smooth muscle, as shown in vivo (52) and in vitro (53, 54). If true, other AEs also might be relevant, as, for example, the high-dose rhEPO-dependent systemic vasoconstriction that impairs the healing of surgical skin flaps in the rat (13).
The observed regional hemodynamic differences of the kidney after acute rhEPO or CEPO injection are of particular importance. For example, a single rhEPO dose acts as a systemic and renal vasoconstrictor and causes salt retention, whereas CEPO lacks acute systemic BP effects and increases renal cortical perfusion, clearance, and sodium elimination. A previous clinical study showed that in normal volunteers maintained on a standard sodium diet, rhEPO administration (150 units/kg of bw) is associated with mild sodium retention (55). A similar effect has been observed in rabbits (56) or in isolated, perfused rat kidneys in which rhEPO mediates antinatriuresis through angiotensin II, with a maximum effect (≈50%) elicited by a concentration of 1,000 units/ml rhEPO in the perfusate (57). The potential differences between rhEPO and CEPO may be important in clinical situations with impaired renal function.
Surprisingly, we observed only small increases in BP after chronic administration of rhEPO, in contrast to much larger changes in BP reported by Vaziri et al. (32) using an identical “low dose” rhEPO protocol and the same rat strain. Our observations also differ from previous publications concerning the acute effects of rhEPO on BP. For example, Vaziri et al. (32) failed to observe acute changes in BP after single large i.v. doses of rhEPO, in contrast to the significant increase we observed. Reasonable explanations of these differences are not obvious, although our data do agree with an acute vasopressive action of rhEPO previously observed in vitro (32). Further, why a larger dose of EPO or CEPO is associated with smaller changes in BP is unclear, but could involve receptor down-regulation or attenuation of signaling induced by chronic treatment.
In summary, these experiments are consistent with the view that treatment with rhEPO at high dose may be associated with potentially clinically relevant AEs that are mediated by (EPOR)2. Nonerythropoietic tissue-protective molecules like CEPO that cannot interact with (EPOR)2 could offer significant advantages for the treatment of disease. This question will require evaluation within the setting of clinical trials.
Materials and Methods
CEPO was prepared as described in ref. 30 by using rhEPO obtained from Dragon Pharmaceuticals (Vancouver). One unit of erythropoietic activity corresponds to ≈8 ng of rhEPO. rhEPO and CEPO have approximately equal molecular weights.
Hematopoietic Colony-Forming Assay.
After informed consent and approval from the Institutional Review Board and Human Research Protection Program at the University of California at San Diego, primary human bone marrow was harvested from postoperative femoral heads obtained during total hip replacement surgery. CD34+ cells capable of differentiation into erythrocytes, leukocytes, and megakaryocytes were isolated using magnetic immunoselection (MACS; Miltenyi Biotec, Auburn, CA). These cells were plated at 5 × 102 per ml in colony-forming assays by using methylcellulose and a cytokine mixture (H4534; StemCell Technologies, Vancouver) with the addition of various concentrations of rhEPO, CEPO, or a combination of rhEPO and a fixed (100 ng/ml) concentration of CEPO. Colonies from duplicate plates were independently counted by two investigators in a blinded fashion using a square grid on the flat surface of the plate, and the results were pooled.
HUVEC Proliferation Assay.
Endothelial cells were harvested from human umbilical veins and cultured as described in ref. 58 for 48 h on fibronectin-coated glass coverslips placed in 24-well plates in MCDB 131 with 20% FBS (GIBCO). Cells were washed in medium without serum and cultured for another 24 h in medium containing 1% BSA. Fresh medium with 1% BSA was then added containing either VEGF (80 ng/ml; PeproTech, Rocky Hill, NJ), rhEPO (80 ng/ml), or CEPO (80 ng/ml), as indicated. Twenty hours later, bromodeoxyuridine (BrdU; 30 μM) was added for 4 h, and the cells were then fixed with 3% paraformaldehyde and stained with anti-BrdU antibody (Ab) (Amersham Pharmacia) followed by tetramethylrhodamine B isothiocyanate (TRITC)-conjugated Ab in the presence of Hoechst 33258 according to the methodology of Lampugnani et al. (58).
Chronic Dosing BP Studies.
Male Sprague–Dawley rats (≈250 g) were habituated to BP measurements obtained via tail cuff plethysmography (Kent Scientific, Torrington, CT), and collected data were digitized (Powerlab; A. D. Instruments, Milford, MA). Ambient room temperature was carefully controlled at 28°C. After animals were habituated to handling and restraining within the device, compounds were administered i.p. in a “low” (150 units/kg of bw twice weekly) or “high” dose (5,000 units/kg of bw three times weekly) regimen (1.2 or 40 μg/kg, respectively), and systolic–diastolic BPs were obtained weekly as an average over a 15-min acquisition period.
Drug Effects on the Bone Marrow.
Sprague–Dawley rats or wild-type C57BL/6J mice were administered CEPO or rhEPO s.c. twice a week at 10 μg/kg of bw (1,250 units/kg of bw) for 2 weeks. A peripheral blood sample was then analyzed for hemoglobin, hematocrit, and platelet count. At death, the number of EPCs in the bone marrow was determined by coexpression of the markers CD34 and VEGF receptor 2 (Flk-1), using fluorescence-activated cell sorting with a FITC-conjugated monoclonal Ab (mAb) against CD34 and a phycoerythrin (PE)-conjugated mAb against Flk-1 (BD Pharmingen).
Renal Studies.
With the approval of the Institutional Animal Utilization Committee, 12 weight-matched adult male Sprague–Dawley rats were divided into two groups of 6 animals each, and basal tail BPs were recorded. Animals were anesthetized with isoflurane (ISO), tracheotomized, and placed on a heated board to maintain core temperature at 37°C by using a rectal thermistor. Arterial BP was continuously monitored with a pressure strain transducer (Grass Instruments, Quincy, MA), and jugular vein cannulation for infusions and blood sampling was established. After laparotomy, the left renal artery was bluntly dissected, and a BF probe (TransSonic TS 420 Flowmeter; Linton Instrumentation, Norfolk, U.K.) was placed on the artery and held with a mircomanipulator. A laser-Doppler flow probe (PeriFlux Laser Doppler System 5000; Perimed AB, Jarfalla, Sweden) for monitoring renal cortical BF was positioned on the surface of the left kidney opposite the renal hilus using a second micromanipulator. The bladder was cannulated by means of a suprapubic approach. The abdomen was covered with sterile gauze saturated with warm normal saline, followed by Parafilm. Animals were allowed to stabilize for 45 min before baseline measurements were obtained.
After 60 min, animals were administered either CEPO or rhEPO at 50 μg/kg of bw, and hemodynamic measurements were obtained every 10 min for 1 h. Urine was collected for 60 min, and blood samples were obtained at 30 and 60 min and replaced isovolumetrically by using heparinized blood obtained from syngeneic rats. Cr and electrolytes were determined by routine methods; urine volume was determined gravimetrically, and Cr clearance, urine flow, and electrolyte excretion then were calculated.
BD Inflammation Model.
Adult male Fisher 344 rats (260–300 g; Harlan, Zeist, The Netherlands) were housed in groups of five or six under standard conditions at the animal research facility of the University Medical Center Groningen with free access to drinking water and rat chow. The experiments were in accordance with institutional and legislator regulations and were approved by the local Committee for Animal Experiments. Rats were pretreated 30 min before induction of BD with 10 μg/kg of bw CEPO, rhEPO, or saline (i.v.) and placed on a heating table (Inventum HNK 513; Martex Holland B.V., Veenendaal, The Netherlands) to maintain a constant body temperature of 37°C. Before induction of BD, rats were anesthetized by using O2/N2O/ISO 5%. During BD induction, anesthesia ISO was reduced to 2%. Rats were intubated and mechanically ventilated (Zoovent model CWC 600 AP; Triumph Technical Services Ltd., Milton Keynes, U.K.). Systemic BP was continuously monitored with an intra-arterial BP sensor (Truwave; Edwards Lifesciences, Irvine, CA) and regulated by infusion of a 10% hydroxyethyl starch 37°C solution as needed, when the mean arterial BP dropped to <80 mmHg. A balloon catheter (0.75 ml 4F EMB; Model No. 120404F; Edwards Lifesciences) was inserted through a frontolateral trepanation lateral to the bregma. The balloon was slowly inflated over 30 min with 0.5 ml of water by using a syringe pump (Terufusion; Model STC-521; Terumo Medical, Somerset, NJ). After ≈27 min, the rats became apneic. BD was confirmed by the absence of brainstem reflexes. After BD induction, anesthesia was stopped, and the rats were ventilated with 100% O2 for 30 min, subsequently switched to O2/air. The control group of six rats consisted of sham-operated rats, which were trepanned without inserting the balloon catheter. The sham-operated rats remained ventilated with oxygen and 5% ISO throughout the entire experiment. Ten minutes before retrieval of organs, the rats were ventilated with N2O/O2/ISO 0.5% to allow muscle relaxation and a laparotomy. Kidneys were retrieved after a flush with saline through the abdominal aorta and stored at −80°C before analysis.
Real-Time RT-PCR.
Snap-frozen tissue samples were homogenized (Ika Ultra Turrax T25; Ika Werke, Staufen, Germany), and total RNA was isolated by using TRIzol reagent (GIBCO). DNase I treatment was performed to remove genomic DNA (Invitrogen). One microgram of total RNA was reverse-transcribed into cDNA by using 1 μl (200 units/μl) of Moloney murine leukemia virus (M-MLV) reverse-transcriptase priming and 1 μl (0.5 μg/μl) of oligo(dT) (Invitrogen). When possible, primer sets were designed to lie in distant exons separated by long introns based on the published sequences by using primer express 2.0 software (Applied Biosystems). The primers and amplified fragment sizes were as follows: GAPDH, forward (fw), 5′-CGCTGGTGCTGAGTATGTCG-3′, and reverse (rv), 5′-CTGTGGTCATGAGCCCTTCC-3′ (266 bp); VCAM-1, fw, 5′-TGTGGAAGTGTGCCCGAAA-3′, and rv, 5′-ACGAGCCATTAACAGACTTTAGCA-3′ (84 bp); E-selectin, fw, 5′-GTCTGCGATGCTGCCTACTTG-3′, and rv, 5′-CTGCCACAGAAAGTGCCACTAC-3′ (73 bp); P-selectin, fw, 5′-TCTCTGGGTCTTCGTGTTTCTTATCT-3′, and rv, 5′-GTGTCCCCCTAGTACCATCTGAA-3′ (80 bp); PAI-1, fw, 5′-GCACAGGAAGGTAACGTGAATCTA-3′, and rv, 5′-TTTTTTTCCAGTGGAGATGTAACG-3′ (72 bp).
Amplification and detection were performed with the ABI Prism 7900-HT Sequence Detection System using emission from SYBR Green system (Applied Biosystems). The PCR reaction mixtures were performed in triplicate and contained 5 μl (10 ng) of cDNA, 10 μl of SYBR Green Universal PCR Master Mix, 900 nM of each primer in a total reaction volume of 20 μl. The reaction profile was 50°C, 2 min; 95°C, 10 min; 40 cycles, 95°C, 15 sec; 60°C, 1 min. Dissociation curve analyses were performed for each reaction, and real-time PCR data were plotted as the normalized relative fluorescence (ΔRn) vs. the cycle number. An arbitrary threshold was set to the mid-linear of the logRn cycle plot. The threshold cycle (CT) was defined as the cycle number at which the Rn crosses this threshold. For each gene the expression was normalized relative to the mean CT value of the GAPDH gene. Results were finally expressed as 2−ΔCT, which is an index of the relative amount of mRNA expressed in each tissue. The SD of the triplicates of the CT values was accepted, if the coefficient of variation (SD/mean) was <3%.
Statistics.
Data are shown as means ± SEM or ± SD as indicated, and n is the number of animals. Data were analyzed by ANOVA with Bonferroni’s correction used for multiple comparisons or by paired or unpaired two-tailed Student’s t test as appropriate. P < 0.05 between data means was considered to be significant.
Acknowledgments
We thank Deborah Diaz and Daniel Gomez for critical technical support, Dr. Michael Yamin for helpful discussions, and Dr. Tianxin Yang for providing us access to the instruments that were required to perform the hemodynamic and renal studies. This work was supported in part by Warren Pharmaceuticals and National Institutes of Health Grant R01 CA31615 (to K.K.).
Abbreviations
- EPO
erythropoietin
- rhEPO
recombinant human EPO
- CEPO
carbamylated rhEPO
- EPOR
EPO receptor
- (EPOR)2
homodimeric EPOR
- βc
βcommon
- AE
adverse effect
- HUVEC
human umbilical vein endothelial cell
- VCAM-1
vascular cell adhesion molecule 1
- PAI-1
plasminogen activator inhibitor-1
- Cr
creatinine
- BF
blood flow
- BD
brain dead
- ISO
isoflurane
- bw
body weight
- EPC
endothelial progenitor cell
- BP
blood pressure.
Footnotes
Conflict of interest statement: T.R.C., A.C., and M.B. are employees of Warren Pharmaceuticals, which is developing tissue-protective cytokines.
References
- 1.Eschbach J. W., Egrie J. C., Downing M. R., Browne J. K., Adamson J. W. N. Engl. J. Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 2.Grossman H. A., Goon B., Bowers P., Leitz G. J. Acquired Immune Defic. Syndr. 2003;34:368–378. doi: 10.1097/00126334-200312010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Santini D., Vincenzi B., La Cesa A., Virzi V., Navajas F., Malafarina V., Dicuonzo G., Cassandro R., Esposito V., Montesarchio V., et al. Anticancer Res. 2005;25:669–674. [PubMed] [Google Scholar]
- 4.Rosenzweig M. Q., Bender C. M., Lucke J. P., Yasko J. M., Brufsky A. M. J. Pain Symptom Manage. 2004;27:185–190. doi: 10.1016/j.jpainsymman.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Leyland-Jones B. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 6.Henke M., Laszig R., Rube C., Schafer U., Haase K. D., Schilcher B., Mose S., Beer K. T., Burger U., Dougherty C., Frommhold H. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 7.Khorana A. A., Francis C. W., Culakova E., Lyman G. H. Cancer. 2005;104:2822–2829. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 8.Westenfelder C., Biddle D. L., Baranowski R. L. Kidney Int. 1999;55:808–820. doi: 10.1046/j.1523-1755.1999.055003808.x. [DOI] [PubMed] [Google Scholar]
- 9.Juul S. E., Yachnis A. T., Christensen R. D. Early Hum. Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 10.Westenfelder C. Exp. Nephrol. 2002;10:294–298. doi: 10.1159/000065304. [DOI] [PubMed] [Google Scholar]
- 11.Brines M., Cerami A. Nat. Rev. Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 12.Moon C., Krawczyk M., Paik D., Lakatta E. G., Talan M. I. Cardiovasc. Drugs Ther. 2005;19:243–250. doi: 10.1007/s10557-005-3189-6. [DOI] [PubMed] [Google Scholar]
- 13.Saray A., Ozakpinar R., Koc C., Serel S., Sen Z., Can Z. Laryngoscope. 2003;113:85–89. doi: 10.1097/00005537-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Fisher J. W. Exp. Biol. Med. (Maywood, NJ) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 15.Clyne N., Berglund B., Egberg N. Thromb. Res. 1995;79:125–129. doi: 10.1016/0049-3848(95)91520-u. [DOI] [PubMed] [Google Scholar]
- 16.Stohlawetz P. J., Dzirlo L., Hergovich N., Lackner E., Mensik C., Eichler H. G., Kabrna E., Geissler K., Jilma B. Blood. 2000;95:2983–2989. [PubMed] [Google Scholar]
- 17.Wolf R. F., Peng J., Friese P., Gilmore L. S., Burstein S. A., Dale G. L. Thromb. Haemostasis. 1997;78:1505–1509. [PubMed] [Google Scholar]
- 18.Tassies D., Reverter J. C., Cases A., Calls J., Escolar G., Ordinas A. Am. J. Hematol. 1998;59:105–109. doi: 10.1002/(sici)1096-8652(199810)59:2<105::aid-ajh1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Fuste B., Serradell M., Escolar G., Cases A., Mazzara R., Castillo R., Ordinas A., Diaz-Ricart M. Thromb. Haemostasis. 2002;88:678–685. [PubMed] [Google Scholar]
- 20.Wolf R. F., Gilmore L. S., Friese P., Downs T., Burstein S. A., Dale G. L. Thromb. Haemostasis. 1997;77:1020–1024. [PubMed] [Google Scholar]
- 21.Wun T., Law L., Harvey D., Sieracki B., Scudder S. A., Ryu J. K. Cancer. 2003;98:1514–1520. doi: 10.1002/cncr.11700. [DOI] [PubMed] [Google Scholar]
- 22.Nomura S., Nakamura T., Cone J., Tandon N. N., Kambayashi J. Cytometry. 2000;40:173–181. [PubMed] [Google Scholar]
- 23.Aguilera A., Selgas R., Ruiz-Caravaca M. L., Bajo M. A., Cuesta M. V., Plaza M. A., Hernanz A. Peritoneal Dialysis Int. 1999;19(Suppl. 2):S161–S166. [PubMed] [Google Scholar]
- 24.Ando M., Iwata A., Ozeki Y., Tsuchiya K., Akiba T., Nihei H. Kidney Int. 2002;62:1757–1763. doi: 10.1046/j.1523-1755.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa A., Suzuki K., Fujita K. Urol. Res. 1999;27:312–314. doi: 10.1007/s002400050156. [DOI] [PubMed] [Google Scholar]
- 26.Abraham P. A., Macres M. G. J. Am. Soc. Nephrol. 1991;2:927–936. doi: 10.1681/ASN.V24927. [DOI] [PubMed] [Google Scholar]
- 27.Buckner F. S., Eschbach J. W., Haley N. R., Davidson R. C., Adamson J. W. Am. J. Hypertens. 1990;3:947–955. doi: 10.1093/ajh/3.12.947. [DOI] [PubMed] [Google Scholar]
- 28.Tokish J. M., Kocher M. S., Hawkins R. J. Am. J. Sports Med. 2004;32:1543–1553. doi: 10.1177/0363546504268041. [DOI] [PubMed] [Google Scholar]
- 29.Brines M., Grasso G., Fiordaliso F., Sfacteria A., Ghezzi P., Fratelli M., Latini R., Xie Q. W., Smart J., Su-Rick C. J., et al. Proc. Natl. Acad. Sci. USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leist M., Ghezzi P., Grasso G., Bianchi R., Villa P., Fratelli M., Savino C., Bianchi M., Nielsen J., Gerwien J., et al. Science. 2004;305:239–242. [Google Scholar]
- 31.Creutzig A., Caspary L., Nonnast-Daniel B., Bahlmann J., Kuhn K., Brunkhorst R., Reimers E., Koch K. M., Alexander K. Eur J. Clin. Invest. 1990;20:219–223. doi: 10.1111/j.1365-2362.1990.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 32.Vaziri N. D., Zhou X. J., Smith J., Oveisi F., Baldwin K., Purdy R. E. Am. J. Physiol. 1995;269:F838–F845. doi: 10.1152/ajprenal.1995.269.6.F838. [DOI] [PubMed] [Google Scholar]
- 33.Nowicki M. J. Hum. Hypertens. 1995;9:81–88. [PubMed] [Google Scholar]
- 34.Cotter D. J., Stefanik K., Zhang Y., Thamer M., Scharfstein D., Kaufman J. J. Clin. Epidemiol. 2004;57:1086–1095. doi: 10.1016/j.jclinepi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Thamer M., Stefanik K., Kaufman J., Cotter D. J. Am. J. Kidney Dis. 2004;44:866–876. [PubMed] [Google Scholar]
- 36.Besarab A., Bolton W. K., Browne J. K., Egrie J. C., Nissenson A. R., Okamoto D. M., Schwab S. J., Goodkin D. A. N. Engl. J. Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 37.Ehrenreich H., Hasselblatt M., Dembowski C., Cepek L., Lewczuk P., Stiefel M., Rustenbeck H. H., Breiter N., Jacob S., Knerlich F., et al. Mol. Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 38.Gorio A., Madaschi L., Di Stefano B., Carelli S., Di Giulio A. M., De Biasi S., Coleman T., Cerami A., Brines M. Proc. Natl. Acad. Sci. USA. 2005;102:16379–16384. doi: 10.1073/pnas.0508479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin A., Ryu J., Harvey D., Sieracki B., Scudder S., Wun T. Gynecol. Oncol. 2006 doi: 10.1016/j.ygyno.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 40.Ley K. Cardiovasc. Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 41.Kahraman S., Yilmaz R., Kirkpantur A., Genctoy G., Arici M., Altun B., Erdem Y., Yasavul U., Turgan C. Nephrology (Carlton) 2005;10:264–269. doi: 10.1111/j.1440-1797.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 42.Villa P., Bigini P., Mennini T., Agnello D., Laragione T., Cagnotto A., Viviani B., Marinovich M., Cerami A., Coleman T. R., et al. J. Exp. Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlini R. G., Alonzo E. J., Dominguez J., Blanca I., Weisinger J. R., Rothstein M., Bellorin-Font E. Kidney Int. 1999;55:546–553. doi: 10.1046/j.1523-1755.1999.00266.x. [DOI] [PubMed] [Google Scholar]
- 44.Aghi M., Chiocca E. A. Mol. Ther. 2005;6:994–1005. doi: 10.1016/j.ymthe.2005.07.693. [DOI] [PubMed] [Google Scholar]
- 45.Byrd N., Grabel L. Trends Cardiovasc. Med. 2004;14:308–313. doi: 10.1016/j.tcm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda Y., Masuda S., Chikuma M., Inoue K., Nagao M., Sasaki R. J. Biol. Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda Y., Musha T., Tanaka H., Fujita Y., Fujita H., Utsumi H., Matsuo T., Masuda S., Nagao M., Sasaki R., Nakamura Y. Br. J. Cancer. 2001;84:836–843. doi: 10.1054/bjoc.2000.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaquet K., Krause K., Tawakol-Khodai M., Geidel S., Kuck K. H. Microvasc. Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 49.Hale S. A., Wong C., Lounsbury K. M. Gynecol. Oncol. 2005;100:14–19. doi: 10.1016/j.ygyno.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 50.Huang C., Davis G., Johns E. J. Clin. Sci. (London) 1992;83:453–459. doi: 10.1042/cs0830453. [DOI] [PubMed] [Google Scholar]
- 51.Heidenreich S., Rahn K. H., Zidek W. Kidney Int. 1991;39:259–265. doi: 10.1038/ki.1991.31. [DOI] [PubMed] [Google Scholar]
- 52.Kanagy N. L., Perrine M. F., Cheung D. K., Walker B. R. J. Cardiovasc. Pharmacol. 2003;42:527–533. doi: 10.1097/00005344-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Ammarguellat F., Gogusev J., Drueke T. B. Nephrol. Dial. Transplant. 1996;11:687–692. doi: 10.1093/oxfordjournals.ndt.a027361. [DOI] [PubMed] [Google Scholar]
- 54.Neusser M., Tepel M., Zidek W. Cardiovasc. Res. 1993;27:1233–1236. doi: 10.1093/cvr/27.7.1233. [DOI] [PubMed] [Google Scholar]
- 55.Bunke M., Gleason J. R., Jr, Brier M., Sloan R. Clin. Pharmacol. Ther. 1994;55:563–568. doi: 10.1038/clpt.1994.70. [DOI] [PubMed] [Google Scholar]
- 56.Nushiro N., Sakamaki T., Hoshino J., Nakamura T., Sakamoto H., Imai Y., Seino M., Omata K., Sekino H., Abe K. Hypertens. Res. 1995;18:203–207. doi: 10.1291/hypres.18.203. [DOI] [PubMed] [Google Scholar]
- 57.Brier M. E., Bunke C. M., Lathon P. V., Aronoff G. R. J. Am. Soc. Nephrol. 1993;3:1583–1590. doi: 10.1681/ASN.V391583. [DOI] [PubMed] [Google Scholar]
- 58.Lampugnani M. G., Zanetti A., Corada M., Takahashi T., Balconi G., Breviario F., Orsenigo F., Cattelino A., Kemler R., Daniel T. O., Dejana E. J. Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]