Abstract
Onchocerciasis (river blindness) is a major parasitic disease of humans in sub-Saharan Africa caused by the microfilarial stage of the nematode Onchocerca volvulus. Using Onchocerca ochengi, a closely related species which infects cattle and is transmitted by the same black fly vector (Simulium damnosum sensu lato) as O. volvulus, we have conducted longitudinal studies after either natural field exposure or experimental infection to determine whether, and under what circumstances, protective immunity exists in onchocerciasis. On the basis of the adult worm burdens (nodules) observed, we determined that cattle reared in endemic areas without detectable parasites (putatively immune) were significantly less susceptible to heavy field challenge than age-matched, naïve controls (P = 0.002), whereas patently infected cattle, cured of infection by adulticide treatment with melarsomine, were fully susceptible. Cattle immunized with irradiated third-stage larvae were significantly protected against experimental challenge (100% reduction in median nodule load, P = 0.003), and vaccination also conferred resistance to severe and prolonged field challenge (64% reduction in median nodule load, P = 0.053; and a significant reduction in microfilarial positivity rates and density, P < 0.05). These results constitute evidence of protective immunity in a naturally evolved host–Onchocerca sp. relationship and provide proof-of-principle for immunoprophylaxis under experimental and field conditions.
Keywords: filariasis, irradiation, larvae, ochengi, Onchocerca
Onchocerciasis (river blindness), caused by the filarial nematode Onchocerca volvulus and transmitted by the vector Simulium damnosum sensu lato, is a debilitating disease of humans, affecting large areas of Africa but also endemic in parts of Central and South America. It is responsible for almost 1 million disability-adjusted life years (1). The adult worms live for 10 years or more in infected hosts (2), continually producing first-stage larvae [microfilariae (Mf)], which invade the skin and eyes and in the latter case, provoke ocular pathology resulting in blindness.
Because of its huge socioeconomic significance, onchocerciasis has been the subject of major international control efforts; first by the Onchocerciasis Control Program, which implemented vector control in 11 countries of West Africa, and now by the African Program for Onchocerciasis Control, which is using the microfilaricidal agent ivermectin as its principal control tool (3). Although the Onchocerciasis Elimination Program for the Americas is using ivermectin in attempts to reduce transmission in circumscribed foci to the point of extinction, this approach is not a realistic possibility in Africa. The Conference on the Global Eradicability of Onchocerciasis held in Atlanta in 2002, while affirming the significant progress made in control achieved with insecticide and ivermectin usage, recognized the problems of insecticide resistance and of potential ivermectin resistance and the need for additional control tools (4). Currently there is no safe adulticidal (macrofilaricidal) drug available for mass therapy, although the encouraging results against Onchocerca spp. with antibiotic therapy, presumed to be active via endosymbiotic Wolbachia pipientis bacteria, have opened up new prospects (5, 6).
Cognizant of the need for a broader range of control options, the Edna McConnell Clark Foundation embarked on a program to stimulate vaccine development for onchocerciasis in 1985. The program (reviewed in a series of papers in Trends in Parasitology, see ref. 7) stimulated research to investigate the existence of protective immunity in humans and its mechanisms, to identify candidate vaccine antigens, and to develop animal models and seek proof-of-principle for prophylactic immunization. This paper describes one of the major results of that program. The nature of protective immunity against onchocerciasis is relevant to not only the possible development of vaccines but also to the application of chemotherapy, in which the existence or induction of immunity in treated, exposed, or infected individuals might have profound effects on the long-term consequences of drug interventions. In humans, evidence of protective immunity in onchocerciasis is derived principally and circumstantially from the existence of noninfected individuals living in endemic or hyperendemic areas. These endemic normals are also termed putatively immune (PI). Various studies have shown that the ex vivo immune responses of PI to onchocercal antigen differ from those of infected patent individuals (8–11). To what extent these parasitological and immunological differences may reflect subtle differences in exposure to infected Simulium spp. among individuals is controversial. Although vector attractiveness per se has been discounted as a hypothesis for the apparent absence of parasites in human PI (12), heterogeneity in exposure is sufficient to explain the local population dynamics of onchocerciasis according to a mathematical model (13). Clearly, it is difficult and potentially unethical to test how putative is immunity of human PI or to experimentally induce immunity. Here, we report experiments in cattle with the parasite Onchocerca ochengi to investigate the nature of functional immunity in onchocerciasis. O. ochengi is an excellent analogue of O. volvulus. It is extremely closely related to O. volvulus (14), is transmitted by the same black fly vector as O. volvulus, and because of its intradermal nodular location, is amenable to quantitative estimation of protection in the face of challenge (15). In addition, in areas endemic for O. ochengi, PI cattle occur as a subset within herds of infected animals (16, 17); and for experimental purposes, it is possible to generate large numbers of infective parasites [third-stage larvae (L3)] in black flies obtained from local breeding sites. In a series of experiments involving either exposure to natural field challenge in Cameroon or experimental single-pulse challenge in Liverpool, United Kingdom, we have asked several key questions about the nature of protective immunity in onchocerciasis; namely, are PI actually immune, are infected drug-cured individuals immune to subsequent challenge and, most critically, is it possible to induce immunity?
Results
PI Cattle Are Relatively Resistant to Challenge (Exp. 1).
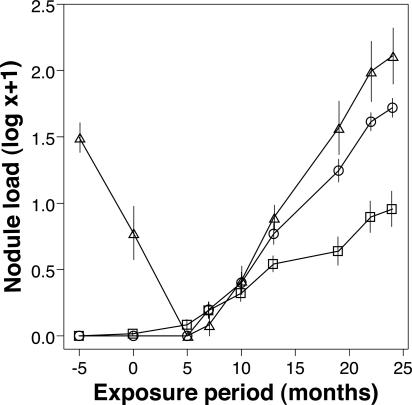
Cattle categorized as PI were resistant to heavy field challenge. During the overall period of exposure, the mean nodule load of PI cattle was significantly lower than that of the originally naïve cattle (P = 0.002; Fig. 1). In addition, PI cattle exhibited a substantially slower rate of nodule acquisition over time relative to the originally naïve group (linear contrast, P < 0.001; Fig. 1). At the termination of the experiment (24 months after exposure), the median nodule load of PI cattle was significantly lower than that in the originally naïve controls (Table 1).
Fig. 1.
Exp. 1: Acquisition of O. ochengi nodules in originally naïve (○, n = 14), melarsomine-treated (Δ, n = 6), and putatively immune (□, n = 18) cattle during 24 months of natural exposure. Data represent the mean ± SE.
Table 1.
Exp. 1: Prevalence and burden of O. ochengi in originally naïve, PI, and melarsomine-treated cattle after 24 months of exposure to natural challenge
| Originally naïve | Putatively immune | Treated | P* | P† | |
|---|---|---|---|---|---|
| Frequency of nodule-positive animals | 14/14 | 18/18 | 6/6 | ||
| Frequency of Mf-positive animals | 14/14 | 15/18 | 6/6 | 0.238 | |
| Median no. of nodules (range) | 57.0 (20–176) | 5.0 (1–229) | 115.0 (26–522) | <0.001 | 0.124 |
| Median Mf density per 100 mg of skin (range) | 1594.2 (75–6,145) | 609.7 (0–4,806) | 1009.8 (309–16,179) | 0.022 | 0.659 |
*Originally naïve vs. putatively immune.
†Originally naïve vs. treated.
The density of Mf per 100 mg of skin was also substantially lower in PI animals compared with originally naïve cattle for the overall exposure period (P = 0.025; data not shown), and Mf densities increased less rapidly in these animals relative to the originally naïve group (linear contrast, P = 0.004; data not shown). At the termination of the experiment, the median Mf density of the PI group was significantly lower than that of the originally naïve group (Table 1).
PI and Naïve Cattle Are Equally Attractive to the Black Fly Vector (Exp. 1).
To determine whether the difference in infection intensity was caused by differential attractiveness to flies, exposure indices were compared in March 1997 and January 1998. There was no difference in median exposure indices of naïve and PI cattle at either time (data not shown). The mean number of flies caught per cow per day was 74.3 in March 1997 and 746.1 in January 1998. There was no correlation between the exposure indices of individual cows in 1997 and in 1998; thus, there was no evidence that certain individuals were more attractive to flies than others.
Previously Infected, Drug-Cured Animals Are Fully Susceptible to Reinfection (Exp. 1).
In treated cattle, melarsomine was macrofilaricidal, totally eliminating adult worms. The group (n = 6) total nodule load fell from 208 at treatment to 50 at time 0. These nodules were all abnormally small and hard by palpation; a sample of eight was removed, and none contained a viable worm. By 5 months after exposure, a point in time too soon for nascent nodules to appear, the palpable nodule count was 0 (Fig. 1). Subsequently, new nodules developed rapidly, and the cumulative mean load exceeded that in the originally naïve group from 7 months after exposure (Fig. 1 and Table 1; not significant).
The Mf density for the treated group fell to 0 by 5 months after exposure and thereafter increased at a similar rate to that observed in the originally naïve group (data not shown). At 24 months after exposure, the median Mf density per 100 mg of skin was not significantly different from that of the originally naïve group (Table 1).
Vaccination with Irradiated L3 of O. ochengi Induces Significant Protection Against Experimental Challenge (Exp. 2).
O. ochengi nodules were first detected in challenge control animals at 153 days after challenge and after 210 days in vaccinated and challenged animals. Vaccination induced a significant reduction both in the positivity rates for O. ochengi nodules and in the median number of nodules detected postmortem (Table 2). The reduction in mean nodule load conferred by vaccination was 84% (mean = 6.1 for challenge controls and 1.0 for vaccinated and challenged animals). Significantly fewer male worms, gravid female worms, and total adult worms were recovered from vaccinated cattle compared with controls (Table 2).
Table 2.
Exp. 2: Protection against experimental challenge induced by vaccination of cattle with irradiated O. ochengi third-stage larvae: postmortem results at 12 months after challenge
| Control | Vaccinated | P | |
|---|---|---|---|
| Frequency of nodule-positive animals | 8/8 | 3/8 | 0.026 |
| Frequency of Mf-positive animals* | 6/8 | 3/8 | 0.315 |
| Median no. of nodules (range) | 4.0 (2–16) | 0.0 (0–3) | 0.003 |
| Median no. of male worms (range) | 4.5 (0–7) | 0.0 (0–2) | 0.009 |
| Median total worm recovery (range) | 8.5 (2–22) | 0.0 (0–5) | 0.002 |
| Median no. of gravid females (range) | 2.0 (0–5) | 0.0 (0–2) | 0.041 |
| Median Mf density per 100 mg of skin (range) | 0.7 (0.0–123.0) | 0.0 (0.0–12.0) | 0.282 |
*As determined by microscopy and/or O-150 diagnostic PCR.
Three of eight vaccinated and challenged animals compared with six of eight challenge control animals developed microfilaridermia as detected by microscopy and diagnostic O-150 PCR (18) (targeting a 150-bp tandem repeat DNA sequence), although there was no significant difference in Mf densities between the two groups (Table 2). Relative to challenge controls, no significant difference was observed in the lengths of male worms. One animal from the vaccinated and challenged group had Mf without a gravid female parasite being identified, suggesting that not all nodules had been found. No nodules or skin Mf were found in vaccine control animals.
The in vitro molting rates of irradiated and nonirradiated O. ochengi L3 were compared to determine whether irradiation reduced the ability of parasites to undergo the third-to-fourth stage molt. Both groups began to molt after 3 days in culture, with the numbers of molting larvae increasing in each group at a comparable rate to a final level of 50% and 42% in the nonirradiated and irradiated groups, respectively, after 10 days (data not shown).
Vaccination with Irradiated L3 of O. ochengi Induces Significant Protection Against Natural Challenge (Exp. 3).
Intradermal nodules were first detected in both control and vaccinated animals at 9 months after exposure. However, by the termination of the experiment at 22 months after exposure, the median nodule load was considerably reduced in the vaccinated group compared with the control, although only one vaccinated animal had no nodules (Table 3). With respect to mean nodule loads, the reduction conferred by vaccination was 53% (mean = 14.6 for the control group and 6.9 for vaccinated animals). The effect on gravid female worms was greater, with a highly significant reduction in the vaccinated group, whereas the recovery of adult male worms was not significantly affected (Table 3).
Table 3.
Exp. 3: Protection against natural challenge (22 months of exposure) induced by vaccination of cattle with irradiated O. ochengi third-stage larvae
| Control | Vaccinated | P | |
|---|---|---|---|
| Frequency of nodule-positive animals | 13/13 | 8/9 | 0.409 |
| Frequency of Mf-positive animals | 13/13 | 3/9 | 0.001 |
| Median no. of nodules (range) | 14.0 (1–45) | 5.0 (0–15) | 0.053 |
| Median no. of male worms (range) | 13.0 (1–34) | 7.0 (0–19) | 0.168 |
| Median total worm recovery (range) | 27.0 (2–79) | 13.0 (0–33) | 0.074 |
| Median no. of gravid females (range) | 3.0 (1–9) | 0.0 (0–3) | 0.009 |
| Median Mf density per 100 mg of skin (range) | 17.5 (0.0–317.2)* | 0.0 (0.0–99.4) | 0.042 |
*One animal was negative at this time point but had been positive on prior occasions.
Although Mf patency was first detected in the control group at 12 months after exposure, the prepatency period in the vaccinated group was 50% longer (data not shown). In accordance with the lower burden of gravid parasites in the vaccinated animals, only one-third of this group’s skin biopsies were positive for Mf by the end of the experiment, in contrast to all of the control cattle (Table 3). Furthermore, the median Mf density was reduced significantly in vaccinated cattle relative to the control animals (Table 3). There was no statistically significant relationship between positive skin biopsies in calves at the termination of the experiment and positive status of dams for Mf before calving (relative risk, 1.2; 95% confidence interval, 0.75–1.97).
Discussion
This paper demonstrates resistance to infection in a defined host subset exposed to a filarial infection under natural conditions. Although the PI cattle in the present study did not exhibit absolute refractoriness to O. ochengi infection, the burden of both adult parasites and Mf in these animals was substantially lower than that in the controls and accumulated at a slower rate. At a minimum, these observations indicate the existence of considerable heterogeneity in susceptibility to O. ochengi among Gudali cattle (19), if not distinct “resistant” and “permissive” populations. In regions that are hyperendemic for O. volvulus, human PI subpopulations have been identified in both West Africa (20) and Latin America (21). This phenomenon has been the focus of intensive immunological study (8–10, 22), and although several characteristic features have been identified in PI, including a discrete distribution of HLA-D alleles (20), it has not been possible to categorically exclude the influence of risk factors such as occupation, social status, and migration. A human bait catch study conducted in Guinea concluded that PI and those with generalized onchocerciasis did not differ significantly in vector attractiveness, although fly catches were performed over a single 2-day period, and the vectors were caught before biting for ethical reasons (12). In the current study, the counterpart experiment in cattle was performed over two 10-day periods separated by 10 months, with the black flies being caught after engorgement, and clearly showed that the parasitological results were not related to vector attractiveness. Furthermore, behavior-related differences in the history of exposure can be effectively excluded in cattle. This is particularly important because a mathematical model that analyzed Mf burdens in different endemic regions concluded that heterogeneity of exposure can explain the observed age- and sex-specific profiles in human onchocerciasis (13).
The induction of protective immunity against helminths by inoculation with irradiated L3 has a long history (23) and is the basis of the currently available, commercially licensed vaccine against Dictyocaulus viviparus (lungworm) in cattle (24). Accordingly, in several hosts challenged with a range of filarial species, 50–100% protective efficacy has been reported after inoculation with irradiated L3. Thus, O. volvulus (25), Brugia pahangi (26), and Litomosoides sigmodontis (27) have been used in murine (surrogate) hosts. Natural host–filaria systems in which significant protection has been demonstrated include Loa loa in mandrills (28), Acanthocheilonema viteae in jirds (29), and Dirofilaria immitis (30) in dogs. However, in this report, the irradiated L3 vaccine is shown to reduce the burden of a filarial infection under conditions of intensive field challenge and in a natural Onchocerca sp. host–parasite relationship. Although the control animals in our field trial were also included in the evaluation of a recombinant vaccine (parallel experiment not reported here) and consequently were injected with adjuvants, any nonspecific enhancement of immunity in these calves would have been expected to reduce the apparent efficacy of the irradiated vaccine in statistical comparisons. In fact, the observed levels of parasitosis in the adjuvant-treated controls were typical for unprotected animals exposed for 2 years at this site (31).
To determine whether protective immunity could be induced by patent infection and revealed by chemotherapeutic elimination of existing parasites, we used the antifilarial drug melarsomine (which is too toxic for macrofilaricidal therapy in humans). In marked contrast to PI and vaccinated animals, melarsomine-treated cattle were fully susceptible to reinfection. This finding is in accordance with observations in O. volvulus-infected humans treated with the macrofilaricidal drug suramin, who became reinfected after chemotherapy and developed Mf loads at pretreatment levels (32).
The immunology of filarial infections is exceedingly complex because of the presence of two sexes and four life-cycle stages in the definitive host: the L3, fourth-stage larva, fifth-stage larva (which matures into the adult), and Mf. The majority of studies have focused on the L3 because this is the target that would prevent initial establishment of infection. The fact that during a primary infection, the majority of L3 are rapidly killed by innate inflammatory processes [probably involving neutrophils (33)] is commonly overlooked. In contrast, a clear consensus has emerged that immunity induced by irradiated L3 is mediated by activated eosinophils (29, 34, 35) and that killing of challenge larvae occurs rapidly, with a major reduction in survival before the molt to the fourth stage (27, 29). The immune responses in PI that prevent or ameliorate infection are similarly assumed to operate against the infective L3, and indeed, there is evidence that human PI recognize a specific subset of larval antigens (9, 36). However, targeting of stages other than L3 should not be discounted in the understanding of antifilarial immunity because distinct reactivity against adult male worm antigen has also been identified in human PI (22).
The development of a patent filarial infection culminates in an immunologically hyporesponsive state, in which T helper lymphocyte responses to adult female and Mf antigens are suppressed (37, 38) by T regulatory cells (39). The existence of concomitant immunity against superinfection with L3 during the patent phase is controversial. In contrast to responses to adult female and Mf antigens, lymphoproliferation in response to O. volvulus L3 antigen has been reported to increase with host age, consistent with the development of partial protection against this stage (40). However, in a statistical model based on nodulectomy data collected from hyperendemic areas of West Africa, adults were shown to acquire far more mature parasites per year than children acquired, and this finding was attributed to the immunosuppressive influence of female worms (41). In the current study, failure of radical cure to elicit resistance to reinfection may have been due to “conditioning” of the immune system toward hyporesponsiveness during patency (39), which outlived the presence of viable worms.
The significance of natural immunity against Mf in human onchocerciasis is also unclear (13). An analysis of the relationship between host age, adult worm burden, female worm fecundity, and Mf density in natural O. ochengi infection of cattle indicated a reduction of microfilaridermia in older animals, and this reduction could not be explained by impaired embryogenesis in female worms, suggesting the existence of anti-Mf immunity (16). Such an interpretation has been challenged for human onchocerciasis by a mathematical modeling approach, in which Mf density increased with host age and independently of the worm burden, presumably as a result of parasite-induced immunosuppression (42). In this case, the observed density dependence between adult worm burden and Mf load could be a result of intraspecific competition (42, 43). However, because anti-Mf immunity is an irrefutable aspect of the sowda form of onchocerciasis (44), a complete absence of immunological control against Mf in the generalized disease seems unlikely.
Immunity against Mf is of great importance to onchocerciasis control, as Mf is the stage that is associated with pathology. In this context, the significant reductions in Mf positivity rates and density in vaccinated animals reported here are particularly noteworthy. These effects are unlikely to be simply a result of reduced adult worm burdens in the vaccinated cattle because density dependence would suggest a compensatory increase in Mf output by female worms. Anti-Mf immunity against the bovine parasite Onchocerca lienalis has been successfully induced in mice by homologous immunization by using live L3 (45), presumably as a result of antigenic crossreactivity between these life-cycle stages. Furthermore, inoculation of mandrills with irradiated L3 of Loa loa resulted in the delayed onset of microfilaraemia after challenge (28). However, it is also possible that immune-mediated damage directed against challenge larvae in the current study resulted in impaired fecundity of the adult “breakthrough” infections, as has been reported in the L. sigmodontis model (46), and this is certainly consistent with the reduced numbers of gravid females recovered from vaccinated animals.
The presently reported experiments, taken with other published studies using O. ochengi in cattle, allow a description of the characteristics of functional protective immunity in a natural Onchocerca sp. host–parasite relationship in an outbred population. As such, it can provide an authentic paradigm for O. volvulus (15). In the host population, there is a minority subset of individuals genuinely resistant to infection. In the susceptible majority, protection can be induced by irradiated L3 larvae but not by normal infective larvae because after natural infection and radical drug treatment, no protective immunity exists. Whether this lack of immunity is due to the persistence of adult worm-mediated down-regulation (38) of an otherwise competent response is unclear, but it is revealing that chemical attenuation of larval infections by ivermectin does not induce protection either (31). This evidence indicates the unique immunological characteristics of irradiated L3 and strongly supports the feasibility of immunoprophylaxis against Onchocerca spp. However, because irradiated L3 could never be used in humans because of constraints on mass production, stability, and safety, the next step is to reproduce significant protection using defined antigens (47).
Materials and Methods
Experiments, Ethical Approval, and Parasitology.
The study was divided into three experiments that investigated the susceptibility of cattle to O. ochengi: Exp. 1 involved simultaneous exposure of naïve, PI, and drug-cured Bos indicus to natural transmission in Cameroon; Exp. 2 evaluated the efficacy of an irradiated L3 vaccine in experimentally challenged Bos taurus calves housed in the United Kingdom; and Exp. 3 used an equivalent vaccination protocol in B. indicus calves that were subsequently exposed to natural challenge in Cameroon. Institutional approval for Exp. 2 was obtained from the University of Liverpool, and all procedures were performed in accordance with the directives contained in a UK Home Office Project License [Animals (Scientific Procedures) Act 1986]. Similar protocols were used in Cameroon (Exps. 1 and 3), which has no domestic legislation to regulate the experimental use of animals. In all experiments, parasitological observations were recorded at predetermined intervals: intradermal nodules were enumerated by palpation, and microfilaridermia was quantified by microscopic examination of skin biopsies removed from the ventral midline as described in ref. 48.
Field Site (Exps. 1 and 3).
The Adamawa Plateau is a Guinea savannah, upland area in northern Cameroon with a high cattle population where both human onchocerciasis (O. volvulus) and bovine onchocerciasis (O. ochengi) are endemic. Rates of O. ochengi transmission vary locally but are extremely high close to riverine breeding sites of the simuliid vector.
The studies were based at the laboratories of the Institut de Recherche Agricole pour le Développement (IRAD) (lat 7°12′33′′N, long 13°34′20′′E), which is located in the Adamawa Province. The prevalence of O. ochengi infection among cattle and its transmission potential at IRAD is known to be very low. For exposure to high levels of natural challenge, cattle were moved to pasture bordering the perennial river Vina du Sud, which has numerous breeding sites for Simulium squamosum, a member of the S. damnosum species complex. In this hyperendemic area, the annual transmission potential for O. ochengi has been estimated at 74,000 L3 per animal (49). Transmission is seasonal, and daily transmission potentials average nearly 400 L3 in the dry season (early November through late March), when their numbers are 36 times higher than during the rainy season. In each experiment, groups were grazed together as a single herd and were continuously exposed to fly challenge.
Selection of Animals and Field Exposure (Exp. 1).
From herds of Gudali cattle around Ngaoundéré on the Adamawa Plateau, healthy cows 5–8 years old (herdsmen’s evidence of age was confirmed in each cow by the presence of a fourth incisor tooth, degree of dental wear, and general physical condition of the cow) were recruited into PI or infected, treated groups according to the following criteria. PI animals had lived for 5 years in the same herd or area. In PI animals, whole-body palpation detected no nodules, three skin biopsies detected no O. ochengi Mf, and the prevalence and intensity of O. ochengi infection in the herd was consistent with hyperendemicity (>80% prevalence of nodules in cattle >5 years old and some individuals with nodule loads >20). For the infected, treated group (destined for chemotherapeutic elimination of O. ochengi infections), animals each had >9 palpable O. ochengi nodules and Mf-positive skin biopsies. To obtain animals that had never been exposed to O. ochengi for use as noninfected, age-matched, naïve controls, it was necessary to seek cattle from beyond the Adamawa Plateau. Massa and Fulbé breed cows, 5–8 years old, were recruited from herds around Yagoua, Extreme North Province, Cameroon, because there are no suitable vector breeding sites in the region and previous visits did not identify endemic O. ochengi infection. These three groups of cattle, PI (n = 18), previously patently-infected, drug-cured animals (n = 6), and naïve controls (n = 14), were exposed to natural field challenge for 24 months.
Chemotherapy (Exp. 1).
Infected cows were each treated with three doses of 4 mg/kg melarsomine hydrochloride (Cymelarsan; Rhône-Mérieux, Lyon, France) in aqueous solution by slow i.v. injection on alternate days, 5 months before exposure. Total doses administered varied from 3.72 to 4.59 g. This regime has been proven to be macrofilaricidal in an experiment involving serial nodulectomies and worm viability assays (V.L.T., V.N.T., and A.J.T., unpublished data). Five months were allowed to elapse after treatment and before exposure to verify the macrofilaricidal effect and to ensure the elimination of all drug residues.
Comparisons of Vector Attractiveness (Exp. 1).
In two, 10-day periods during field exposure, black flies biting and feeding on each animal were caught and counted. On successive days, flies were caught from two animals per group (i.e., six animals per day) until all animals had been included (thus, for the smaller treated group, repeat catches were made with the same individuals). From 1530 to 1730 hours local time [coinciding with peak diurnal biting activity (50)], feeding flies (judged by abdominal distension) were collected by pooter (mouth-operated aspirator) by a single catcher at each cow. To control for catcher variability, each person caught for an equal time from each cow, moving from one animal to the next every 20 min. The total daily catch per cow was expressed as a percentage of the day’s total catch on all six cattle (the exposure index). The identity of 23 flies taken at different catching times was determined by multivariate morphotaxonomy (51) to be S. squamosum (J. B. Davies, personal communication), a confirmed vector of both O. ochengi and O. volvulus in this region (49).
Vaccine Preparation and Protocol (Exps. 2 and 3).
Cattle naturally infected with O. ochengi were used as bait to obtain infected Simulium spp. at the River Mungo, South-West Province, Cameroon. Blood-fed flies were housed individually at 27°C, 85% humidity and fed 30% (wt/vol) sucrose supplemented with 200 units/ml penicillin and 200 μg/ml streptomycin for 7 days. The L3 dissected from flies were cryopreserved following a protocol modified from ref. 52. The larvae were suspended in 4 mM polyvinylpyrrolidone (Sigma), 9% (vol/vol) DMSO, and 10% (vol/vol) FCS (GIBCO) in Grace’s Insect Medium (Sigma) and incubated at 0°C for 30 min. By using Bio-Cool computerized cryopreservation equipment (FTS Systems, Stone Ridge, NY) larvae were frozen at a rate of 1°C/min for 40 min, maintained at −40°C for 30 min, and then transferred to liquid nitrogen. Ultra-frozen larvae were transported to the United Kingdom, thawed at 37°C, and washed three times by centrifugation (100 × g for 5 min) in supplemented media [45% (vol/vol) NCTC-135 medium (GIBCO), 45% (vol/vol) Iscove’s modified Dulbecco’s medium (GIBCO), 10% (vol/vol) FCS, 200 units/ml penicillin, and 200 μg/ml streptomycin]. Larvae were suspended in supplemented media and γ-irradiated at 350 grays from a 137caesium source (Gammacell 1000; MDS Nordion, Ottawa, Canada) operated at room temperature. The vaccination protocol for both Exps. 2 and 3 comprised an initial inoculation with 300 irradiated L3 injected s.c. around the umbilicus, followed by two identical booster immunizations administered at two-weekly intervals, without adjuvants in all cases. Samples of both irradiated and nonirradiated L3 were cultured in vitro to assess molting rates. Single larvae in individual wells of a 96-well, flat-bottom tissue culture plate (Nunc), each containing 1.5 × 104 bovine peripheral blood mononuclear cells in 150 μl of supplemented media, were incubated at 37°C in a 5% CO2 atmosphere and examined every 24 h.
Experimental Challenge (Exp. 2).
Twenty Belgian Blue × Jersey crossbred female calves were maintained indoors in fly-proof accommodations near Liverpool. Cattle were randomly assigned into three groups: vaccinated and challenged (n = 8); unvaccinated and challenged (challenge controls, n = 8); and vaccinated and unchallenged (vaccine controls, n = 4). The animals received the primary vaccination at 4–6 months of age and were challenged with 1,000 nonirradiated L3 at 2 weeks after the final immunization. Animals were killed at 12 months after challenge and the complete hides were searched under code. Putative nodules were matched against antemortem records and removed, and duplicate skin biopsies were examined by microscopy for Mf or frozen (−80°C) for subsequent Onchocerca spp.-specific PCR (18) as described in ref. 38.
Field Challenge (Exp. 3).
Pregnant Gudali cows were recruited from the area around Ngaoundéré, and their calves were reared in fly-proof accommodations from birth. The calves were assigned to two groups, vaccinated (n = 9) and control (n = 13), which were matched for age at the start of immunization [median (range) = 12 (7–14) weeks for the vaccinated group and 11 (7–16) weeks for the controls] and O. ochengi infection status of the dams (67% Mf-positive for the vaccinated group and 77% for the controls).
The control group was also used in a parallel recombinant vaccine trial (experiment not reported here), and thus injections with Freund’s complete adjuvant at 14 weeks preexposure, Freund’s incomplete adjuvant at 10 weeks preexposure, and alum adjuvant at 12, 8, and 4 weeks preexposure were necessary. In the vaccinated group, the final immunization with irradiated L3 was administered at 2 weeks preexposure, and both groups were concurrently exposed to natural field challenge for 22 months. At the termination of the experiment, all nodules were surgically removed under local anaesthetic.
Examination of Adult Parasites (Exps. 2 and 3).
Nodule contents were extirpated into PBS, and the gravid status of female worms was determined by microscopic examination for developing embryos or Mf. The female mass was dissected to release any male worms, which were counted and measured.
Statistical Analysis.
In Exp. 1, parasitological comparisons between groups of cattle over the entire period of exposure were based on data normalized by the log10(x + 1) transformation and analyzed by repeated-measures ANOVA. This test is appropriate where a particular variable is sampled in the same group of subjects on multiple occasions (53). Time (duration of exposure in months) was the within-subjects factor and group was the between-subjects factor; the interaction between time and group was analyzed by polynomial contrasts, and the significance of the difference between groups was determined by Tamhane’s T2 post hoc test. Differences in median parasitological loads were also assessed at individual time points by using the Mann–Whitney U test (with exact significance). Black fly exposure indices were compared between groups by using the Mann–Whitney U test, and indices of vector attractiveness for individual animals in 1997 and 1998 were analyzed by the Pearson correlation coefficient.
In Exps. 2 and 3, vaccine efficacy was assessed by comparison of median parasitological loads at the termination of the experiments by using the Mann–Whitney U test (with exact significance), whereas frequency (positivity rates) data were analyzed by Fisher’s exact test.
All analyses were performed by using spss 11.0.1 software (SPSS, Chicago), with significance accepted at P < 0.05.
Acknowledgments
We thank the herdsmen, watchmen, and technical staff of Institut de Recherche Agricole pour le Développement for invaluable support and Nigel Jones and colleagues for animal husbandry at the University of Liverpool. We are also very grateful to the L3 production team at the Tropical Medicine Research Station, which was managed on-site by Tracy Tierney and Kristine Bennett. This work was supported by the Edna McConnell Clark Foundation and the European Union (International Cooperation with Developing Countries Contract ICA4-CT-1999-10002); and L.M.N. received a Research Training grant from the World Health Organization Special Programme for Research and Training in Tropical Diseases (M8/181/4/N.194).
Abbreviations
- L3
third-stage larva
- Mf
microfilaria
- PI
putatively immune.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Remme J. H., Blas E., Chitsulo L., Desjeux P. M., Engers H. D., Kanyok T. P., Kengeya Kayondo J. F., Kioy D. W., Kumaraswami V., Lazdins J. K., et al. Trends Parasitol. 2002;18:421–426. doi: 10.1016/s1471-4922(02)02387-5. [DOI] [PubMed] [Google Scholar]
- 2.Plaisier A. P., Van Oortmarssen G. J., Remme J., Habbema J. D. Acta Trop. 1991;48:271–284. doi: 10.1016/0001-706x(91)90015-c. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux D. H. Trends Parasitol. 2005;21:525–529. doi: 10.1016/j.pt.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Dadzie Y., Neira M., Hopkins D. Filaria J. 2003;2:2. doi: 10.1186/1475-2883-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoerauf A., Volkmann L., Hamelmann C., Adjei O., Autenrieth I. B., Fleischer B., Büttner D. W. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 6.Langworthy N. G., Renz A., Mackenstedt U., Henkle-Dührsen K., de Bronsvoort M. B., Tanya V. N., Donnelly M. J., Trees A. J. Proc. R. Soc. London Ser. B; 2000. pp. 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook J. A., Steel C., Ottesen E. A. Trends Parasitol. 2001;17:555–558. doi: 10.1016/s1471-4922(01)02115-8. [DOI] [PubMed] [Google Scholar]
- 8.Ward D. J., Nutman T. B., Zea-Flores G., Portocarrero C., Lujan A., Ottesen E. A. J. Infect. Dis. 1988;157:536–543. doi: 10.1093/infdis/157.3.536. [DOI] [PubMed] [Google Scholar]
- 9.Nutman T. B., Steel C., Ward D. J., Zea-Flores G., Ottesen E. A. J. Infect. Dis. 1991;163:1128–1133. doi: 10.1093/infdis/163.5.1128. [DOI] [PubMed] [Google Scholar]
- 10.Elson L. H., Calvopina M., Paredes W., Araujo E., Bradley J. E., Guderian R. H., Nutman T. B. J. Infect. Dis. 1995;171:652–658. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 11.Soboslay P. T., Geiger S. M., Weiss N., Banla M., Luder C. G., Dreweck C. M., Batchassi E., Boatin B. A., Stadler A., Schulz-Key H. Immunology. 1997;90:592–599. doi: 10.1046/j.1365-2567.1997.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruppa T. F., Burchard G. D. Trans. R. Soc. Trop. Med. Hyg. 1999;93:365–367. doi: 10.1016/s0035-9203(99)90117-7. [DOI] [PubMed] [Google Scholar]
- 13.Filipe J. A., Boussinesq M., Renz A., Collins R. C., Vivas-Martinez S., Grillet M. E., Little M. P., Basanez M. G. Proc. Natl. Acad. Sci. USA. 2005;102:15265–15270. doi: 10.1073/pnas.0502659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H., Bain O., Williams S. A. Parasite. 1994;1:141–151. doi: 10.1051/parasite/1994012141. [DOI] [PubMed] [Google Scholar]
- 15.Trees A. J. Parasitol. Today. 1992;8:337–339. doi: 10.1016/0169-4758(92)90068-d. [DOI] [PubMed] [Google Scholar]
- 16.Trees A. J., Wahl G., Klager S., Renz A. Parasitology. 1992;104:247–252. doi: 10.1017/s0031182000061680. [DOI] [PubMed] [Google Scholar]
- 17.Wahl G., Achukwi M. D., Mbah D., Dawa O., Renz A. Vet. Parasitol. 1994;52:297–311. doi: 10.1016/0304-4017(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 18.Meredith S. E., Lando G., Gbakima A. A., Zimmerman P. A., Unnasch T. R. Exp. Parasitol. 1991;73:335–344. doi: 10.1016/0014-4894(91)90105-6. [DOI] [PubMed] [Google Scholar]
- 19.Achukwi M. D., Harnett W., Bradley J., Renz A. Vet. Parasitol. 2004;122:35–49. doi: 10.1016/j.vetpar.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Meyer C. G., Gallin M., Erttmann K. D., Brattig N., Schnittger L., Gelhaus A., Tannich E., Begovich A. B., Erlich H. A., Horstmann R. D. Proc. Natl. Acad. Sci. USA. 1994;91:7515–7519. doi: 10.1073/pnas.91.16.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elson L. H., Guderian R. H., Araujo E., Bradley J. E., Days A., Nutman T. B. J. Infect. Dis. 1994;169:588–594. doi: 10.1093/infdis/169.3.588. [DOI] [PubMed] [Google Scholar]
- 22.Turaga P. S., Tierney T. J., Bennett K. E., McCarthy M. C., Simonek S. C., Enyong P. A., Moukatte D. W., Lustigman S. Infect. Immun. 2000;68:1905–1911. doi: 10.1128/iai.68.4.1905-1911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould S. E., Gomberg H. J., Bethell F. H., Villella J. B., Hertz C. S. Am. J. Pathol. 1955;31:933–963. [PMC free article] [PubMed] [Google Scholar]
- 24.Bain R. K. Int. J. Parasitol. 1999;29:185–191. doi: 10.1016/s0020-7519(98)00187-8. [DOI] [PubMed] [Google Scholar]
- 25.Lange A. M., Yutanawiboonchai W., Lok J. B., Trpis M., Abraham D. Am. J. Trop. Med. Hyg. 1993;49:783–788. doi: 10.4269/ajtmh.1993.49.783. [DOI] [PubMed] [Google Scholar]
- 26.Bancroft A., Devaney E. Parasite Immunol. 1993;15:153–162. doi: 10.1111/j.1365-3024.1993.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 27.LeGoff L., Marechal P., Petit G., Taylor D. W., Hoffmann W., Bain O. Trop. Med. Int. Health. 1997;2:1170–1174. doi: 10.1046/j.1365-3156.1997.d01-218.x. [DOI] [PubMed] [Google Scholar]
- 28.Ungeheuer M., Elissa N., Morelli A., Georges A. J., Deloron P., Debre P., Bain O., Millet P. Parasite Immunol. 2000;22:173–183. doi: 10.1046/j.1365-3024.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 29.Bleiss W., Oberlander U., Hartmann S., Adam R., Marko A., Schonemeyer A., Lucius R. J. Parasitol. 2002;88:264–270. doi: 10.1645/0022-3395(2002)088[0264:PIIBIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida M., Nakagaki K., Nogami S., Harasawa R., Maeda R., Katae H., Hayashi Y. J. Vet. Med. Sci. 1997;59:1115–1121. doi: 10.1292/jvms.59.1115. [DOI] [PubMed] [Google Scholar]
- 31.Njongmeta L. M., Nfon C. K., Gilbert J., Makepeace B. L., Tanya V. N., Trees A. J. Int. J. Parasitol. 2004;34:1069–1074. doi: 10.1016/j.ijpara.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Duke B. O. Bull. W. H. O. 1968;39:307–309. [PMC free article] [PubMed] [Google Scholar]
- 33.Martin C., Saeftel M., Vuong P. N., Babayan S., Fischer K., Bain O., Hoerauf A. Infect. Immun. 2001;69:7067–7073. doi: 10.1128/IAI.69.11.7067-7073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeGoff L., Martin C., Oswald I. P., Vuong P. N., Petit G., Ungeheuer M. N., Bain O. Parasitology. 2000;120:271–280. doi: 10.1017/s0031182099005533. [DOI] [PubMed] [Google Scholar]
- 35.Abraham D., Leon O., Schnyder-Candrian S., Wang C. C., Galioto A. M., Kerepesi L. A., Lee J. J., Lustigman S. Infect. Immun. 2004;72:810–817. doi: 10.1128/IAI.72.2.810-817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irvine M., Johnson E. H., Lustigman S. Ann. Trop. Med. Parasitol. 1997;91:67–77. doi: 10.1080/00034983.1997.11813113. [DOI] [PubMed] [Google Scholar]
- 37.Soboslay P. T., Dreweck C. M., Taylor H. R., Brotman B., Wenk P., Greene B. M. J. Immunol. 1991;147:346–353. [PubMed] [Google Scholar]
- 38.Graham S. P., Trees A. J., Collins R. A., Moore D. M., Guy F. M., Taylor M. J., Bianco A. E. Infect. Immun. 2001;69:4313–4319. doi: 10.1128/IAI.69.7.4313-4319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maizels R. M., Balic A., Gomez-Escobar N., Nair M., Taylor M. D., Allen J. E. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald A. J., Turaga P. S., Harmon-Brown C., Tierney T. J., Bennett K. E., McCarthy M. C., Simonek S. C., Enyong P. A., Moukatte D. W., Lustigman S. Infect. Immun. 2002;70:2796–2804. doi: 10.1128/IAI.70.6.2796-2804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duerr H. P., Dietz K., Schulz-Key H., Büttner D. W., Eichner M. Trans. R. Soc. Trop. Med. Hyg. 2003;97:242–250. doi: 10.1016/s0035-9203(03)90132-5. [DOI] [PubMed] [Google Scholar]
- 42.Duerr H. P., Dietz K., Schulz-Key H., Büttner D. W., Eichner M. Int. J. Parasitol. 2004;34:463–473. doi: 10.1016/j.ijpara.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Beaver P. C., Orihel T. C., Johnson M. H. J. Parasitol. 1974;60:310–315. [PubMed] [Google Scholar]
- 44.Hoerauf A., Kruse S., Brattig N. W., Heinzmann A., Mueller-Myhsok B., Deichmann K. A. Microbes Infect. 2002;4:37–42. doi: 10.1016/s1286-4579(01)01507-6. [DOI] [PubMed] [Google Scholar]
- 45.Townson S., Bianco A. E., Doenhoff M. J., Muller R. Tropenmed. Parasitol. 1984;35:202–208. [PubMed] [Google Scholar]
- 46.Babayan S., Attout T., Specht S., Hoerauf A., Snounou G., Renia L., Korenaga M., Bain O., Martin C. Med. Microbiol. Immunol. 2005;194:151–162. doi: 10.1007/s00430-004-0226-1. [DOI] [PubMed] [Google Scholar]
- 47.Lustigman S., James E. R., Tawe W., Abraham D. Trends Parasitol. 2002;18:135–141. doi: 10.1016/s1471-4922(01)02211-5. [DOI] [PubMed] [Google Scholar]
- 48.Renz A., Trees A. J., Achukwi D., Edwards G., Wahl G. Trop. Med. Parasitol. 1995;46:31–37. [PubMed] [Google Scholar]
- 49.Wahl G., Enyong P., Ngosso A., Schibel J. M., Moyou R., Tubbesing H., Ekale D., Renz A. Parasitology. 1998;116:349–362. doi: 10.1017/s003118209700228x. [DOI] [PubMed] [Google Scholar]
- 50.Wahl G., Ekale D., Schmitz A. Parasitology. 1998;116:327–336. doi: 10.1017/s0031182097002333. [DOI] [PubMed] [Google Scholar]
- 51.Wilson M. D., Post R. J., Gomulski L. M. Ann. Trop. Med. Parasitol. 1993;87:65–82. doi: 10.1080/00034983.1993.11812739. [DOI] [PubMed] [Google Scholar]
- 52.Lowrie R. C., Jr. Am. J. Trop. Med. Hyg. 1983;32:138–145. doi: 10.4269/ajtmh.1983.32.138. [DOI] [PubMed] [Google Scholar]
- 53.Looney S. W., Stanley W. B. Am. Stat. 1989;43:220–225. [Google Scholar]