Abstract
The reasons for the cellular specificity and slow progression of motoneuron diseases such as ALS are still poorly understood. We previously described a motoneuron-specific cell death pathway downstream of the Fas death receptor, in which synthesis of nitric oxide (NO) is an obligate step. Motoneurons from ALS model mice expressing mutant SOD1 showed increased susceptibility to exogenous NO as compared with controls. Here, we report a signaling mechanism whereby NO leads to death of mutant, but not control, motoneurons. Unexpectedly, exogenous NO triggers expression of Fas ligand (FasL) in cultured motoneurons. In mutant SOD1G93A and SOD1G85R, but not in control motoneurons, this up-regulation results in activation of Fas, leading through Daxx to phosphorylation of p38 and further NO synthesis. This Fas/NO feedback amplification loop is required for motoneuron death in vitro. In vivo, mutant SOD1G93A and SOD1G85R mice show increased numbers of positive motoneurons and Daxx nuclear bodies weeks before disease onset. Moreover, FasL up-regulation is reduced in the presence of transgenic dominant-negative Daxx. We propose that chronic low-level activation of the Fas/NO feedback loop may underlie the motoneuron loss that characterizes familial ALS and may help to explain its slowly progressive nature.
Keywords: cell death, motoneuron disease, NO, p38 kinase, neurodegeneration
Amyotrophic lateral sclerosis (ALS) is the most frequent adult-onset motoneuron disease in humans. ALS is characterized by the selective degeneration of motoneurons in spinal cord, brainstem, and cerebral cortex leading to muscle atrophy and paralysis and ultimately to death. About 1 to 2% of all human ALS forms are caused by dominantly inherited mutations in the Cu/Zn superoxide dismutase (SOD1) gene. Mice transgenic for the ALS-linked SOD1 mutations G37R (1), H46R/H48Q (2), G85R (3), and G93A (4) develop an adult-onset motoneuron disorder that remarkably resembles human ALS.
Despite much intensive study, many questions remain concerning the mechanism(s) by which mutant SOD1 triggers specific motoneuron death. One unresolved issue is the cellular site of action of the gain-of-function mutations. Clement et al. (5) generated chimeric mice carrying a mixture of WT and mutant SOD1-expressing cells in the spinal cord. In these mice, WT motoneurons eventually showed stigmata of degeneration, whereas some mutant SOD1 motoneurons were protected from degeneration when surrounded by WT nonneuronal cells. These results suggest that mutant SOD1 in both motoneurons and surrounding cells may play a role in the disease process.
Our earlier studies using cultures of purified embryonic motoneurons reached similar conclusions. We found that motoneurons from SOD1G93A, SOD1G37R, and SOD1G85R mice survived normally in the presence of optimal trophic support or when challenged by excitotoxic agonists. In marked contrast, compared with controls, they displayed a 10- to 100-fold increase in sensitivity to extracellular agonists of the Fas receptor or to exogenous nitric oxide (6). Thus, motoneurons expressing mutant SOD1 have an intrinsic susceptibility that is only revealed when challenged with specific extrinsic agents. Only motoneurons, and no other cell type from mutant SOD1 mice, showed enhanced susceptibility. In motoneurons, we showed that the Fas receptor signals through two synergistic pathways involving Fadd/Caspase-8 and Daxx/Ask1/p38/nNOS, respectively (6).
Another open question in ALS relates to the kinetics of the neurodegenerative process. Mutant SOD1 mice display multiple features of programmed cell death at presymptomatic stages. Examples are proteolytic activation of caspases-1, -3, -7, -8, and -9 in the spinal cord (7–9), mitochondrial release of cytochrome c (10), and translocation of Bax from the cytosol to mitochondria (11). These modifications are manifest weeks to months before there is significant loss of motoneuron cell bodies or axons. One aspect of Fas-triggered motoneuron death seemed relevant to these slow kinetics. Whereas Fas activation in vitro kills lymphocytes within hours, motoneuron death in the same conditions takes days (12). The first aim of this study was therefore to identify the molecular mechanisms that underlie this exceptionally protracted cell death.
Our results linking Fas signaling to mutant SOD1 were obtained by using purified motoneurons in vitro. A second important aim of this study was therefore to investigate the significance of this signaling pathway in mutant SOD1 mice in vivo. Since our earlier publication (6), other groups studying mutant SOD1 mice have reported activation of certain intermediates in the Fas pathway we described. In the spinal cord of SOD1G93A mice, Tortarolo et al. (13), Hu et al. (14), and Ackerley et al. (15) observed increased p38 activation, whereas Wengenack et al. (16) reported increased levels of Ask1. However, these results concern intermediates that are common to several signaling mechanisms. We therefore focused our attention on Fas and Daxx, which are specific to this pathway.
We report here that nitric oxide (NO), an end-product of the Fas pathway in motoneurons, unexpectedly induces expression of the endogenous Fas ligand (FasL), which in mutant SOD1 motoneurons then triggers further chronic activation of the pathway downstream of Fas. This feedback loop is necessary for motoneuron death induced by NO or FasL in vitro. Furthermore, because all elements of this feedback loop show perturbed expression in mutant SOD1 mice at presymptomatic stages, it is possible that this pathway is linked to the slowly progressive nature of motoneuron loss in vivo.
Results
Identification of a Fas/NO Feedback Loop in Mutant SOD1 Motoneurons.
Because purified mutant SOD1 motoneurons show increased susceptibility to activation of the Fas death pathway, we asked whether expression of key signaling molecules differed between mutant SOD1 and control mice. Levels of Fas receptor and Fas ligand (FasL) were estimated by Western blotting. Levels of Fas were similar between C57BL/6 and mutant SOD1 motoneurons and unchanged by the presence of NO donors such as Detanonoate {Z-1-[2-(2-aminoethyl)-N-(2-aminonioethyl)amino]diazen-1-ium-1,2-diolate} (data not shown). The levels of the 38-kDa form of FasL (17), however, were increased 10- to 15-fold within 6–12 h of addition of Detanonoate (20 μM) to cultures of SOD1G85R and SOD1G93A motoneurons (Fig. 1A and B). FasL was up-regulated to a similar degree in motoneurons from C57BL/6 mice (Fig. 1C), indicating that this event was independent of the presence of mutant SOD1. FasL up-regulation was concentration-dependent in the range from 0 to 20 μM Detanonoate and motoneuron-specific (Figs. 6 A–D and 7A, which are published as supporting information on the PNAS web site). FasL up-regulation was also observed after addition of the NO donor sodium nitroprusside (Fig. 6E). FasL can exist in both membrane-bound and soluble forms (18). However, by using a sensitive ELISA assay (detection limit 3.6 pg/ml), no soluble FasL could be detected in media conditioned by Detanonoate-treated motoneurons. This result suggests that the FasL expressed after NO exposure is mostly membrane-bound, and therefore more likely to exert autocrine than paracrine effects under these experimental conditions.
Fig. 1.
Regulation of FasL and p38 kinase by NO in cultured motoneurons. Motoneurons were cultured for 16 h and then treated for 6 or 12 h with the NO donor Detanonoate (20 μM). (A and B) Western blots of SOD1G85R and SOD1G93A motoneuron protein extracts demonstrated that the 38 kDa form of FasL protein was strongly up-regulated by NO within 6–12 h. (C) Similar up-regulation of FasL protein was seen in C57BL/6 motoneuron extracts after Detanonoate addition. Control protein extracts were prepared immediately before Detanonoate addition (0). Blots were reprobed with an antibody against neurofilament medium chain (NF-M). (D) Immunocytochemistry revealed a strong increase in phosphorylated p38 kinase in SOD1G93A motoneurons exposed for 6 h to Detanonoate as compared with SOD1G93A motoneurons cultured under basal conditions or motoneurons from C57BL/6 mice. Motoneurons are colabeled with anti MAP-2 antibodies and DAPI. (E) Histograms showing that Detanonoate increased the phospho-p38 immunoreactivity (IR) in mutant SOD1G93A and SOD1G85R motoneurons by 2- and 2.8-fold, respectively, although having no significant effect on C57BL/6 or SOD1WT motoneurons. Detanonoate-induced phosphorylation of p38 kinase in mutant motoneurons was blocked by preincubation with Fas-Fc. Values for phospho-p38 kinase IR were normalized to MAP-2 IR; error bars represent SEM.
We next asked whether the up-regulated FasL was capable of activating the Fas receptor expressed on the same motoneurons. As a reporter for Fas activation, we quantified phosphorylation of p38 kinase, a key event in the Fas pathway in cultured motoneurons (6), by immunolabeling of phospho-p38 (Tyr-180/Tyr-182) followed by quantitative confocal microscopy. Phospho-p38 was only weakly detected in untreated motoneurons, irrespective of the genotype (mutant SOD1, SOD1WT, or nontransgenic). However, in mutant SOD1 motoneurons treated with 20 μM Detanonoate, phospho-p38 kinase became clearly apparent in the nucleus and cytoplasm (Fig. 1D). Quantitative image analysis demonstrated that, after treatment with Detanonoate, immunoreactivity for phospho-p38 kinase increased by 2- and 2.8-fold in SOD1G93A and SOD1G85R motoneurons, respectively (P < 0.0005; Fig. 1E). In contrast, identical treatment of control motoneurons from C57BL/6 or SOD1WT mice gave no significant increase in p38 activation (Fig. 1E).
To confirm that p38 kinase activation was a result of FasL up-regulation, we preincubated motoneurons for 2 h with Fas-Fc (19), an extracellular decoy that competes with interactions between Fas and FasL, before exposing them to NO donors. Fas-Fc inhibited the NO-induced phosphorylation of p38 kinase in both SOD1G93A and SOD1G85R motoneurons by >70% (Fig. 1E). These findings indicate that in both control and mutant SOD1 motoneurons, exogenous NO leads to up-regulation of FasL. However, only in the presence of G93A or G85R mutant forms of SOD1 does this mechanism lead to a Fas-dependent increase in phosphorylation of p38 kinase.
Functional Evidence for a Role of the NO/Fas Feedback Loop in Mutant Motoneuron Death.
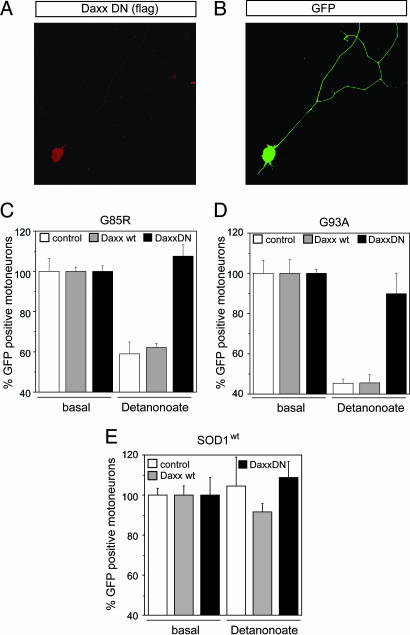
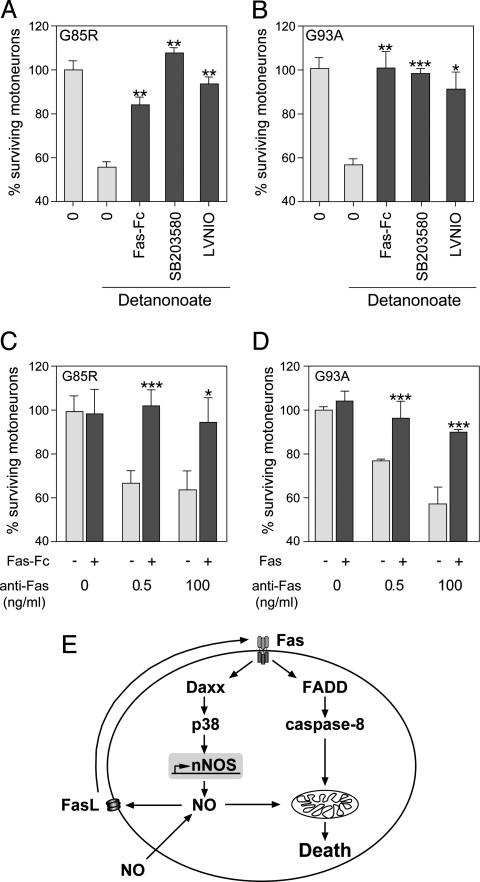
To confirm that FasL-Fas interactions and p38 activation were functionally involved in NO-triggered death of mutant SOD1 motoneurons, we used dominant-negative constructs and pharmacological inhibitors. We previously reported that 20 μM Detanonoate triggers death of ≈45% of mutant SOD1G85R and SOD1G93A motoneurons, whereas it does not affect survival of motoneurons from C57BL/6 mice (6) or SOD1WT mice (Fig. 2E). To detect potential involvement of Daxx in the NO/Fas feedback loop, we electroporated purified SOD1 motoneurons with vectors encoding either a dominant-negative form of Daxx (Daxx-DN), WT Daxx, or a control vector. An EGFP vector was coelectroporated to monitor survival of transduced motoneurons (Fig. 2 A and B). After administration of 20 μM Detanonoate, the survival of SOD1G85R and SOD1G93A motoneurons transduced with WT Daxx or control vector was reduced by 42% and 55%, respectively (Fig. 2 C and D). These figures are close to those for nonelectroporated cells, demonstrating that the transduced neurons are representative of the whole population. In contrast, expression of Daxx-DN almost completely protected mutant SOD1G85R and mutant SOD1G93A motoneurons against NO-induced cell death (Fig. 2 C and D). Mutant motoneuron death induced by exogenous NO was also strongly inhibited by Fas-Fc, SB203588, an inhibitor of p38 kinase, and L-VNIO, an inhibitor of nNOS (Fig. 3A and B). Thus, in agreement with the expression data, FasL-Fas interactions, p38 kinase, and nNOS are all required for NO-triggered death.
Fig. 2.
Role of Daxx in mutant SOD1 motoneuron death. (A and B) Plasmid electroporation was used to coexpress a dominant negative FLAG-tagged form of Daxx (Daxx-DN) and EGFP in cultured motoneurons. (C and D) SOD1G85R or SOD1G93A motoneurons were electroporated with the EGFP expression vector in combination with an empty control vector or vectors encoding either WT Daxx or Daxx-DN. At 1 day in vitro (DIV), motoneurons from each electroporation were either exposed to the NO donor Detanonoate or kept under basal conditions. Motoneuron survival at 3 DIV was expressed as the percentage of EGFP-positive cells in the presence versus absence of NO. Daxx-DN protected mutant SOD1G85R and SOD1G93A motoneurons from NO-triggered cell death. Expression of Daxx-WT had no effect on survival. The differences between the effects of Daxx-DN and Daxx-WT were statistically significant: P < 0.005 for SOD1G85R; P < 0.01 for SOD1G93A, Student's t test. (E) Survival of SOD1WT motoneurons transduced with control, Daxx-WT, or Daxx-DN vectors with or without NO challenge. Histograms represent means and SEM from two independent experiments.
Fig. 3.
Role of FasL, Fas, p38 kinase, and nNOS in mutant SOD1 motoneuron death. (A–D) Mutant SOD1 motoneurons were treated (or not treated) at 1 DIV with Detanonoate (A and B) or agonistic anti-Fas antibodies (C and D). Cell survival was quantified at 3 DIV by phase-contrast microscopy and expressed relative to the number of motoneurons alive under basal conditions (0). Addition of the extracellular part of Fas receptor, Fas-Fc, the p38 kinase inhibitor SB203580 (5 μM), or the nNOS inhibitor L-VNIO (10 μM) significantly reduced the NO-triggered death of SOD1G85R (A) or SOD1G93A (B) motoneurons. Mutant SOD1 motoneuron death mediated by agonistic anti-Fas antibodies at 0.5 ng/ml or 100 ng/ml was prevented or significantly reduced by Fas-Fc. Error bars show SEM. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, Student's t test. (E) Model illustrating the Fas/NO feedback loop in mutant SOD1 motoneurons.
These results provided strong functional evidence for a feedback loop triggered by NO in mutant SOD1 motoneurons. However, it remained possible that NO from the exogenous donor was provided at levels in excess of those generated by the Fas/NO pathway, and thereby led to nonphysiological activation of Fas. We therefore looked for evidence of the feedback loop in neurons triggered to die by another element of the pathway, the Fas receptor itself. Mutant SOD1 motoneurons were exposed to agonistic anti-Fas antibodies in the absence or presence of Fas-Fc (Fig. 3 C and D). Fas-Fc does not functionally interact with the Fas antibodies used to trigger death (Fig. 7C). Blockade of FasL/Fas interactions by Fas-Fc gave nearly complete protection against cell death (Fig. 3 C and D). This result demonstrates that even when the pathway is triggered by the endogenous Fas receptor, further activation of Fas by FasL is required for cell death to occur (Fig. 3E).
FasL Is Up-Regulated in Mutant SOD1 Spinal Cord.
Our in vitro results showed that, to kill a mutant SOD1 motoneuron, Fas or NO need to trigger a feedback loop. The involvement of such a loop, together with the requirement for transcriptional up-regulation of nNOS (6) may explain the relatively slow time course of motoneuron death when compared with other cellular models of Fas-triggered apoptosis. We reasoned that it might also be related to the late onset and prolonged time course of the neurodegenerative process in mutant SOD1 mice in vivo. We therefore asked whether the elements of the Fas/NO feedback loop were expressed in the spinal cord and whether they showed alterations during the period leading up to the onset of clinical symptoms.
Disease course varies between different lines of mutant SOD1 mice: SOD1G93A mice expressing a catalytically active form of human SOD1 display an early disease onset ≈100 days of age (4), whereas SOD1G85R mice express an inactive form of SOD1 leading to late onset around day 200 (3). We therefore chose to analyze these mouse lines at comparable presymptomatic stages when the total number of motoneurons is still normal: day 75 for SOD1G93A mice and day 120 for SOD1G85R mice (7, 20) (Supporting Materials and Methods, which is published as supporting information on the PNAS web site). We found that not only the Fas receptor, as shown in ref. 21, but also its endogenous ligand FasL and its signaling intermediate Daxx were expressed in control spinal cord (Fig. 4A). We therefore quantified the expression of different elements of the pathway in presymptomatic mutant mice.
Fig. 4.
Up-regulation of FasL in lumbar motoneurons of mutant SOD1 mice. (A) Transverse sections of L4 lumbar spinal cords of C57BL/6 (control), 75-day-old SOD1G93A, and 120-day-old SOD1G85R mice immunostained for Fas, Daxx, FasL, and choline acetyl transferase (ChAT). Fas and Daxx displayed a scattered expression pattern in various cell types of the gray and white matter. In control mice, FasL was mainly detected in dorsal and ventral horn neurons, including a few motoneurons. In SOD1G93A and SOD1G85R mice, a high proportion of motoneurons, as identified by ChAT staining on adjacent sections, was positive for FasL. (Scale bar: 100 μm.) (B) Histograms showing the percentage of FasL-positive motoneurons in serial sections of L4 lumbar spinal cord. Note the increased percentage of FasL-positive motoneurons in SOD1G93A and SOD1G85R mice as compared with C57BL/6 and SOD1WT mice. (C) Immunolabeling reveals coexpression of FasL and Fas in spinal cord motoneurons in a 75-day-old SOD1G93A mouse. (Scale bar: 50 μm.)
In nontransgenic mice, 31 ± 2% of all choline acetyl transferase (ChAT)-positive motoneurons in the lumbar spinal cord segment L4 expressed significant levels of FasL at day 75 (n = 3 mice) and 29 ± 4% were stained at day 120 (n = 2). In SOD1WT mice, the number of FasL positive motoneurons (26.5 ± 3.2%, n = 3) was close to that in nontransgenic mice. Strikingly, the proportion of FasL-positive motoneurons was at least two-fold higher in SOD1G93A mice at 75 days (70 ± 1.9%, n = 4) and in SOD1G85R mice at 120 days (60.2 ± 6.6%, n = 4; Fig. 4B; P < 0.001, Student's t test). We therefore asked whether FasL might be able to engage Fas receptor signaling in motoneurons in vivo. Double immunofluorescence labeling of presymptomatic SOD1G93A spinal cord showed that all FasL-positive motoneurons coexpressed significant levels of Fas (Fig. 4C).
Daxx Accumulates in Nuclear Speckles in Motoneurons of Mutant SOD1 Mice.
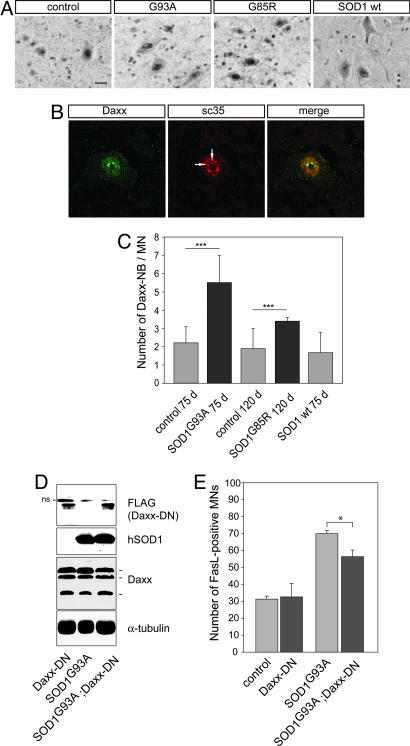
Immunostaining for Daxx in control and mutant SOD1 lumbar spinal cords revealed a diffuse cytoplasmic and nuclear localization in a broad range of neurons in the ventral and dorsal horn (Figs. 4A and 5A). Interestingly, in motoneurons, Daxx accumulated in discrete subnuclear domains (Fig. 5B) that stained positive for sc35, a general splicing factor and marker of nuclear speckles. Only a subpopulation of nuclear speckles were stained for Daxx. Colocalization of Daxx and sc35 was confirmed by Z-scan confocal analysis (data not shown). These nuclear domains (Daxx-NBs) are reminiscent of promyelocytic leukemia nuclear bodies (PML-NB) which, in other cell types, have been shown to contain sc35 (22–24). We quantified Daxx-NBs in control and mutant SOD1 motoneurons (Fig. 5C). Lumbar motoneurons contained 2.2 ± 0.9 Daxx-NBs and 1.9 ± 1.1 Daxx-NBs per section in C57BL/6 mice aged 75 and 120 days, respectively, and 1.8 ± 1.2 Daxx-NBs per section in SOD1WT mice aged 75 days. In contrast, the number of Daxx-NBs was increased to 5.5 ± 1.5 per section in SOD1G93A mice and 3.4 ± 0.2 in SOD1G85R mice (mean ± SD, n = 3, P < 0.001, Student's t test). In conclusion, therefore, two key intermediates of the Fas/NO signaling loop, FasL and Daxx, are activated in motoneurons of presymptomatic ALS mice.
Fig. 5.
Daxx activation in lumbar motoneurons of mutant SOD1 mice and its functional relevance in the Fas/NO loop. (A) Immunolabeling reveals that Daxx accumulates in the nuclei of motoneurons and forms nuclear bodies. SOD1G93A,C57BL/6 littermates and SOD1WT mice were analyzed at age 75 days; SOD1G85R and C57BL/6 litter mice (C.R., data not shown) were analyzed at age 120 days. (Scale bar: 20 μm.) (B) Daxx nuclear bodies (arrows) are associated with a subpopulation of nuclear speckles, as detected by confocal analysis of Daxx- and sc35-immunostained SOD1G93A lumbar spinal cords at age 75 days. (C) The number of Daxx nuclear bodies (Daxx-NBs) in L4 motoneurons was higher in SOD1G93A and SOD1G85R mice than in C57BL/6 or SOD1WT mice of the same age. Values represent means ± SD from three mice per genotype; ∗∗∗, P < 0.001, Student's t test. (D and E) Analysis of double transgenic SOD1G93A;Daxx-DN mice. (D) Western blots of protein extracts from lumbar spinal cords show that the dominant negative form of Daxx (Daxx-DN) and the human SOD1G93A are expressed at similar levels in double transgenic mice and in the parental mice at age 75 days. Daxx-DN was revealed with an anti-FLAG antibody that also detects a nonspecific (ns) upper band. Daxx-DN expression did not modify expression of endogenous Daxx because the three known Daxx isoforms of 70, 97, and 110 kDa (43) were detected at similar levels in all genotypes. (E) Transgenic Daxx-DN expression significantly reduced the percentage of FasL-positive motor neurons in double transgenic mutant SOD1G93A;Daxx-DN mice as compared with values in age-matched 75-day-old SOD1G93A mice. Values are means ± SD, n = 3 per genotype; ∗∗∗, P < 0.001, one-way ANOVA followed by Newman–Keuls post hoc analysis.
A Dominant-Negative Form of Daxx Inhibits FasL Up-Regulation in Vivo.
To address the functional relevance of Fas-Daxx signaling in mutant SOD1-linked motoneuron disease, we crossbred SOD1G93A mice with transgenic mice expressing a dominant negative form of Daxx. These Daxx-DN mice (25) show a weaker phenotype than the Daxx null mutants, which are embryonically lethal (26). Western blot analysis demonstrated that SOD1 and Daxx-DN transgenes were expressed in lumbar spinal cord of double transgenic SOD1G93A;Daxx-DN mice at levels similar to those in the parental strains (Fig. 5D). Endogenous Daxx expression was not influenced by Daxx-DN (Fig. 5D). Interestingly, in SOD1G93A;Daxx-DN mice, only 56.4 ± 3.9% of L4 motoneurons were FasL-positive, as compared with 70 ± 1.9% in SOD1G93A and 32.6 ± 7.9% in Daxx-DN mice (mean ± SD, n = 3 each, P < 0.001; Fig. 5E). Thus, expression of Daxx-DN leads to a reduction of 36% in the mutant SOD1G93A-induced increase in FasL-positive motoneurons. These data are consistent with a model in which chronic cycling of the mutant SOD1-dependent Fas feedback loop is required to build up signaling intermediates to levels at which they can trigger neurodegeneration.
Discussion
Amplification mechanisms play an important role in intracellular signaling pathways. The best studied examples are posttranslational modifications, e.g., protein phosphorylation and proteolysis, but signal amplification can also be achieved by induction of gene expression. We previously showed that Fas activation in cultured motoneurons results in transcriptional up-regulation of nNOS (6). Here we provide the evidence that this signal needs to be amplified by a positive feedback loop. In particular, we show that NO is able to up-regulate FasL, thereby retrogradely inducing Fas signaling. In mutant SOD1 motoneurons, this feedback loop leads to phosphorylation of p38 kinase and activation of Daxx and nNOS in the slowest cell death process yet reported downstream of the much-studied Fas receptor. Several key elements of this loop are activated in vivo in mutant SOD1 mice. We show that SOD1G93A and SOD1G85R mice display presymptomatically an increased number of FasL-positive motoneurons in comparison with control mice, as well as an increased accumulation of the Fas adaptor protein Daxx in nuclear bodies.
These findings extend reports concerning early activation of Ask-1 (16) and of several protein kinases including p38 (refs. 13–15 and 27; C.R., unpublished results) in SOD1G93A mice. Taken together, these findings provide a molecular means for comparing the disease process in these mouse models of familial ALS with that in human patients with sporadic ALS unlinked to SOD1 mutations. Indeed, Bendotti et al. (27) report that sporadic ALS cases show the same characteristic skein-like staining pattern for phospho-p38 as that observed in mutant SOD1G93A mice.
A particularly striking aspect of the Fas/NO feedback loop is that its activation is absolutely required for motoneuron cell death over the normal time span of motoneuron cultures. Even triggering the pathway by using agonistic Fas antibodies does not lead to death when interactions between FasL and Fas are blocked by using Fas-Fc. Therefore, the amplification provided by the loop is not simply a reinforcement mechanism. It is necessary to transform a subliminal activation of the pathway into an effective death stimulus. This result presumably reflects a low-intensity stimulation of the death-inducing signaling complex (DISC) complex in motoneurons compared with thymocytes or hepatocytes, perhaps because levels of Fas and its signaling intermediates are relatively low in these cells (12).
One prediction of our data is that blocking the Fas/NO loop in mutant SOD1 mice should affect disease course. Indeed, inhibition of Daxx in mutant SOD1G93A mice by transgenic Daxx-DN expression blocked FasL up-regulation in about one-third of the sensitive motoneurons. This figure probably underestimates the role of the Fas/NO loop because transgenic Daxx-DN mice are hypomorphs rather than complete nulls (25). The same is true for the majority of existing mouse mutants in which Fas signaling is still present at levels sufficient to trigger this loop: Fas knockout mice produce a truncated Fas protein that conserves part of its intracellular domain (28, 29) lpr mice are regulatory mutants of the Fas gene and express significant levels of Fas protein (ref. 30; C.R. and B.P., unpublished data), and gld mice bear a point mutation in FasL that does not completely inactivate the ligand (31). It will thus be critical to test the effects of complete inactivation of Fas or FasL on pathological motoneuron death.
In the past, crossing mutant SOD1 mice with mice carrying a loss-of-function allele of the nNOS gene did not prolong survival (32). Nevertheless, the nNOS enzyme can exist in three forms resulting from alternative splicing (33) and specific increases in the β and γ forms have been reported in spinal cords from sporadic ALS patients (34). The nNOS mutant mice used for the cross display residual NOS activity of ≈15% of control and, strikingly, produce the same β and γ forms of nNOS as those found in ALS patients (32). In support of our model, administration of the nNOS inhibitor AR-R 17.477 significantly prolonged the lifespan of SOD1G93A mutant mice (32). In the future, it will be important to determine the precise role of NO synthases in this process, because a requirement for NO also has been demonstrated in death of motoneurons induced by avulsion (35) or neurofilament gene mutations (36).
Feedback amplification loops involving other intermediates in the Fas signaling, such as caspase-8 and caspase-3 or Bid have been reported in acellular systems and nonneuronal cells (37, 38). In the context of ALS, the Fas/NO feedback loop is of particular interest because it involves an extracellular step and diffusible factors. The cell death trigger NO is known to be produced not only by motoneurons but also by microglia and activated astrocytes (34, 39) and Fas agonists have been detected in sera of patients with sporadic ALS (40). “Community effects” may thus allow for cellular neighbors to accelerate or to inhibit motoneuron death (5) and also underlie the clinical finding that ALS often progresses locally, between adjacent muscles or motor pools. Further studies are required to better understand the molecular and cellular basis of these phenomena.
We believe that chronic cycling of feedback loops of the type described here may provide a general approach to understanding the delayed onset and relatively slow progression of many neurodegenerative diseases. As has been proposed for nucleation of mutant proteins with polyglutamine expansions (41), the initial insult produced by the feedback loop may be subliminal and without phenotype. However, as levels of toxic intermediates and death signals build up, they may reach a threshold that can trigger the pathological process. If this model is correct, then therapeutic intervention at the level of “death receptors” and cell death pathways should be envisioned at much earlier stages in the disease process than is generally imagined.
Materials and Methods
See Supporting Materials and Methods for details.
Animals and Reagents.
SOD1G85R mice, line 148 (3), were maintained as homozygotes, SOD1G93A mice (4) and SOD1WT mice, line 76 (3) as hemizygotes. All mice were on a pure C57BL/6 background. The following reagents and antibodies were used: brain-derived neurotrophic factor and ciliary neurotrophic factor (R & D Systems), glial cell line-derived neurotrophic factor, sodium nitroprusside (Sigma), Detanonoate, Fas-Fc, L-VNIO (Alexis, San Diego,), SB203580 (Calbiochem), anti-mouse Fas (JO2, Pharmingen); anti Fas (M-20) and anti FasL (N-20 and N-20-G, Santa Cruz Biotechnology); anti-FLAG (M2, Sigma), anti-NF-M (Ab1987, Chemicon), anti-sc35 (S4045, Sigma), anti-phospho-p38 (Cell Signaling Technology, Beverly, MA), anti-MAP2 (Sternberger–Meyer, Jarrettsville, MD), and secondary antibodies (Jackson ImmunoResearch or Molecular Probes).
Immunohistochemistry of Spinal Cord.
Spinal cord cryosections (16 μm) were incubated with primary antibodies [1:100 for anti-FasL, N-20-G, and anti-Fas, M-20; 1:200 for anti-Daxx, M112; 1:500 for anti-sc35, S4045, and anti-choline acetyl transferase (ChAT)], revealed with the ABC staining kit (Vector Laboratories) or with fluorochrome-conjugated secondary antibodies and analyzed by confocal microscopy. FasL-positive motoneurons or fluorescently labeled Daxx nuclear bodies were counted on at least 20 different sections originating from three mice per genotype.
Motoneuron Culture and Analysis.
Male transgenic SOD1 mice were mated with female C57BL/6 mice, embryos harvested at embryonic day 12.5, and genotyped by PCR (6). Motoneurons were purified from ventral spinal cords by using a metrizamide density gradient (42), cultured in the presence of neurotrophic factors and treated at 1 DIV with Detanonoate or anti-Fas antibodies. Electroporation was done as described (6). Cell survival was determined by fluorescence or phase-contrast microscopy. Immunohistochemistry, quantitative confocal microscopy, and Western blot analysis were performed as described in Supporting Materials and Methods. Soluble FasL in conditioned media was measured by ELISA (R & D Systems). All experiments were performed in triplicate or quadruplicate and repeated at least twice.
Supplementary Material
Acknowledgments
We thank S. Corby for animal care and genotyping, Drs. A. O. Hueber (INSERM, Nice, France) for Daxx plasmids, I. Medina (INSERM, Marseille, France) for advice on quantitive imaging, S. Przedborski (Columbia University, New York) for providing SOD1WT mice, and G. Tanackovic and T. Abbas-Terki (both of EPFL, Lausanne, Switzerland) for sc35 antibodies and helpful comments on the manuscript. This work was funded by grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Association Française Contre les Myopathies, French Ministère de la Recherche et de la Technologie, American ALS Association, and Swiss National Scientific Foundation.
Abbreviations
- DIV
day(s) in vitro
- NB
nuclear bodies.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wong P. C., Pardo C. A., Borchelt D. R., Lee M. K., Copeland N. G., Jenkins N. A., Sisodia S. S., Cleveland D. W., Price D. L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Xu G., Gonzales V., Coonfield M., Fromholt D., Copeland N. G., Jenkins N. A., Borchelt D. R. Neurobiol. Dis. 2002;10:128–138. doi: 10.1006/nbdi.2002.0498. [DOI] [PubMed] [Google Scholar]
- 3.Bruijn L. I., Becher M. W., Lee M. K., Anderson K. L., Jenkins N. A., Copeland N. G., Sisodia S. S., Rothstein J. D., Borchelt D. R., Price D. L., Cleveland D. W. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 4.Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X., et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 5.Clement A. M., Nguyen M. D., Roberts E. A., Garcia M. L., Boillee S., Rule M., McMahon A. P., Doucette W., Siwek D., Ferrante R. J., et al. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 6.Raoul C., Estevez A. G., Nishimune H., Cleveland D. W., deLapeyriere O., Henderson C. E., Haase G., Pettmann B. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 7.Pasinelli P., Houseweart M. K., Brown R. H., Jr, Cleveland D. W. Proc. Natl. Acad. Sci. USA. 2000;97:13901–13906. doi: 10.1073/pnas.240305897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Ona V. O., Guegan C., Chen M., Jackson-Lewis V., Andrews L. J., Olszewski A. J., Stieg P. E., Lee J. P., Przedborski S., Friedlander R. M. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 9.Inoue H., Tsukita K., Iwasato T., Suzuki Y., Tomioka M., Tateno M., Nagao M., Kawata A., Saido T. C., Miura M., et al. EMBO J. 2003;22:6665–6674. doi: 10.1093/emboj/cdg634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu S., Stavrovskaya I. G., Drozda M., Kim B. Y., Ona V., Li M., Sarang S., Liu A. S., Hartley D. M., Wu du C., et al. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 11.Guegan C., Vila M., Rosoklija G., Hays A. P., Przedborski S. J. Neurosci. 2001;21:6569–6576. doi: 10.1523/JNEUROSCI.21-17-06569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoul C., Henderson C. E., Pettmann B. J. Cell Biol. 1999;147:1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortarolo M., Veglianese P., Calvaresi N., Botturi A., Rossi C., Giorgini A., Migheli A., Bendotti C. Mol. Cell. Neurosci. 2003;23:180–192. doi: 10.1016/s1044-7431(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 14.Hu J. H., Chernoff K., Pelech S., Krieger C. J. Neurochem. 2003;85:422–431. doi: 10.1046/j.1471-4159.2003.01669.x. [DOI] [PubMed] [Google Scholar]
- 15.Ackerley S., Grierson A. J., Banner S., Perkinton M. S., Brownlees J., Byers H. L., Ward M., Thornhill P., Hussain K., Waby J. S., et al. Mol. Cell. Neurosci. 2004;26:354–364. doi: 10.1016/j.mcn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Wengenack T. M., Holasek S. S., Montano C. M., Gregor D., Curran G. L., Poduslo J. F. Brain Res. 2004;1027:73–86. doi: 10.1016/j.brainres.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 17.Suda T., Takahashi T., Golstein P., Nagata S. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M., Suda T., Takahashi T., Nagata S. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alderson M. R., Tough T. W., Davis-Smith T., Braddy S., Falk B., Schooley K. A., Goodwin R. G., Smith C. A., Ramsdell F., Lynch D. H. J. Exp. Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer L. R., Culver D. G., Tennant P., Davis A. A., Wang M., Castellano-Sanchez A., Khan J., Polak M. A., Glass J. D. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita K., Wu Y., Qiu J., Lang-Lazdunski L., Hirt L., Waeber C., Hyman B. T., Yuan J., Moskowitz M. A. J. Neurosci. 2000;20:6879–6887. doi: 10.1523/JNEUROSCI.20-18-06879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhardt O. G., Boutell C., Orr A., Ullrich E., Haller O., Everett R. D. Exp. Cell Res. 2003;283:36–50. doi: 10.1016/s0014-4827(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 23.Lamond A. I., Spector D. L. Nat. Rev. Mol. Cell Biol. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- 24.von Mikecz A., Zhang S., Montminy M., Tan E. M., Hemmerich P. J. Cell Biol. 2000;150:265–273. doi: 10.1083/jcb.150.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raoul C., Barthelemy C., Couzinet A., Hancock D., Pettmann B., Hueber A. O. J. Neurobiol. 2005;62:178–188. doi: 10.1002/neu.20086. [DOI] [PubMed] [Google Scholar]
- 26.Michaelson J. S., Bader D., Kuo F., Kozak C., Leder P. Genes Dev. 1999;13:1918–1923. doi: 10.1101/gad.13.15.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendotti C., Atzori C., Piva R., Tortarolo M., Strong M. J., DeBiasi S., Migheli A. J. Neuropathol. Exp. Neurol. 2004;63:113–119. doi: 10.1093/jnen/63.2.113. [DOI] [PubMed] [Google Scholar]
- 28.Adachi M., Suematsu S., Kondo T., Ogasawara J., Tanaka T., Yoshida N., Nagata S. Nat. Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 29.Yang X., Khosravi-Far R., Chang H. Y., Baltimore D. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani S. M., Matiba B., Armandola E. A., Krammer P. H. Eur. J. Immunol. 1994;24:3119–3123. doi: 10.1002/eji.1830241231. [DOI] [PubMed] [Google Scholar]
- 31.Karray S., Kress C., Cuvellier S., Hue-Beauvais C., Damotte D., Babinet C., Levi-Strauss M. J. Immunol. 2004;172:2118–2125. doi: 10.4049/jimmunol.172.4.2118. [DOI] [PubMed] [Google Scholar]
- 32.Facchinetti F., Sasaki M., Cutting F. B., Zhai P., MacDonald J. E., Reif D., Beal M. F., Huang P. L., Dawson T. M., Gurney M. E., Dawson V. L. Neuroscience. 1999;90:1483–1492. doi: 10.1016/s0306-4522(98)00492-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Newton D. C., Marsden P. A. Crit. Rev. Neurobiol. 1999;13:21–43. doi: 10.1615/critrevneurobiol.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- 34.Catania M. V., Aronica E., Yankaya B., Troost D. J. Neurosci. 2001;21:RC148. doi: 10.1523/JNEUROSCI.21-11-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin L. J., Chen K., Liu Z. J. Neurosci. 2005;25:6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strong M., Sopper M., He B. P. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2003;4:81–89. doi: 10.1080/14660820310012727. [DOI] [PubMed] [Google Scholar]
- 37.Slee E. A., Keogh S. A., Martin S. J. Cell Death Differ. 2000;7:556–565. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- 38.Suhara T., Kim H. S., Kirshenbaum L. A., Walsh K. Mol. Cell. Biol. 2002;22:680–691. doi: 10.1128/MCB.22.2.680-691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almer G., Vukosavic S., Romero N., Przedborski S. J. Neurochem. 1999;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- 40.Yi F. H., Lautrette C., Vermot-Desroches C., Bordessoule D., Couratier P., Wijdenes J., Preud'homme J. L., Jauberteau M. O. J. Neuroimmunol. 2000;109:211–220. doi: 10.1016/s0165-5728(00)00288-5. [DOI] [PubMed] [Google Scholar]
- 41.Perutz M. F., Windle A. H. Nature. 2001;412:143–144. doi: 10.1038/35084141. [DOI] [PubMed] [Google Scholar]
- 42.Henderson C. E., Bloch-Gallego E., Camu W. In: Nerve Cell Culture: A Practical Approach. Cohen J., Wilkin G., editors. London: Oxford Univ. Press; 1995. pp. 69–81. [Google Scholar]
- 43.Hollenbach A. D., Sublett J. E., McPherson C. J., Grosveld G. EMBO J. 1999;18:3702–3711. doi: 10.1093/emboj/18.13.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.