Abstract
There is a need for better description and heuristic understanding of the sustainability of populations connected over space by a dispersing stage, both for management purposes and to increase our basic knowledge of the dynamics of these populations. We show that persistence of such a population of connected subpopulations depends on whether the sum of the reproductive gains through all possible closed, between-patch reproductive paths through multiple generations, relative to the shortfall in self-persistence in each path, exceeds unity plus extra terms, which only appear if there are four or more patches. These extra terms have the heuristic explanation that they avoid double counting of reproductive paths that arise with four or more patches because there can be nonoverlapping subnetworks. Thus only those patterns of reproduction and connectivity which eventually lead to descendants returning to the patch from which they originate contribute to persistence. This result provides the basis for evaluating connectivity and habitat heterogeneity to understand reserve design, the effects of human fragmentation, the collapse of marine fisheries, and other conservation issues.
Keywords: connectivity, M-matrix, population dynamics, spatial dynamics, marine reserves
Understanding conditions for the persistence of populations that are distributed over space is a central issue in population biology that has received increasing attention in recent years from both a theoretical and empirical perspective (1–20). This general question of persistence can be viewed in a variety of particular contexts. The large body of theory that has been developed to describe metapopulation dynamics poses this question as a balance between colonization and extinction. Yet, in many instances, especially where extinctions do not occur or management depends on limited information, a different question is more useful to ask, namely whether growth rates with spatial heterogeneity are positive, looking only at deterministic rather than stochastic aspects (12). This question, or variants of it, arises in the context of reserve design, both for marine and terrestrial populations, in understanding the consequences of habitat fragmentation and for understanding the dynamics of infectious agents. We address this question by using a particular simple, general system that will allow us to deduce principles of biological interest.
Our goals are to understand how the interplay between connectivity (dispersal) and local population dynamics allows persistence in a network of heterogeneous patches, and to develop a simple general understanding of conditions for persistence. This question is the analogue of the similar question for persistence of a single species in a single patch. For a single species with age structure and no density dependence, dynamics are described by the Leslie matrix. Obviously, the population persists if the growth rate of the population, the largest eigenvalue of the Leslie matrix, is >1. Yet, rather than directly computing this eigenvalue, it is much simpler to use the criterion that the population will persist, and the largest eigenvalue will be >1, if the expected number of offspring per newborn is >1 (21). This quantity has a clear biological interpretation in terms of individuals replacing themselves in their lifetime. We aim to obtain an analogous simple persistence criterion for spatially structured populations. We analyze a simple density independent matrix model that could be derived from the description of a density-dependent spatially structured population at low abundance. Persistence obviously could be determined from the largest eigenvalue, but we seek instead an easily interpretable persistence condition.
The distribution of populations over space, and movement of individuals within them, are essential elements of their dynamics (18–20, 22). Connectivity among subpopulations has been identified as important, but we have no way to compute the general implications of connectivity for sustainability (23). Our understanding of the persistence of populations is essentially limited to two cases: the single, well mixed, nonspatial population and idealized systems of spatially distributed populations without habitat heterogeneity. This limited understanding restricts our ability to manage spatially distributed populations. For example, marine fisheries collapse (24) for a variety of reasons, but increasing and expanding fishing pressure is usually involved. As anthropogenic pressures increase, there are fewer de facto spatial refuges (25). Understanding and accounting for the conditions under which spatially structured marine populations can support sustainable fisheries is essential for improving their management. The developing potential of spatial management (26–29), for example the use of marine reserves and marine protected areas, underscores this need. Marine species need to be treated not as single populations but rather by using an approach that explicitly includes their heterogeneity and connectivity over space.
Understanding the implications of habitat fragmentation in terrestrial habitats also requires a better understanding of the interplay among connectivity, local growth rates, and persistence. Much of the focus has been on the dynamics of metapopulations, where stochasticity plays a dominant role, but of equal importance is an understanding of the fundamental deterministic factors that would lead to positive growth rates when populations are rare. A simple heuristic description of the conditions leading to a positive growth rate would provide valuable information in the evaluation of management options for spatially distributed populations, especially when data-poor management is necessary.
In the fisheries context, there is currently a good understanding of the persistence of single populations, and that information is used in fishery management of marine populations. Deterministic models with age structure and density-dependent recruitment predict that populations persist when lifetime reproduction is greater than a certain value, and that is used to set thresholds in fisheries management (30). However, this result applies only to single populations, and it is not clear how to summarize persistence of a spatially distributed population where reproduction may vary over space. Even though most fished populations consist of adults distributed over space connected by larval dispersal, that spatial distribution is generally not accounted for in fishery management, although specific simulations are possible (31).
In addition to providing a tool for conventional management, our approach also provides guidelines for determining which patches play a critical role in network persistence in spatially explicit management, as in reserve networks (22, 31 –34). Our results complement several previously studied cases of persistence in a spatial setting, including the single patch (35) and equally spaced reserves with fishing completely removing individuals between them in a uniform habitat (36). The important general case of persistence for species with heterogeneous habitat has been approached only in ref. 18. Our results complement and extend the development in ref. 18 by providing a biologically interpretable version of the persistence condition in the simplest possible case with habitat heterogeneity and exploring its applicability.
Here we address the case of a single species with a dispersing juvenile stage (which we call juvenile for simplicity but could, alternatively, be the adult stage) that are sedentary adults (or possibly juveniles). This case includes many plants, butterflies, marine and terrestrial invertebrates, reef-dwelling fishes, and some rockfish. For this case, we provide biologically interpretable conditions for population persistence in a temporally constant, but spatially heterogeneous, patchy environment. These persistence conditions allow us to answer a variety of essential questions. What is the persistence condition if habitats are heterogeneous with different per capita propagule production or survival and nonuniform patterns of dispersal as would arise from physical advective processes such as in wind or water? In a heterogeneous system, what parts of the system would be most important to protect to achieve persistence? How does a network of reserves function to ensure persistence of a species when a single reserve cannot?
We use a general model of the dynamics of a species in an arbitrary habitat, where the habitat is divided into a series of discrete patches, which may be connected by the dispersing stage in any fashion, and may have differing effects on egg production or propagule settlement, and time is discrete. Here, we do not address issues associated with using discrete patches to describe a habitat that is in fact continuous, but we note that data on population dynamics can only be collected in discrete patches, and that there are ways to compare the discrete patch approach to the continuous space approach (35). Moreover, what we are calling a patch in this paper need not necessarily be a small, connected region. We simply need to be able to specify rates of self-retention of juveniles and rates of exchange of juveniles between patches. To simplify our results, we do not include the role of age structure. Although our model is phrased as though there is no adult survival, this effect can be accounted for as part of the term describing self-retention of juveniles. Thus, our model applies directly, as long as the survivorship of adults is not age dependent. We recognize that our approach does not take into account some important aspects that would arise from stochasticity and density dependence.
Model and Analysis
Our focus here is on the conditions for persistence in the neighborhood of the equilibrium where the species would not be present in any patch. In essence, we begin by looking at a model for a population that is distributed among discrete habitat patches of the form
where the sum is over all of the habitat patches in the system, ni(t) is the population level in patch i at time t, fj is a function describing the production of larvae in patch j as a function of the adults in the patch in year t, and gji is a function that describes how the number of larvae produced in patch j and the adults in patch j the previous year contribute to the population level in patch i, taking into account both larval survival and movement. We can linearize this model about the zero equilibrium, leading to a model that does not include density dependence. We are assuming that population sizes are small in each patch and also implicitly assuming that there is no Allee effect in any patch. Thus, we are considering systems where extinction, if it occurs, happens by a steady decline in every part of the habitat. We are not including the effects of stochasticity but developing intuition based on deterministic processes.
This procedure leads to a single-species matrix model that need not include density dependence because our goal is to understand criteria for persistence. For simplicity, we also first consider a model that includes only simple year-to-year survival, which does not change the nature of the results from a model with adult survival, but allows us to emphasize and understand the role of connectivity. Thus, the model we analyze can be written as
where Nt is an n-dimensional vector of population densities in each of the n patches in year t, and C is a matrix with entries cij = bjpijai, where ai is the propagule production per settling recruit in habitat patch i, bj is the fraction of propagules arriving in habitat patch j that successfully recruit (and survive until censused the next year) when the species is rare, and pij is the probability that a propagule produced in habitat j ends up in habitat i. Because C has only nonnegative terms, Perron–Frobenious theory (37) implies that that C has a real eigenvalue equal to its spectral radius, and therefore, the condition for persistence is equivalent to the question of whether C has a real eigenvalue >1. Although the Jury conditions (38) provide an answer to this question directly, they are in a form that is very complex and does not provide a biologically meaningful interpretation. In the analogous situation for density independent growth in a single population, the largest eigenvalue determines persistence, but a much more useful heuristic criterion is whether the expected lifetime reproduction of an individual is >1, as noted above.
The first step toward deriving a biologically interpretable persistence condition is to note that because C has a real eigenvalue equal to its spectral radius, C will have no eigenvalues >1 in magnitude if and only if the real part of all of the eigenvalues of Q = C − I is negative. Thus, determining persistence reduces to the question of whether the matrix Q has any real positive eigenvalues.
We use results for M-matrices (39) to analyze Q. Matrices with all nonpositive off-diagonal elements and only eigenvalues with positive real parts are known as M-matrices. Thus −Q is potentially an M-matrix because it has the correct sign pattern for its entries. Using a standard result about M-matrices (39), that −Q is an M-matrix (and has all eigenvalues with positive real part) and therefore Q has all eigenvalues with negative real part (and the model is not persistent) if and only if all principle minors of Q are negative, i.e.,
where J is any principal submatrix of Q, including Q itself. Consequently, if [2] fails to hold for any submatrix, i.e., if any principle minor is positive, then the population will persist because there will be at least one eigenvalue of Q with positive real part.
One direct implication of the conditions implied by [2] is the biologically reasonable (and trivial) observation that if any subnetwork would be persistent without immigration, then the full network would be persistent, e.g., if any patch is self-sustaining, the population is, by definition, persistent. Thus, the conditions for persistence of a network are of interest only when no single patch could persist without receiving any immigrants and with the same level of emigration, i.e.,
for all i. A similar implication [2] is that we need to check for persistence at the level of smaller subnetworks before looking for persistence for larger networks, again assuming that the level of emigration is held fixed, and the subnetwork is assumed not to receive any immigrants from outside.
Here we distinguish two possible aspects of persistence of a single patch. One (which we call “demographic”) focuses only on the reproductive and settlement phases, and so asks whether ri = aibi is >1, whereas the other (which we call “life cycle”) asks whether the patch could persist in isolation and so would include the self-connectivity, and asks whether cii = piiri is >1 (as in equation [3]). Obviously, at least one patch must be demographic persistent or the population will collapse.
Assuming that no single patch is (life cycle) persistent, the magnitude of qii measures the shortfall in the sustainability of each single patch that would have to be made up by contributions from the other patches in the network (Eq. [3]). Then we can determine persistence for the full network by considering the converse of the condition [2], namely that the system persists if any principle minor is positive. From Eq. [2], by using the alternating sum definition of the determinant (37), we find that a necessary and sufficient condition for persistence in a network of n patches is
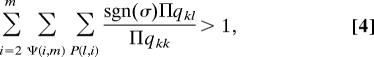 |
where the second sum is over all possible subnetworks Ψ(i,m) of i unique patches among any subset m of the n patches in the original system, and the third sum is over all possible permutations P(l,i) of the indices of the i unique patches except permutations that leave one or more indices unchanged. Finally, for the products in the numerator and in the denominator, the index k runs through the indices of the i unique patches in numerical order and the index l contains the indices in the order given by the permutation in the sum. Finally, sgn (σ) is determined by the sign of the permutation: It is 1 for an “even” permutation, and −1 for an “odd” permutation. Condition [4] follows from the definition of the determinant in terms of a sum of all possible permutations of rows and columns followed by dividing the resulting condition [2] by the product Πqkk, where the product runs from 1 to the number of patches (and, of course, reversing the sign in the inequality as necessary). We thus see that the condition for network persistence is most informatively written in terms of the elements of Q, producing a form with direct biological meaning, as we discuss below.
Our seemingly complex persistence condition [4] becomes much more transparent when we write out the cases of two, three, or four patches explicitly. For two patches, the persistence condition [4] is
whereas for three patches it becomes
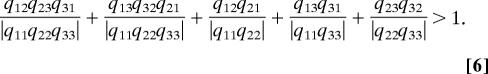 |
For four patches, a new feature appears, so we write out that condition for persistence as
 |
with the new kind of terms on the right-hand side. For the conditions [5–7], the denominators in all cases are products of terms representing shortfalls in persistence in single patches. For condition [5] note that the numerator is the only closed reproductive path through both patches. Similarly, in [6], note that the numerators in the three “two-patch” terms each represent the possible closed reproductive paths through two patches in two years of successive propagule dispersal, and that the two “three-patch” terms represent the possible closed reproductive paths through three patches in three years of successive propagule dispersal.
With four or more patches, a new structure emerges because there can be disjoint subnetworks, such as patches 1 and 2, and also patches 3 and 4 in a four-patch system. Thus this system can persist if any two-patch subnetwork persists, and also if any three-patch subnetwork persists. The persistence due to the two-patch subnetworks needs to be examined first, but then persistence at the four-patch level should not “double” the count of the contribution from the two-patch subnetworks. Thus, the structure of the four-patch condition [7] is similar, but new terms appear on the right-hand side representing the product of paths through disjoint two-patch subnetworks. An example illustrating the role of these terms is given below. With more patches, additional terms representing products of nonoverlapping subnetworks appear. Also the fact that a four-patch network consisting of two disjoint two-patch subnetworks could persist if either one or two of the subnetworks persisted means that one needs multiple criteria for persistence, as we have outlined, and explains why we do not obtain a single number like the reproductive number for a single population.
We note again that a network can be persistent only if at least one patch is demographic persistent, but can persist if no patch is life cycle persistent. Although the two-patch conditions are contained in the three-patch conditions as is seen in [5] and [6], the condition for persistence from interactions among four patches as in [7] may not hold, whereas it does hold for two disjoint two-patch subnetworks. Thus, one needs to check persistence conditions for subnetworks as well as the persistence conditions involving loops with larger numbers of patches.
Examples.
We present here examples that illustrate our general conditions. In general, joint persistence among patches represents a network effect, beyond the combined effects of individual patches. For example, in Eq. [5], assume that c11 = c22 = 0.9, so each population in isolation would decrease by 10% each year, accounting for local population dynamics and losses because of dispersal out. Then, for persistence, the between-patch reproductive gain, q12q21, must exceed (0.1)2. The quantity q12q21 is the product of the per capita production rates in each patch per settling propagule, the fraction of individuals produced in patch 1 that settle in patch 2, and the fraction of individuals produced in patch 2 that settle in patch 1, minus 1. An important characteristic of this expression is that none of the reproductive paths need to provide a reproductive gain >1.0, rather it is only the weighted sum of the reproductive gains in all paths that is important.
Given the form of the persistence conditions, even patches that are sinks (even life cycle sinks) may be essential for the persistence of a network. If there is a two-patch network (patches labeled 1 and 2) that is not persistent, then adding a third patch may ensure persistence even if the third patch is a life cycle sink, with q33 < 1, if the third patch acts as a vital link by contributing enough connectivity.
As another example, we consider a three-patch network where no two-patch subnetwork is persistent and also where the network would not persist if the only connections would be between neighboring patches. We then ask how large the connectivity between the end patches must be for the system to persist. For an example, assume that the connectivity matrix takes the form
 |
We have specified all of the connectivities, except those between patches 1 and 3, and we will examine how persistence may depend on the strength of this connection. Further assume that the population dynamics are the same in each patch, so that aibi = 1.1 for all three patches.
We now compute the persistence conditions. From the left-hand side of [5], we see that, if p13 = p31 = 0, the values of the terms for the two different two-patch subnetworks on the left-hand side of [6] would be 0.283014 and 0.57619, summing to 0.859231, so the system would not persist. However, if p13 = p31 = 0.03, then the left-hand side of [6] would be 1.00156, and the system would be persistent. Note that, in this case, the contribution from the pathway involving patches 1 and 3 would be 0.02183, whereas the two different pathways through all three patches would contribute 0.06671 and 0.05337. The relatively small numerical values of the contributions from the pathways through three patches is typical for many reasonable connectivity matrices.
We now discuss a four-patch example with patches arranged in a line, with dispersal only between neighboring patches, so qij = 0 unless i = j, or i and j differ by 1. To present the persistence condition, it is easiest to first give a name to the two-patch persistence condition, defining
where the order of i and j does not matter and i is different from j. Thus γ(i, j) is a summary of the persistence condition for two patches and is very useful in writing a compact form of the persistence for more than two patches.
Assuming that no single patch is persistent, and that no two-patch subnetwork is persistent, γ(i, j) < 1 for all pairs i and j, the relevant three-patch subnetwork persistence conditions are γ (1, 2) + γ (2, 3) > 1 or γ (2, 3) + γ (3, 4) > 1. Finally the four-patch persistence condition becomes
Thus, for example, if γ (1, 2) = 0.7, γ (2, 3) = 0.2, and γ (3, 4) = 0.7, no two-patch or three-patch subnetwork is persistent, but the four-patch network is persistent. Here the two patches 1 and 2 form one subnetwork, which is only loosely connected to the subnetwork formed by patches 3 and 4. Consequently, if the connection between the two disjoint two-patch subnetworks were weaker, if γ (2, 3) < 0.09, then the four-patch network would not persist either. This example helps illustrate the heuristic role played by the terms on the right-hand side of [7], and explains why we need to look at both the condition for the two-patch subnetworks and the four-patch systems to check for persistence.
Note also that if there is a two-patch subnetwork that is weak, where it is located in a linear array with only nearest neighbor dispersal can be critical for persistence, as would be expected. Thus, if the values of γ for the three two-patch subnetworks of a linear four-patch network were 0.5, 0.4, and 0.25, and if the poorly connected two-patch network represented by the measure γ = 0.25 was at either end, the system would be persistent, whereas if it was in the middle [γ (2, 3) = 0.25] the system would not persist.
Extensions.
Two important extensions to our linear model can be considered in the same framework we have developed here, namely, the role of adult survivorship, and the consistency of our approach when more finely dividing the available habitat into more patches. Our model [1] as defined above does not include adult survivorship, but a model with adult survivorship could be written simply as
where the entries in the diagonal matrix S are the patch-specific adult survivorship rates. If we then define a new matrix C* = C + S, we can then repeat our analysis above and obtain analogous results.
As we have suggested, the division of the habitat into patches can be arbitrary. We therefore consider the consequences of the division of the division of patch i into patches j and k, demonstrating that if the demographic and dispersal parameters are consistent, that the persistence condition is unchanged. We assume that rj = rk = ri, which leads to conditions on the parameters describing per capita larval production and larval survivorship. We also need consistency of the dispersal parameters, so we need to assume that pii = pjk + pkk = pkj + pjj, so retention is the same. We also need to assume that pli = plj = plk, for any other patch l, so the probability of dispersal to patch l is the same. Finally, assuming that the probability of dispersing from patch l is the same leads to the condition pil = pjl + pkl. Under all these conditions, it is a straightforward calculation to show that for the maximal positive eigenvalue (growth rate) λ for the original model with corresponding eigenvector, then the model with the ith patch divided will also have an eigenvalue λ with an analogous corresponding eigenvector where the ith entry is now repeated to correspond to patches j and k. Thus we can increase the resolution of our model in a consistent fashion without changing the persistence of the system. Then we can look at the effects of changing parameters in this system described at a higher level of resolution.
Discussion
We have developed a biologically interpretable quantitative condition for persistence of a spatially structured population, which is also easily understood when written explicitly for small numbers of patches, as in [5–7]. Also we note again that the Jury conditions (37) also provide essentially equivalent necessary and sufficient conditions, but because they do not take into account the structure of the matrix (positive entries) they are not as simple nor are as easy to interpret biologically. The overall advantage of this expression is that it provides a condition that is both easily interpretable and quantitative. Note that this condition, although on the surface similar to earlier results (40, 41), is in fact different, providing a necessary and sufficient condition for persistence (here instability ≈0) that depends critically on the signs of the entries in the model, unlike the earlier results. The underlying model we use is not a conventional metapopulation model that would allow for stochasticity and extinctions and colonization. Instead, we determine a persistence condition for a deterministic model with underlying spatial heterogeneity in growth and specified connectivity. Kritzer and Sale (42) have discussed this kind of development of metapopulation models for marine populations, where frequent extinctions and recolonizations are not the primary issue. Similar questions arise in understanding the dynamics of diseases and the role of habitat fragmentation (43). We also need to emphasize that the deterministic conditions we derive in the absence of density dependence need to be used as a guide in more complex situations where other issues are included.
Our condition for persistence when no patch can persist in isolation, Eqs. [4–7], has a simple heuristic interpretation: Each term essentially represents the gain in number of individuals that are contributed to a patch through successive generations by dispersal among a set of patches (the numerator), relative to a power of the geometric mean of the shortfall in each patch below what would be necessary for that patch to be self-sustaining (the denominator). The form of each term in the sum indicates that the shortfall in individual patches can be made up by reproductive paths through other patches. This form clearly implies that only patches that truly both receive and contribute propagules to the entire system play a role in the sustainability of the system, although the effect of adding an additional patch to a network is quite complex, as we showed above.
The results obtained here can contribute to our intuition regarding spatially structured populations. The form of the terms in the persistence condition [4], [5], [6], or [7] describe the currency by which we should measure a patch’s contribution to persistence. Each term can be envisioned as an element contributing to persistence, which will have a connectivity component, divided by the local shortfall in persistence. Note that when considering different subnetworks here, we are assuming that adding or removing patches does not change the rate of movement among the patches, as would be the case for larvae that are not actively choosing settlement sites. Furthermore, ecologists commonly think about the dynamics of spatially distributed populations in terms of their source/sink nature. Although the notion of source and sink has a variety of definitions (18, 33, 44), our result makes explicit the important consideration of connectivity (45). It is not simply the number of propagules being exported to other patches that is important but also the number returning.
A second general conclusion (obvious from the form of Eqs. [4–7]) is that in the persistence condition the population dynamic description appears only as ri = aibi, namely the per capita production and propagule survival in each patch enter into the condition for persistence only as the product. (The denominators only contain terms of the form qii, and in the numerator, if there is a qki, then there also is a qij as well, where k and j may be the same.) Thus, when considering a network, the key patches are those that are good for both production and propagule settlement.
For any number of patches, the interpretation of the form is similar, a sum of the product of reproductive gain divided by individual shortfall in persistence, summed over all possible reproductive paths relative to unity and terms that essentially prevent double counting of contributions from smaller subnetworks. Increasing the number of patches in the same area increases resolution, but understanding the effect on the persistence condition is complex as this increase will change the connectivity (pij) terms. For example, dividing a single patch in two will typically change the pii term for the two new patches relative to the original one, unless there is no dispersal at all. As we have shown, increasing the resolution does not introduce inconsistencies in the persistence condition, and changes in persistence if the connectivities are defined in a consistent fashion.
From a practical standpoint, although the exact values of parameters that appear in the formulae exhibited in this paper, specifically the propagule distribution patterns, are currently highly uncertain in most systems, the results clearly delineate the kind of information that is needed to make a quantitative determination of persistence. However, in the marine realm, attempts to obtain information on connectivity are increasing by using techniques like chemical analysis of otoliths of fish (46), and analogous approaches may be applied to some mollusks. The analysis in ref. 46 provides some initial estimates of a connectivity matrix. A variety of techniques can be used to determine connectivity and spatial scale (45), including approaches based on genetics and on chemical signatures, each having advantages and pitfalls. This question of population connectivity is a difficult, but important, question to address.
The major result of this paper does provide intuitive guidance for overfishing, marine reserves, essential fish habitat, understanding habitat fragmentation, and delisting criteria for endangered species. In each of these applications, although the values of pij may currently be uncertain, we can control the values of ri. In fisheries, the parameters ri will be heterogeneous over space because of an uneven distribution of effort in addition to possible heterogeneous habitat. The single population condition used in fishery management (30) is our condition for life cycle persistence. Our results show that it can be unmet for all individual subpopulations, yet the full population may persist. This situation may exist in heavily fished populations. In deciding which fish habitat should be included as essential fish habitat, the condition given here emphasizes that connectivity is as essential to consider as productivity. For example, consideration of connectivity might tend to lead to several protected areas near each other, rather than many spread further apart. Some other results for persistence of marine reserves are special cases of the condition derived here, if one considers all reserve areas to be one habitat type and all fished area to be another type (36, 47).
Although we have used a standard definition of persistence here, that a persistent population increases when rare in a deterministic model, other definitions may be desirable (e.g., including a condition for minimal abundance at all locations). Such definitions may be useful for marine reserves. These other definitions will likely include some elements similar to the conclusions drawn here, in the sense that areas that include patches that contribute significantly to persistence as defined here are likely to be areas of higher density under other definitions. Extending the results here to include these effects, and also stochasticity and species with highly mobile adults, is an important challenge for future work. As we have shown, extending the results here to include adult survivorship is also relatively straightforward and does not change the nature of the results, as doing so involves only changing the terms involving the self-recruitment. However, the results from our modeling approach obviously can be taken only as a guide to understanding, and the effects of stochasticity of various kinds and other aspects we do not consider would need to be taken into account when looking at more complex systems.
In summary, the results obtained here provide a valuable tool for understanding and managing populations distributed in space. Currently used approaches to persistence cannot deal with the acknowledged dynamically important spatial structure and internal connectivity of these populations. Further examination of these results will allow us to determine the potential effects of areas of current uncertainty and may lead to refinements of the definition of persistence.
Acknowledgments
We thank Steve Gaines and two anonymous referees for comments on the manuscript. This research was supported by a subcontract from American Museum of Natural History (to A.H. at the University of California, Davis), under National Science Foundation Biocomplexity Grant OCE-0119976 and through a grant to A.H. and L.W.B. for the Designing Marine Reserves Working Group from the National Center for Ecological Analysis and Synthesis, a Center funded by National Science Foundation Grant DEB-0072909, the University of California, and the Santa Barbara campus.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.DeWoody Y. D., Feng Z. L., Swihart R. K. Am. Nat. 2005;166:42–55. doi: 10.1086/430639. [DOI] [PubMed] [Google Scholar]
- 2.Akcakaya H. R., Franklin J., Syphard A. D., Stephenson J. R. Ecol. Appl. 2005;15:521–531. [Google Scholar]
- 3.Kallimanis A. S., Kunin W. E., Halley J. M., Sgardelis S. P. Conserv. Biol. 2005;19:534–546. [Google Scholar]
- 4.Akcakaya H. R., Radeloff V. C., Mlandenoff D. J., He H. S. Conserv. Biol. 2004;18:526–537. [Google Scholar]
- 5.King A. A., Hastings A. Theor. Popul. Biol. 2003;64:431–438. doi: 10.1016/s0040-5809(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 6.Krawchuk M. A., Taylor P. D. Oikos. 2003;103:153–161. [Google Scholar]
- 7.Kuras T., Benes J., Fric Z., Konvicka M. Popul. Ecol. 2003;45:115–123. [Google Scholar]
- 8.Cronin J. T. Ecology. 2003;84:1179–1188. [Google Scholar]
- 9.Smedbol R. K., Wroblewski J. S. Fish. Res. 2002;55:161–174. [Google Scholar]
- 10.Park A. W., Gubbins S., Gilligan C. A. Oikos. 2001;94:162–174. [Google Scholar]
- 11.Ellner S. P., McCauley E., Kendall B. E., Briggs C. J., Hosseini P. R., Wood S. N., Janssen A., Sabelis M. W., Turchin P., Nisbet R. M., Murdoch W. W. Nature. 2001;412:538–543. doi: 10.1038/35087580. [DOI] [PubMed] [Google Scholar]
- 12.Johnson M. P. Mar. Ecol. Prog. Ser. 2001;211:215–224. [Google Scholar]
- 13.Baguette M., Petit S., Queva F. J. Appl. Ecol. 2000;37:100–108. [Google Scholar]
- 14.Thomas C. D., Kunin W. E. J. Anim. Ecol. 1999;68:647–657. [Google Scholar]
- 15.Smith D. L., Dushoff J., Perencevich E. N., Harris A. D., Levin S. A. Proc. Natl. Acad. Sci. USA. 2004;101:3709–3714. doi: 10.1073/pnas.0400456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earn D. J. D., Levin S. A., Rohani P. Science. 2000;290:1360–1364. doi: 10.1126/science.290.5495.1360. [DOI] [PubMed] [Google Scholar]
- 17.Levin S. A. Ecology. 1992;73:1943–1967. [Google Scholar]
- 18.Armsworth P. R. Ecology. 2002;83:1092–1104. [Google Scholar]
- 19.James M. K., Armsworth P. R., Mason L. B., Bode L. Proc. R. Soc. London Ser. B; 2002. pp. 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hixon M. A., Pacala S. W., Sandin S. A. Ecology. 2002;83:1490–1508. [Google Scholar]
- 21.Caswell H. Matrix Population Models: Construction, Analysis, and Interpretation. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 22.Dahlgren C. P., Sobel J. Bull. Mar. Sci. 2000;66:707–719. [Google Scholar]
- 23.Roberts C. M. Science. 1997;278:1454–1457. doi: 10.1126/science.278.5342.1454. [DOI] [PubMed] [Google Scholar]
- 24.Hutchings J. A. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- 25.Orensanz J. M. L., Armstrong J., Armstrong D., Hilborn R. Rev. Fish Biol. Fish. 1998;8:117–176. [Google Scholar]
- 26.Roberts C. M. Fisheries. 1998;23:16–19. [Google Scholar]
- 27.Roberts C. M., Polunin N. V. C. Ambio. 1993;22:363–368. [Google Scholar]
- 28.Lubchenco J., Palumbi S. R., Gaines S. D., Andelman S. Ecol. Apps. 2003;13:S3–S7. [Google Scholar]
- 29.Sala E., Aburto-Oropeza O., Paredes G., Parra I., Barrera J. C., Dayton P. K. Science. 2002;298:1991–1993. doi: 10.1126/science.1075284. [DOI] [PubMed] [Google Scholar]
- 30.Sissenwine M. P., Shepherd J. G. Can. J. Fish. Aquat. Sci. 1987;44:913–918. [Google Scholar]
- 31.Murray S. N., Ambrose R. F., Bohnsack J. A., Botsford L. W., Carr M. H., Davis G. E., Dayton P. K., Gotshall D., Gunderson D. R., Hixon M. A., et al. Fisheries. 1999;24:11–25. [Google Scholar]
- 32.Airame S., Dugan J. E., Lafferty K. D., Leslie H., McArdle D. A., Warner R. R. Ecol. Apps. 2003;13:S170–S184. [Google Scholar]
- 33.Tuck G., Possingham H. Mar. Ecol. Prog. Ser. 2000;192:89–101. [Google Scholar]
- 34.Lipcius R. N., Stockhausen W. T., Eggleston D. B. Mar. Freshw. Res. 2001;52:1589–1598. [Google Scholar]
- 35.VanKirk R. W., Lewis M. A. Bull. Math. Biol. 1997;59:107–137. [Google Scholar]
- 36.Botsford L. W., Hastings A., Gaines S. D. Ecol. Lett. 2001;4:144–150. [Google Scholar]
- 37.Horn R. A., Johnson C. R. Matrix Analysis. Cambridge, U.K.: Cambridge Univ. Press; 1985. [Google Scholar]
- 38.Elaydi S. An Introduction to Difference Equations. New York: Springer; 1996. [Google Scholar]
- 39.Horn R. A., Johnson C. R. Topics in Matrix Analysis. Cambridge, U.K.: Cambridge Univ. Press; 1991. [Google Scholar]
- Levins R. In: Ecology and Evolution of Communities. Cody M. L., Diamond J. M., editors. Cambridge, MA: Harvard Univ. Press; 1975. pp. 16–50. [Google Scholar]
- 41.Lewis E. R. Ecology. 1976;57:33–47. [Google Scholar]
- 42.Kritzer J. P., Sale P. F. Fish Fish. 2004;5:131–140. [Google Scholar]
- 43.Fahrig L., Merriam G. Conserv. Biol. 1994;8:50–59. [Google Scholar]
- 44.Pulliam H. R. Am. Nat. 1988;132:652–661. [Google Scholar]
- 45.Sale P. F., Kritzer J. P. Fish. Res. 2003;65:153–172. [Google Scholar]
- 46.Thorrold S. R., Latkoczy C., Swart P. K., Jones C. M. Science. 2001;291:297–299. doi: 10.1126/science.291.5502.297. [DOI] [PubMed] [Google Scholar]
- 47.Lockwood D. R., Hastings A., Botsford L. W. Theor. Popul. Biol. 1976;61:297–309. doi: 10.1006/tpbi.2002.1572. [DOI] [PubMed] [Google Scholar]


