Abstract
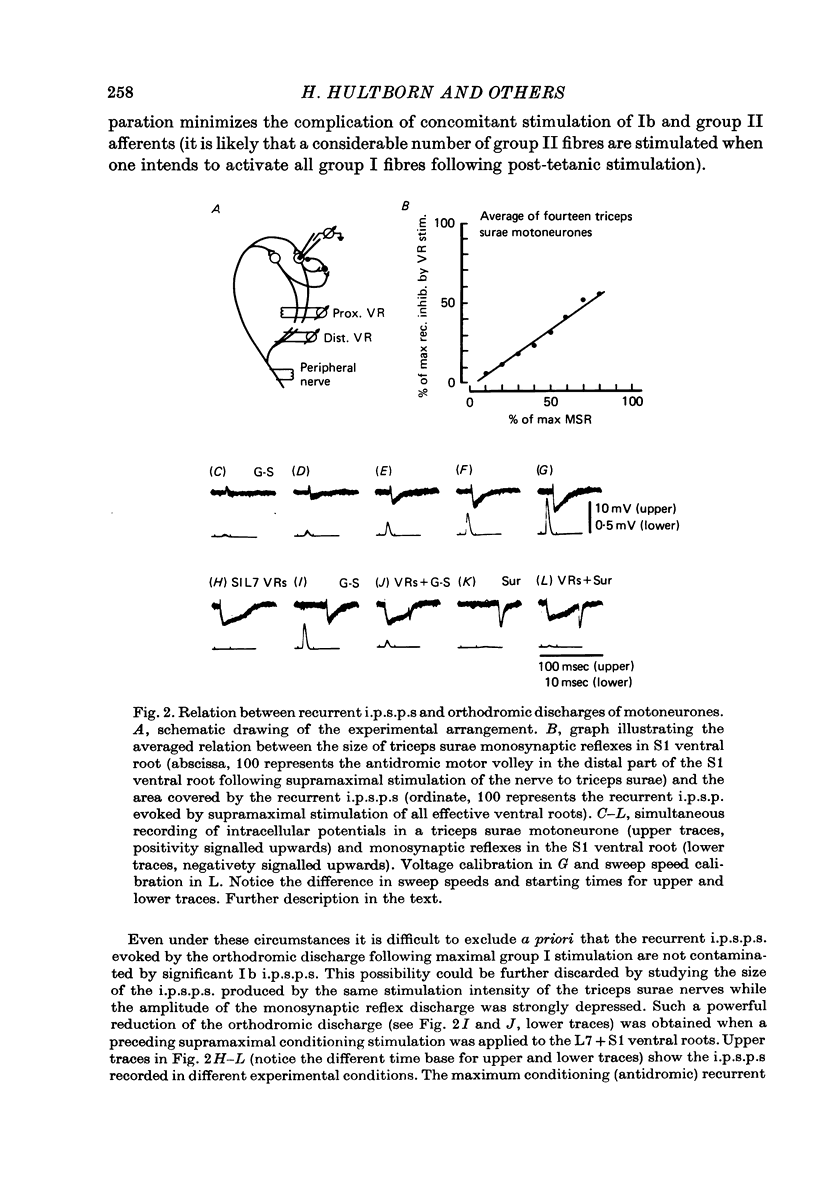
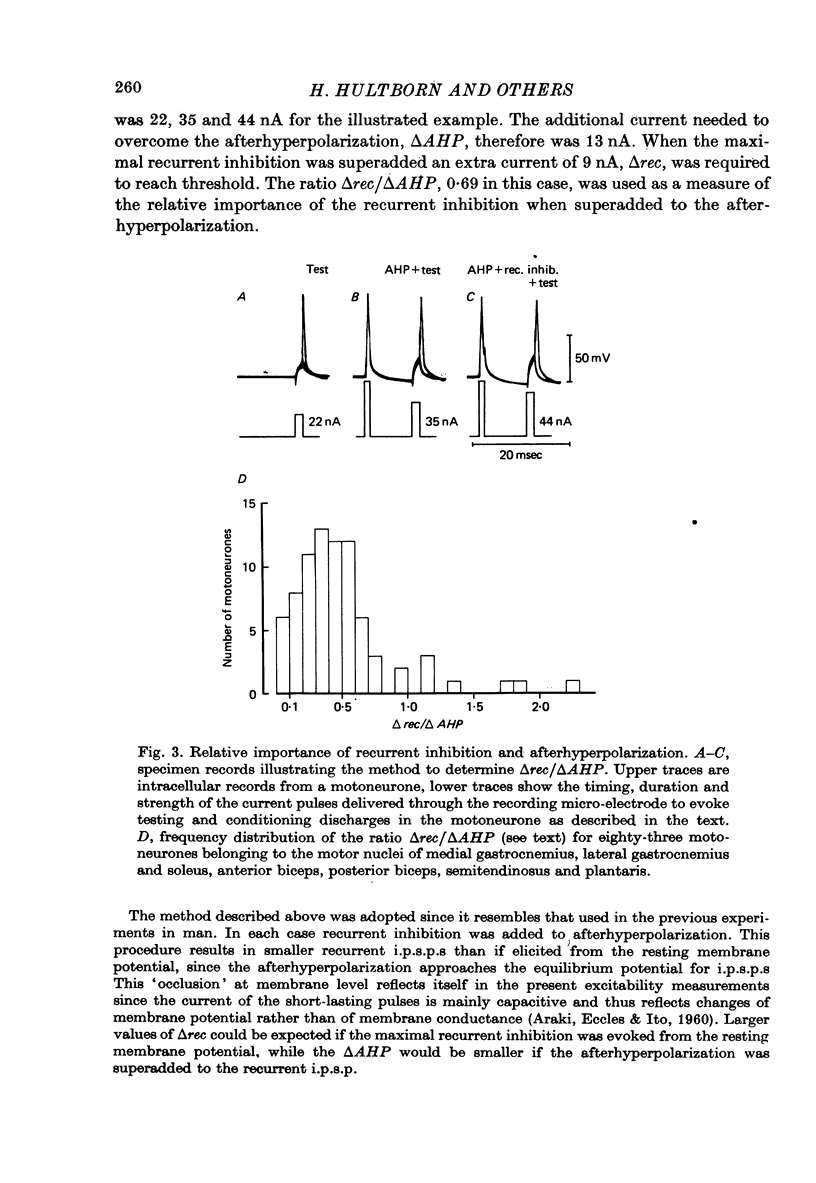
1. The relation between the size of a monosynaptic reflex (varying from the smallest values to the maximal motor response) and the output from Renshaw cells was investigated. This relation was extremely variable from one Renshaw cell to another. However, a linear relation between the reflex size and the early discharge emerged when the responses of all the Renshaw cells were averaged or when the summed activity of a pool of Renshaw cells was estimated by recording the recurrent inhibition in their target motoneurones. It was concluded that the lowest threshold motoneurones were efficient in producing recurrent inhibition. 2. In motoneurones, recorded intracellularly, the size of the depression caused by the afterhyperpolarization was compared to the maximum autogenetic recurrent inhibition. Under the particular experimental conditions used to mimic human experiments (Hultborn & Pierrot-Deseilligny, 1979a), it was found that recurrent inhibition had the same order of magnitude as the depression caused by afterhyperpolarization. 3. The additional depression caused by the summation of afterhyperpolarizations of two consecutive spikes was measured. It was shown that a summation of importance equal to the maximum autogenic recurrent inhibition required a mean interspike interval of 25 msec.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand. 1974 Sep;92(1):27–47. doi: 10.1111/j.1748-1716.1974.tb05720.x. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Schmalbruch H. Contraction times and fibre types in intact human muscle. Acta Physiol Scand. 1970 Aug;79(4):435–452. doi: 10.1111/j.1748-1716.1970.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel B., Pierrot-Deseilligny E. Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol. 1977 Jul;269(2):319–339. doi: 10.1113/jphysiol.1977.sp011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamann H. P., Henneman E. Electrical measurement of axon diameter and its use in relating motoneuron size to critical firing level. J Neurophysiol. 1976 Jul;39(4):844–851. doi: 10.1152/jn.1976.39.4.844. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different hind-limb muscles. J Physiol. 1978 Aug;281:285–299. doi: 10.1113/jphysiol.1978.sp012422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological investigations on Renshaw cells. J Physiol. 1961 Dec;159:461–478. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Illert M., Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. I. Disynaptic Ia inhibition of Ia inhibitory interneurones. Acta Physiol Scand. 1976 Feb;96(2):193–201. doi: 10.1111/j.1748-1716.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979 Dec;297(0):229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Input-output relations in the pathway of recurrent inhibition to motoneurones in the cat. J Physiol. 1979 Dec;297(0):267–287. doi: 10.1113/jphysiol.1979.sp013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Lundberg A., Rudomin P., Sykova E. Effects of 4-aminopyridine on transmission in excitatory and inhibitory synapses in the spinal cord. Brain Res. 1977 Nov 11;136(2):387–392. doi: 10.1016/0006-8993(77)90816-2. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Fukushima K. Effect of differential blocking of motor axons on antidromic activation of Renshaw cells in the cat. Exp Brain Res. 1974;20(2):135–143. doi: 10.1007/BF00234008. [DOI] [PubMed] [Google Scholar]
- Ross H. G., Cleveland S., Haase J. Quantitative relation of Renshaw cell discharges to monosynaptic reflex height. Pflugers Arch. 1972;332(1):73–79. doi: 10.1007/BF00603815. [DOI] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C., Goldfarb J. Excitation of Renshaw cells in relation to orthodromic and antidromic excitation of motoneurons. J Neurophysiol. 1972 Jan;35(1):137–148. doi: 10.1152/jn.1972.35.1.137. [DOI] [PubMed] [Google Scholar]


