Abstract
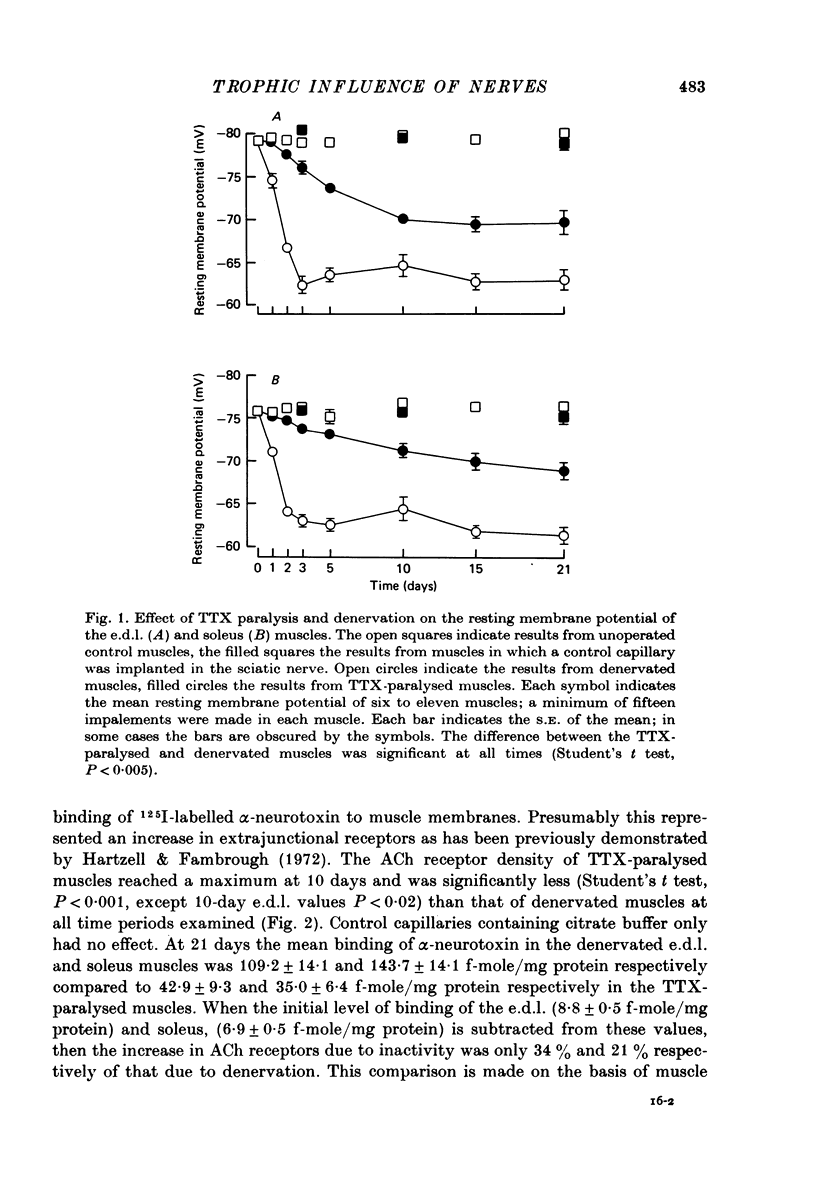
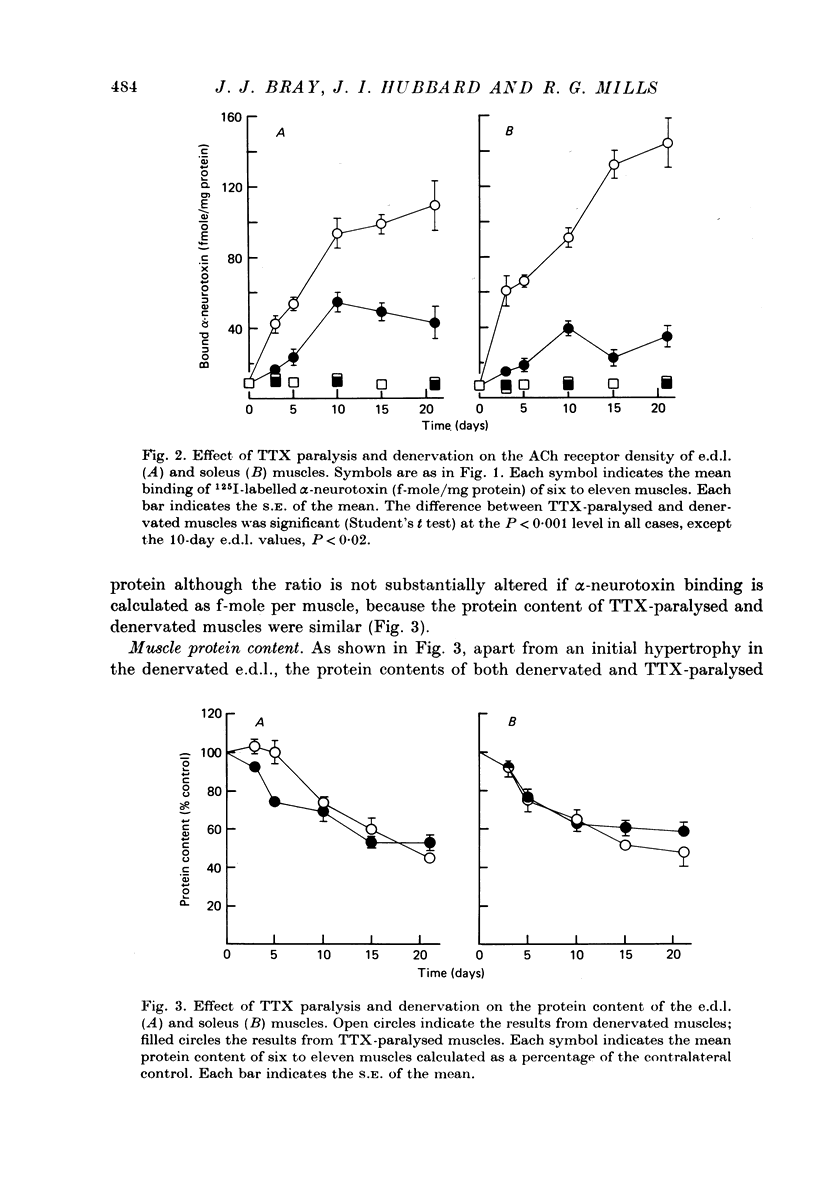
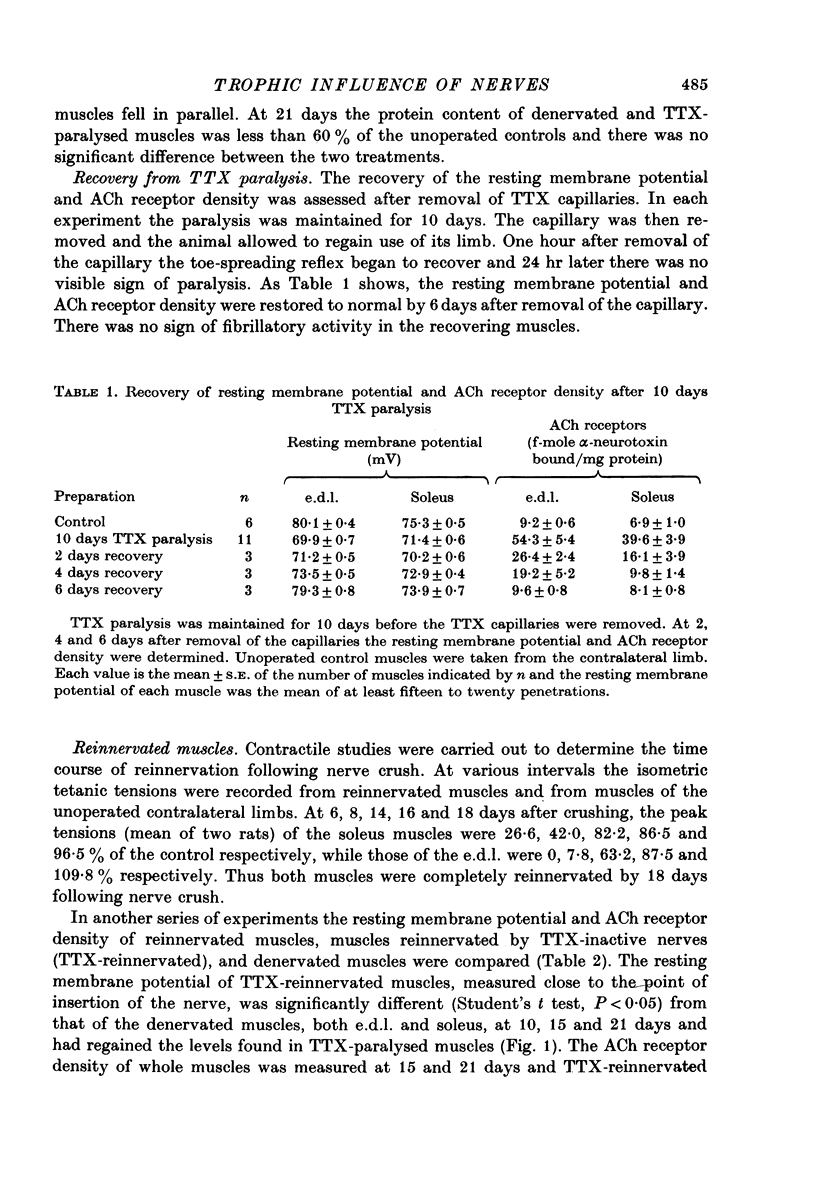
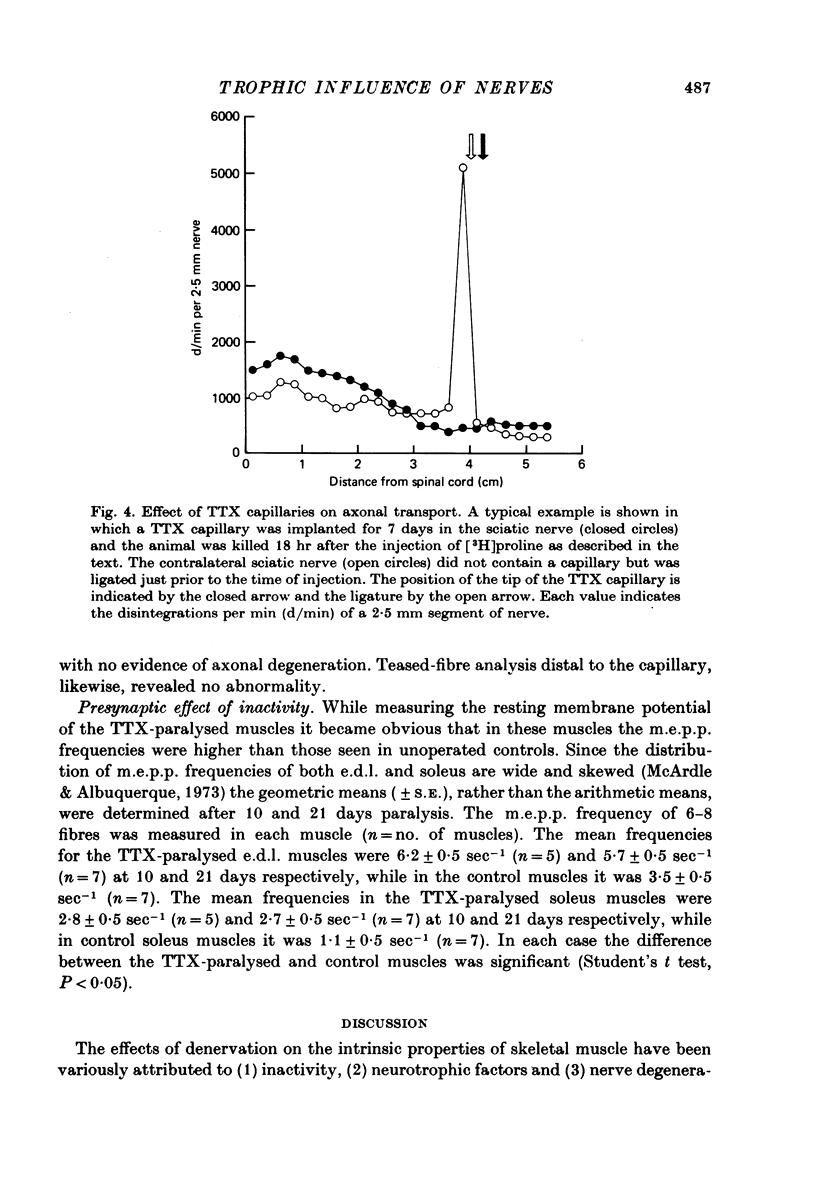
1. Nerve impulses in the rat sciatic nerve were blocked for long periods by tetrodotoxin (TTX) released from capillary implants. The TTX capillaries did not block axonal transport, nor did they cause any sign of nerve degeneration. 2. A comparison of the effects of TTX paralysis and denervation was made on both extensor digitorium longus (e.d.l.) and soleus muscles over 21 days, a time when the products of nerve degeneration were unlikely to contribute to the changes associated with denervation. The resting membrane potential of TTX-paralysed muscles was significantly different (P less than 0.005) from that of the denervated muscles at all periods and at 21 days the decrease that can be attributed to inactivity was 61% (e.d.l.) and 49% (soleus) of that which follows denervation. This disparity was even more pronounced for the ACh receptor density where the increase in receptors due to inactivity was only 34% (e.d.l.) and 21% (soleus) of that due to denervation. 3. A similar comparison was made on muscles which had been reinnervated by TTX-inactive nerves. These muscles were found to have a significantly higher resting membrane potential and lower ACh receptor density than the denervated muscles (P less than 0.05). 4. The experiments on reinnervated muscles preclude the possibility that nerve degeneration products are solely responsible for the difference between the TTX-paralysed and denervated muscles and suggest that the difference can be attributed to the trophic influence of the nerve. 5. An observed increase in the m.e.p.p. frequency of the TTX-paralysed muscles indicated that nerve action potentials play a role in regulating the spontaneous release from nerve terminals.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E., Tasse J. R., Sansone F. M. Effects of vinblastine and colchicine on neural regulation of the fast and slow skeletal muscles of the rat. Exp Neurol. 1972 Dec;37(3):607–634. doi: 10.1016/0014-4886(72)90103-3. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. J., Harris A. J. Dissociation between nerve-muscle transmission and nerve trophic effects on rat diaphragm using type D botulinum toxin. J Physiol. 1975 Dec;253(1):53–77. doi: 10.1113/jphysiol.1975.sp011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Degenerating nerve products affect innervated muscle fibres. Nature. 1978 Oct 19;275(5681):652–654. doi: 10.1038/275652a0. [DOI] [PubMed] [Google Scholar]
- Cangiano A., Fried J. A. The production of denervation-like changes in rat muscle by colchicine, without interference with axonal transport or muscle activity. J Physiol. 1977 Feb;265(1):63–84. doi: 10.1113/jphysiol.1977.sp011705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Lutzemberger L., Nicotra L. Non-equivalence of impulse blockade and denervation in the production of membrane changes in rat skeletal muscle. J Physiol. 1977 Dec;273(3):691–706. doi: 10.1113/jphysiol.1977.sp012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Lutzemberger L. Partial denervation affects both denervated and innervated fibers in the mammalian skeletal muscle. Science. 1977 Apr 29;196(4289):542–545. doi: 10.1126/science.850797. [DOI] [PubMed] [Google Scholar]
- Deak I. I. Demonstration of sensory neurones in the ectopic cuticle of spineless-aristapedia, a homoeotic mutant of Drosophila. Nature. 1976 Mar 18;260(5548):252–254. doi: 10.1038/260252a0. [DOI] [PubMed] [Google Scholar]
- Deshpande S. S., Albuquerque E. X., Guth L. Neurotrophic regulation of prejunctional and postjunctional membrane at the mammalian motor endplate. Exp Neurol. 1976 Oct;53(1):151–165. doi: 10.1016/0014-4886(76)90289-2. [DOI] [PubMed] [Google Scholar]
- Ferdandez H. L., Inestrosa N. C. Role of axoplasmic transport in neurotrophic regulation of muscle end plate acetylcholinesterase. Nature. 1976 Jul 1;262(5563):55–56. doi: 10.1038/262055a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliatt R. W., Westgaard R. H., Williams I. R. Acetylcholine-sensitivity of denervated and inactivated baboon muscle fibres [proceedings]. J Physiol. 1977 Oct;271(2):21P–22P. [PubMed] [Google Scholar]
- Gilliatt R. W., Westgaard R. H., Williams I. R. Extrajunctional acetylcholine sensitivity of inactive muscle fibres in the baboon during prolonged nerve pressure block. J Physiol. 1978 Jul;280:499–514. doi: 10.1113/jphysiol.1978.sp012397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. W., Price D. L., Drachman D. B., Engel W. K. Axonal transport to and from the motor nerve ending. Ann N Y Acad Sci. 1976;274:31–45. doi: 10.1111/j.1749-6632.1976.tb47674.x. [DOI] [PubMed] [Google Scholar]
- Gutmann E. Neurotrophic relations. Annu Rev Physiol. 1976;38:177–216. doi: 10.1146/annurev.ph.38.030176.001141. [DOI] [PubMed] [Google Scholar]
- Harris A. J. Inductive functions of the nervous system. Annu Rev Physiol. 1974;36:251–305. doi: 10.1146/annurev.ph.36.030174.001343. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCO J. V., EYZAGUIRRE C. Fibrillation and hypersensitivity to ACh in denervated muscle: effect of length of degenerating nerve fibers. J Neurophysiol. 1955 Jan;18(1):65–73. doi: 10.1152/jn.1955.18.1.65. [DOI] [PubMed] [Google Scholar]
- Lasek R. J. Axoplasmic transport of labeled proteins in rat ventral motoneurons. Exp Neurol. 1968 May;21(1):41–51. doi: 10.1016/0014-4886(68)90032-0. [DOI] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenehouse A. Comparison of alpha-bungarotoxin binding to skeletal muscles after inactivity or denervation. Nature. 1976 Mar 25;260(5549):349–350. doi: 10.1038/260349a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C., Chiu T. H., Chiu P. J., Tseng T. C., Lee S. Y. Pharmacological properties of cardiotoxin isolated from Formosan cobra venom. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(4):360–374. doi: 10.1007/BF00536909. [DOI] [PubMed] [Google Scholar]
- Lomo T. Neurotrophic control of colchicine effects on muscle? Nature. 1974 May 31;249(456):473–474. doi: 10.1038/249473a0. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973 Jan;61(1):1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R. G., Bray J. J., Hubbard J. I. Effects of inactivity on membrane potentials in rat muscle. Brain Res. 1978 Jul 21;150(3):607–610. doi: 10.1016/0006-8993(78)90824-7. [DOI] [PubMed] [Google Scholar]
- Ochs S., Hollingsworth D. Dependence of fast axoplasmic transport in nerve on oxidative metabolism. J Neurochem. 1971 Jan;18(1):107–114. doi: 10.1111/j.1471-4159.1971.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Vrbová G. Induction of an extrajunctional chemosensitive area in intact innervated muscle fibres. J Physiol. 1967 Jul;191(1):20P–21P. [PubMed] [Google Scholar]


