Abstract
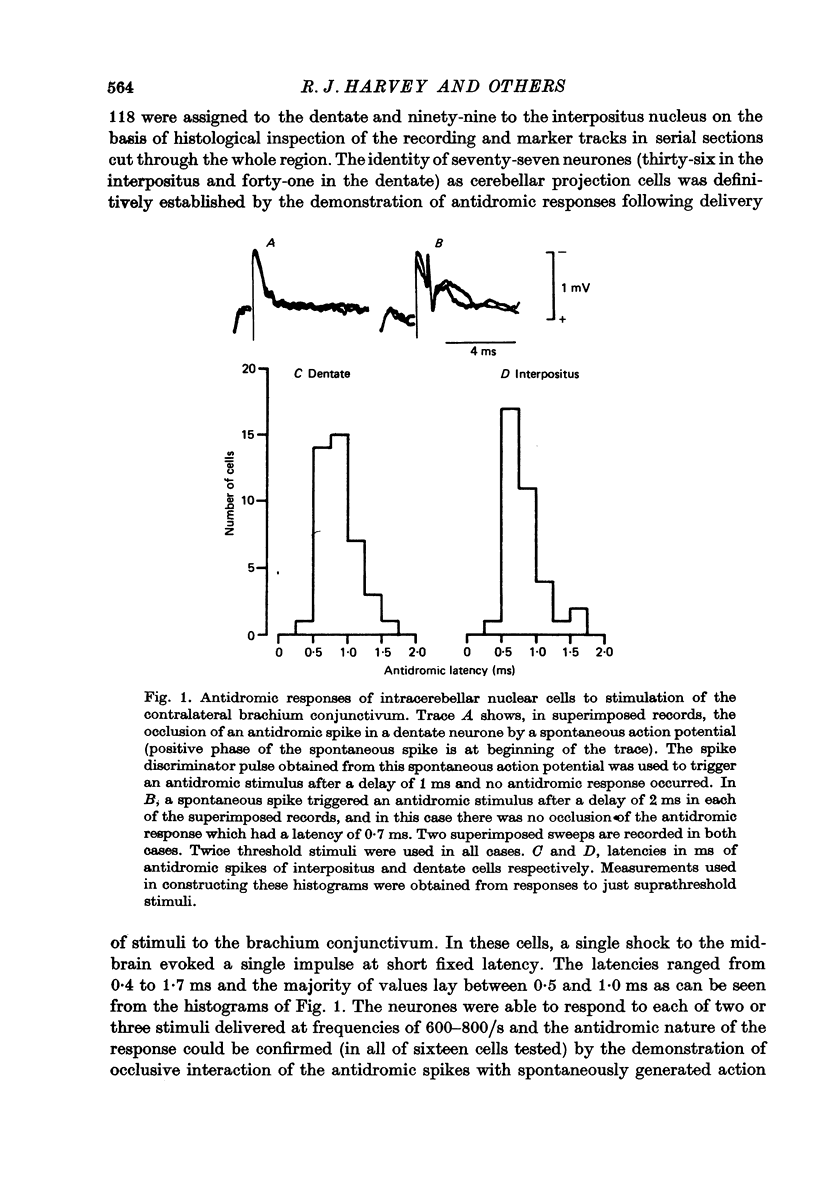
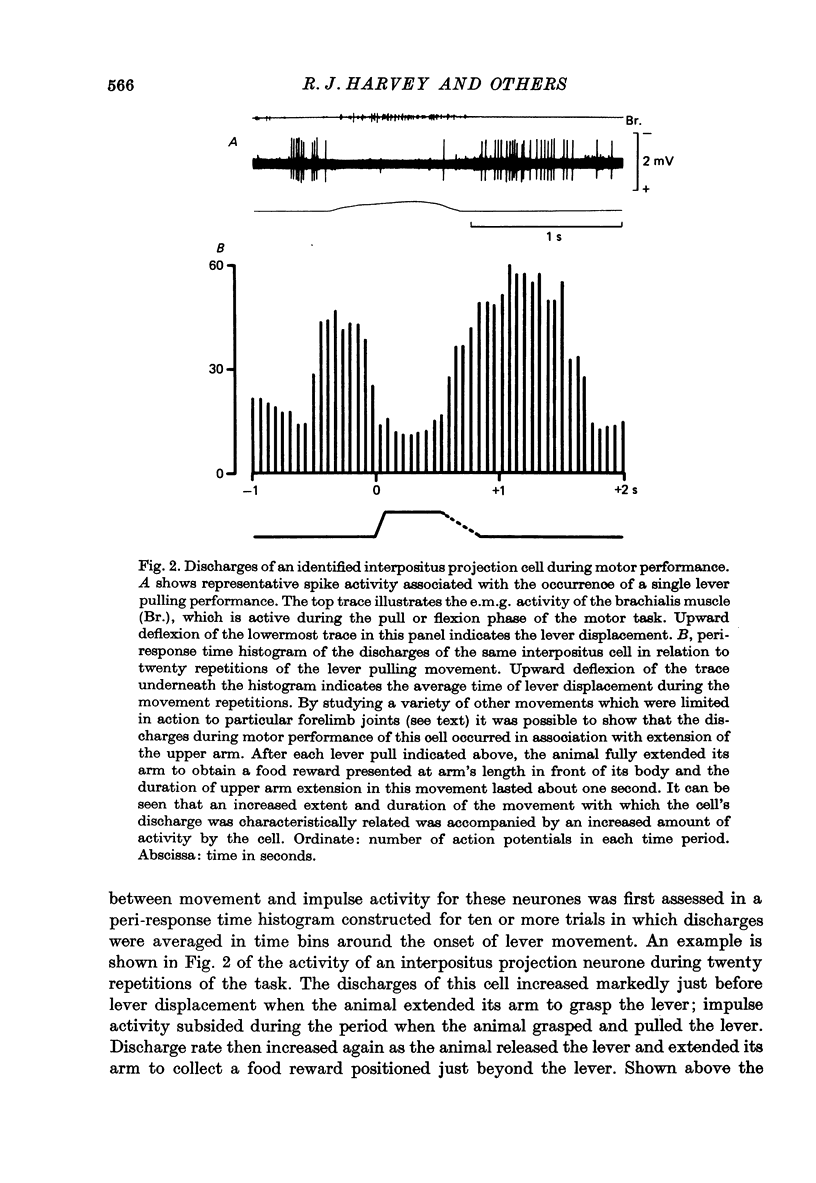
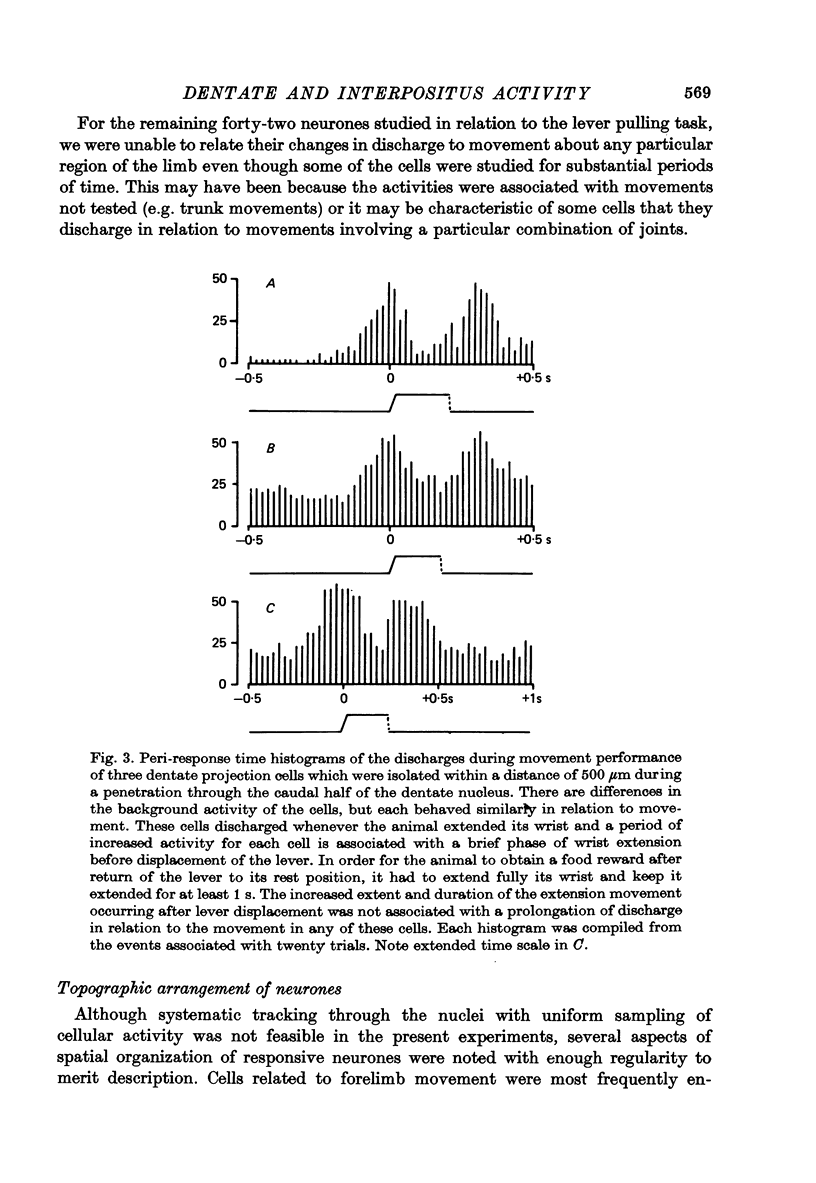
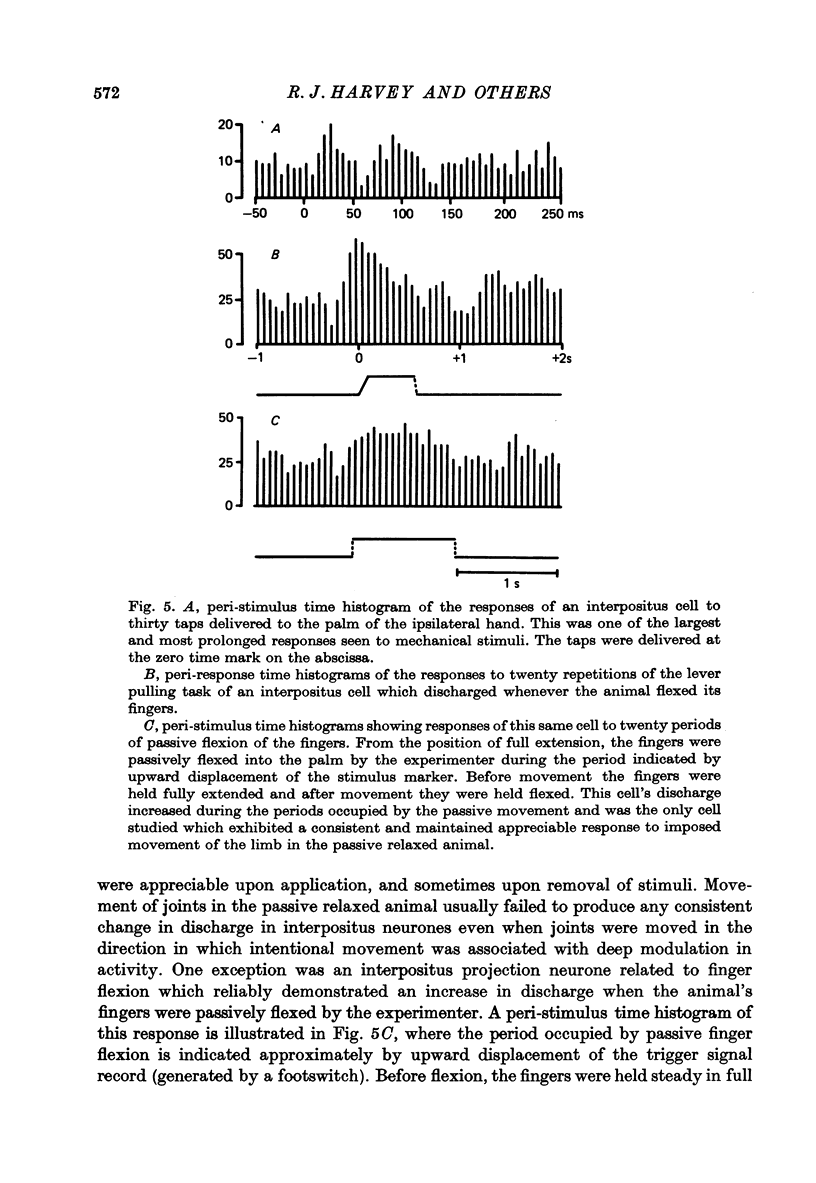
1. Conscious monkeys were trained with food rewards to perform movement tasks with the left forelimb and to accept manipulation of the joints and muscles and natural non-noxious stimulation of the skin of all four limbs. 2. Recordings were made from 217 cells situated in the left interpositus and dentate nuclei of the cerebellum. The identity of seventy-seven cells as cerebellar projection neurones was definitively established by activating them antidromically from the brachium conjunctivum near the contralateral red nucleus. 3. Modulation in the natural activity of 129 of these crebellar nuclear cells (sixty in interpositus; sixty-nine in dentate) occurred in a reproducible manner in temporal association with a phase of the self-paced movement tasks performed by the animal using the ipsilateral arm and hand. The discharges during motor performance of forty-two dentate and forty-five interpositus cells were shown to be associated with movement about a particular joint or region of the forelimb whenever that movement occurred. 4. Cells whose discharges were related to proximal joint movements (shoulder, elbow) and cells related to distal joint movements (wrist, fingers) were encountered in both the dentate and interposed nuclei. 5. The cells were tonically active at rest. Most commonly, accelerations in the discharge were related to movement of a joint or the limb in one direction and a reduction or cessation of activity accompanied movement in the opposite direction. 6. For some cells, variation of the amount of discharge demonstrated during movement performance could be related to the range of the movement or its duration, more activity being characteristic of more prolonged movement performance through larger angles of joint displacement. 7. The dentate and interpositus cells whose discharges were most strongly and consistently related to movements of the forelimb were concentrated in the mid region and caudal half of either nucleus. 8. None of seventy-three dentate neurones examined showed appreciable responses to stimulation of the skin or manipulation of joints and muscles of the fore- or hind limbs and only two cells responded to unexpected perturbation of movement performance. 9. No influence resulting from peripheral afferent input from the ipsilateral forelimb was detected in any interpositus cell whose firing was unchanged during ipsilateral arm movements. 10. Of the sixty interpositus cells whose discharge rates changed during motor performance, twenty-eight were demonstrated to be in receipt of input from receptors in the ipsilateral hand or arm, which could be activated by brisk tapping of the skin and sometimes by gentle squeezing of the forearm. 11. In the passive relaxed animal, manipulation of joints was ineffective in modifying the discharges of most interpositus neurones and, in all cases, prolonged pressure upon the skin elicited only transient responses...
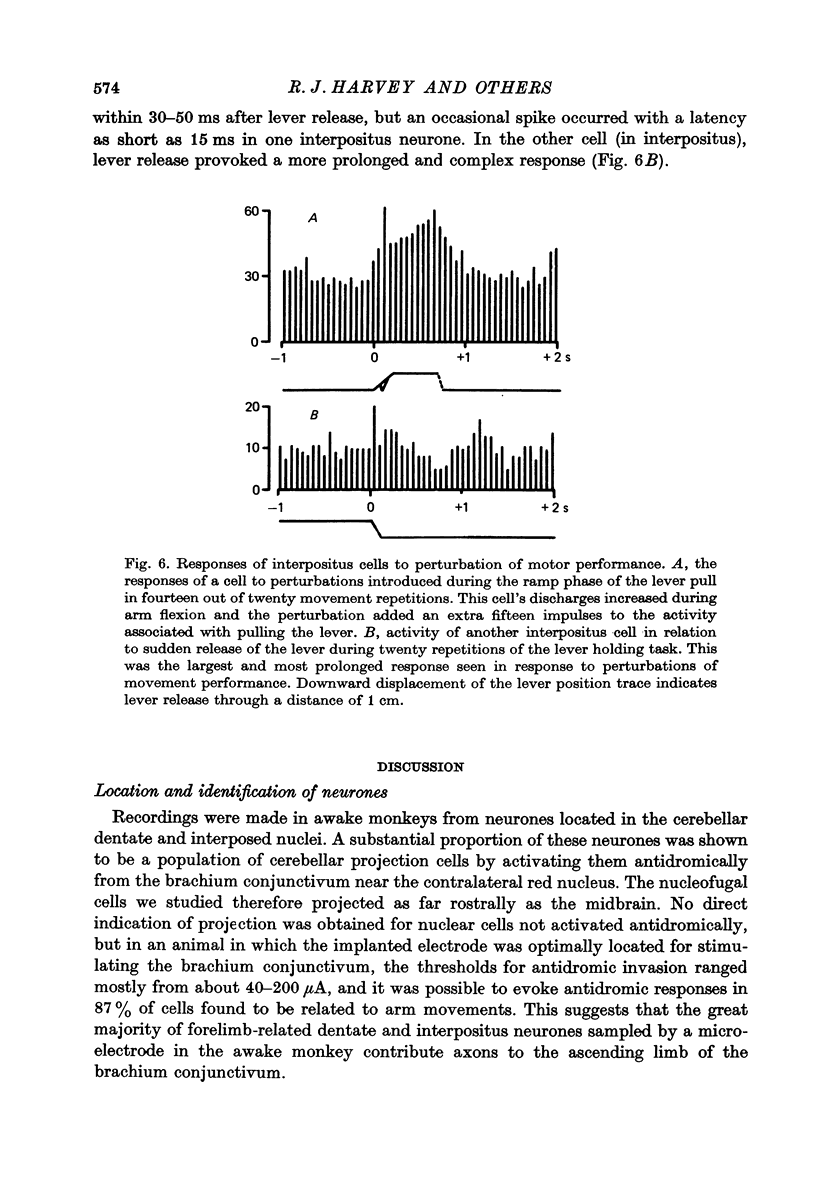
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Gilbert P. F., Marini R., Schultz W., Yin T. C. Integration of cerebral and peripheral inputs by interpositus neurons in monkey. Exp Brain Res. 1977 Jan 18;27(1):81–99. doi: 10.1007/BF00234827. [DOI] [PubMed] [Google Scholar]
- Allen G. I., Gilbert P. F., Yin T. C. Convergence of cerebral inputs onto dentate neurons in monkey. Exp Brain Res. 1978 Jun 19;32(2):151–170. doi: 10.1007/BF00239724. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. J Physiol. 1979 Apr;289:403–423. doi: 10.1113/jphysiol.1979.sp012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantli H., Bloedel J. R. Characteristics of the output from the dentate nucleus to spinal neurons via pathways which do not involve the primary sensorimotor cortex. Exp Brain Res. 1976 May 28;25(2):199–200. doi: 10.1007/BF00234903. [DOI] [PubMed] [Google Scholar]
- Bloedel J. R. Cerebellar afferent systems: a review. Prog Neurobiol. 1973;2(1):3–68. doi: 10.1016/0301-0082(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Brooks V. B. Roles of cerebellum and basal ganglia in initiation and control of movements. Can J Neurol Sci. 1975 Aug;2(3):265–277. doi: 10.1017/s0317167100020369. [DOI] [PubMed] [Google Scholar]
- Burton J. E., Onoda N. Dependence of the activity of interpositus and red nucleus neurons on sensory input data generated by movement. Brain Res. 1978 Aug 18;152(1):41–63. doi: 10.1016/0006-8993(78)90133-6. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955 Aug;103(1):105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955 Dec;74(6):653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- Clendenin M., Ekerot C. F., Oscarsson O. The lateral reticular nucleus in the cat. III. Organization of component activated from ipsilateral forelimb tract. Exp Brain Res. 1974;21(5):501–513. doi: 10.1007/BF00237168. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. Circuits in the cerebellar control of movement. Proc Natl Acad Sci U S A. 1967 Jul;58(1):336–343. doi: 10.1073/pnas.58.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts E. V., Thach W. T. Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–498. doi: 10.1146/annurev.ph.31.030169.002315. [DOI] [PubMed] [Google Scholar]
- Grimm R. J., Rushmer D. S. The activity of dentate neurons during an arm movement sequence. Brain Res. 1974 May 17;71(2-3):309–326. doi: 10.1016/0006-8993(74)90974-3. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey's cerebellum. J Physiol. 1977 Oct;271(2):515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12(3):317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Porter R., Rack P. M. Timing of the responses in the motor cortex of monkeys to an unexpected disturbance of finger position. Brain Res. 1976 Feb 20;103(2):201–213. doi: 10.1016/0006-8993(76)90794-0. [DOI] [PubMed] [Google Scholar]
- Robertson L. T., Grimm R. J. Responses of primate dentate neurons to different trajectories of the limb. Exp Brain Res. 1975 Nov 14;23(5):447–462. doi: 10.1007/BF00234914. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Kawaguchi S., Oka H., Sakai M., Mizuno N. Electrophysiological studies on the cerebellocerebral projections in monkeys. Exp Brain Res. 1976 Mar 15;24(5):495–507. doi: 10.1007/BF00234966. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol. 1978 May;41(3):654–676. doi: 10.1152/jn.1978.41.3.654. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Toyama K., Tsukahara N., Kosaka K., Matsunami K. Synaptic excitation of red nucleus neurones by fibres from interpositus nucleus. Exp Brain Res. 1970;11(2):187–198. doi: 10.1007/BF00234322. [DOI] [PubMed] [Google Scholar]



