Abstract
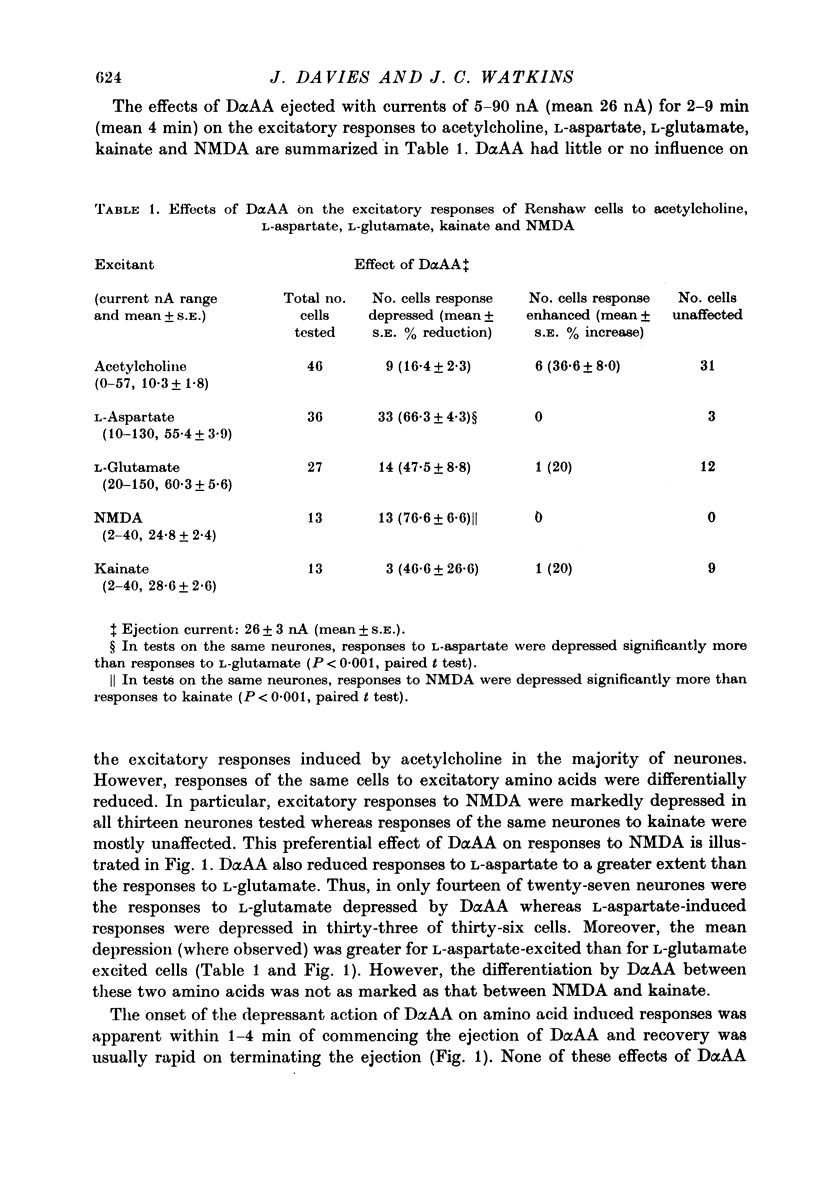
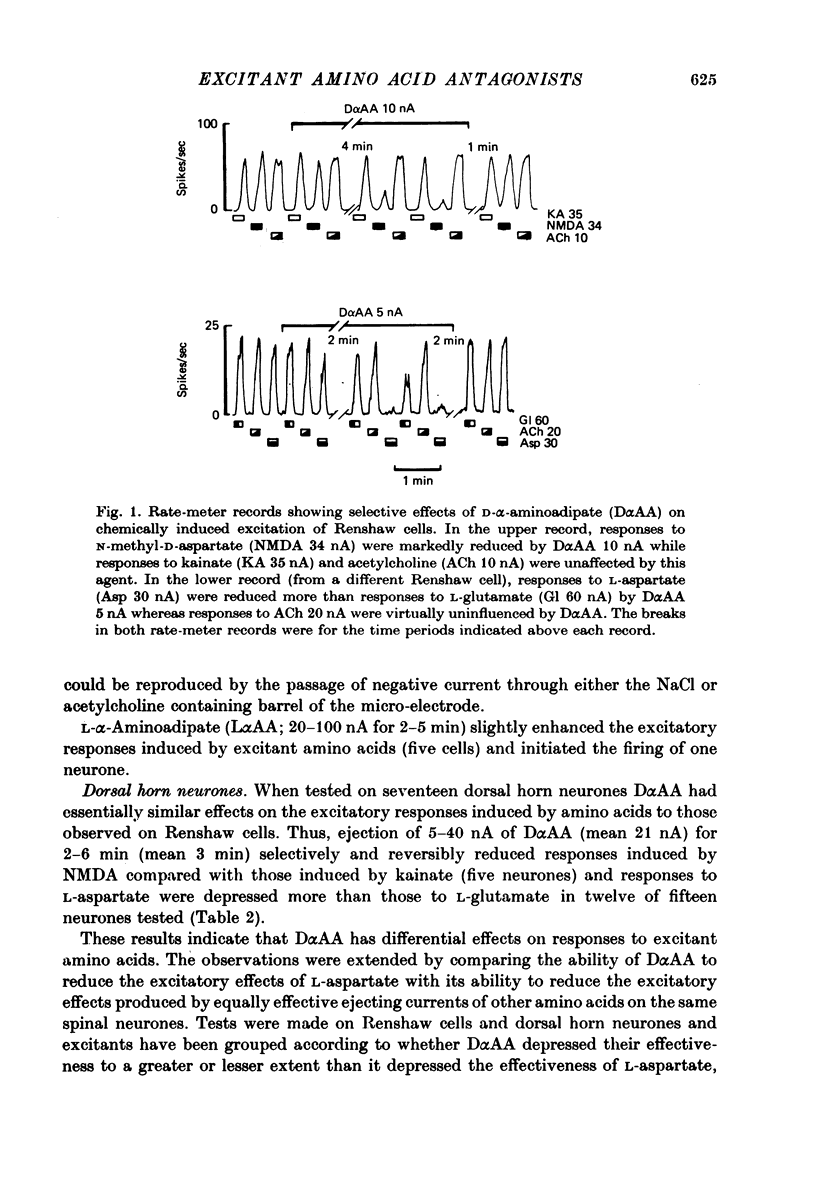
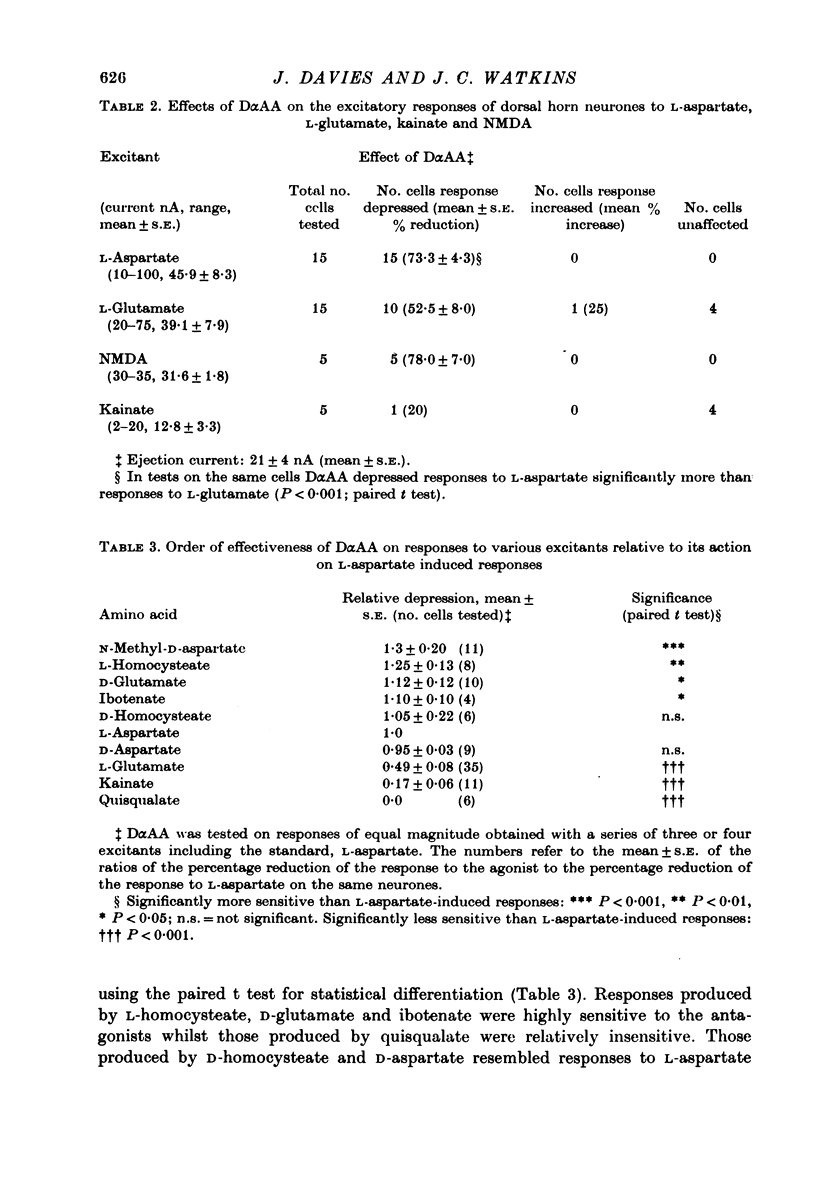
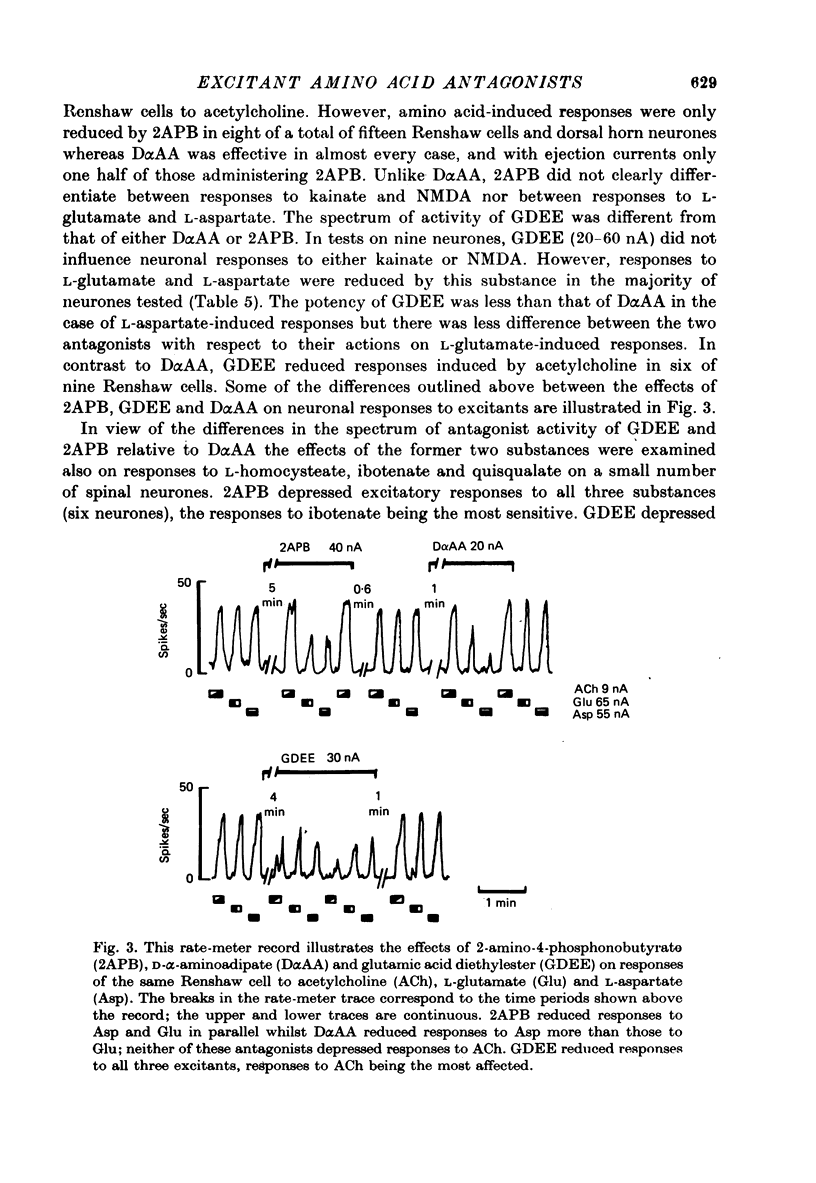
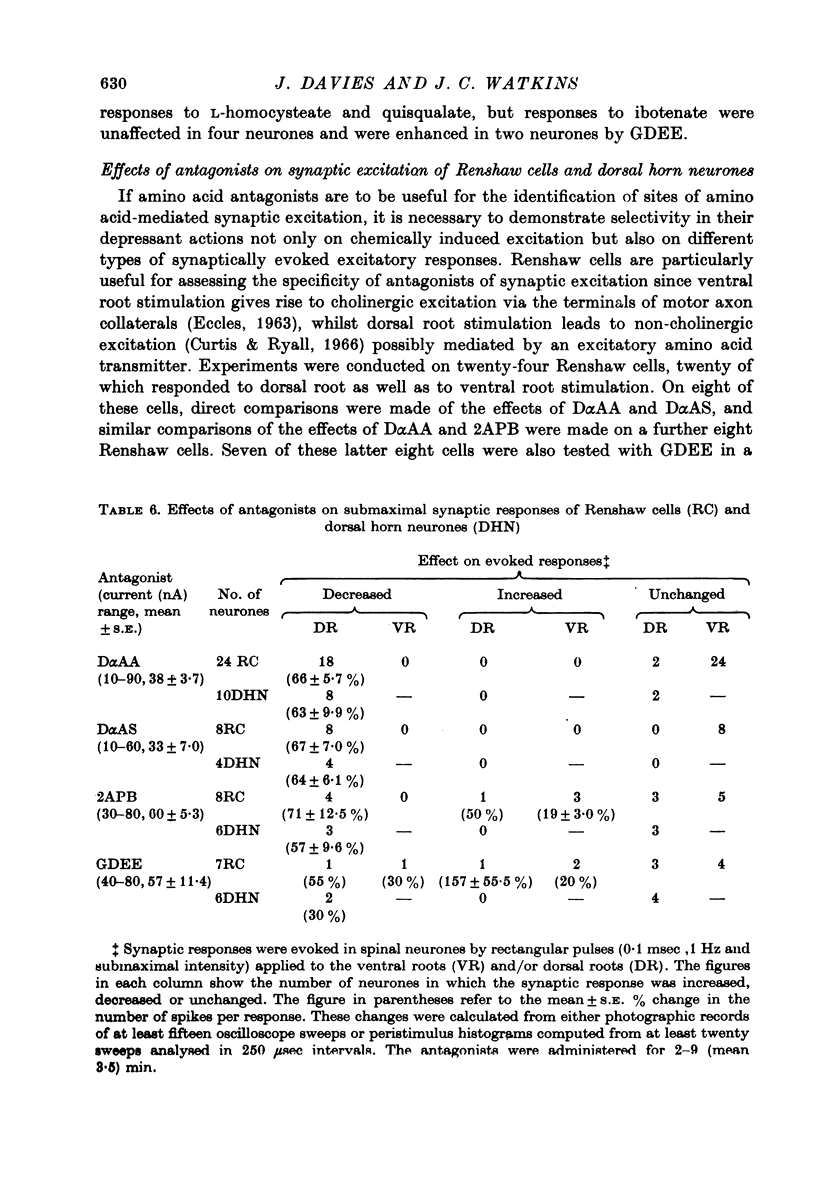
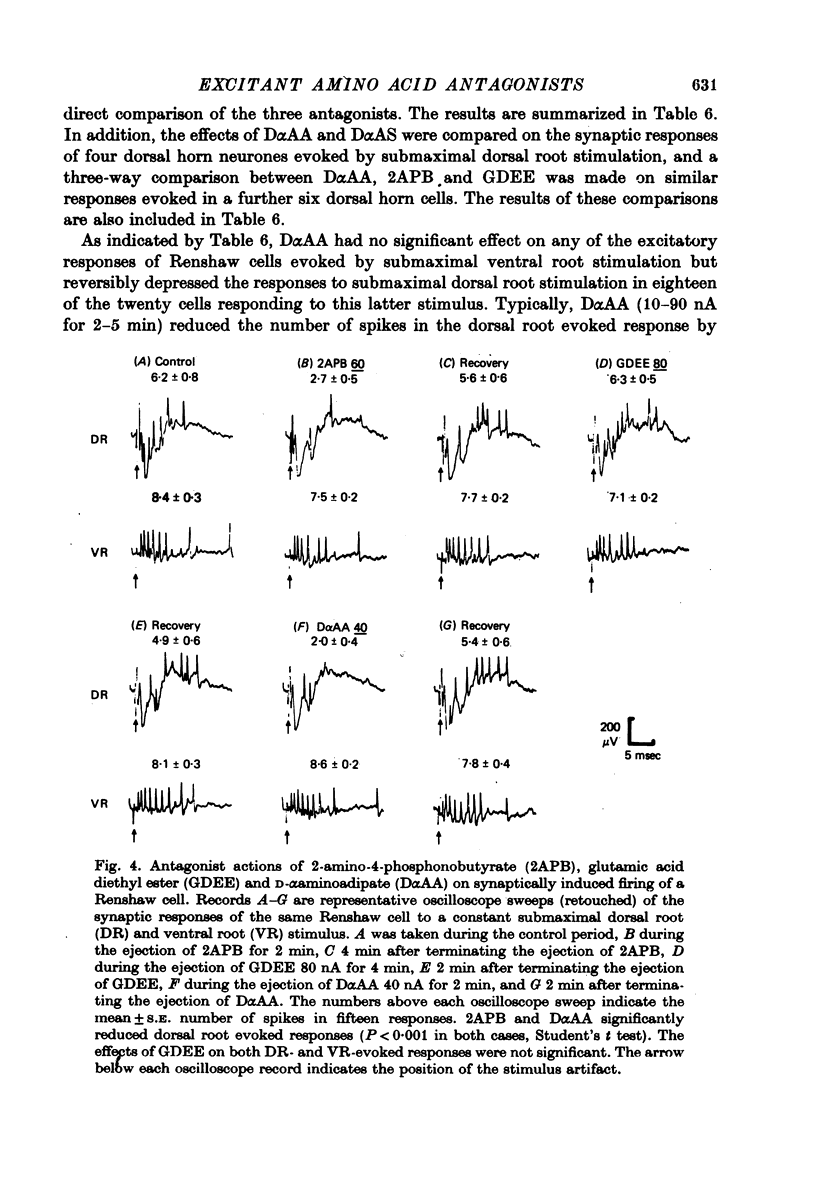
1. The effects of D-alpha-aminoadipate (DalphaAA), D-alpha-aminosuberate (DalphaAS) and other excitatory amino acid antagonists have been compared on the excitatory responses of neurones of the cat spinal cord to acetylcholine, a range of glutamate-related amino acids and stimulation of appropriate excitatory synaptic pathways. The ionophoretic technique was used for administration of excitants and antagonists. 2. DalphaAA and DalphaAS had little or no effect on acetylcholine-induced excitation of Renshaw cells. Responses of either Renshaw cells or dorsal horn neurones in the spinal cord to excitatory amino acids were depressed in the order: N-methyl-D-aspartate (NMDA), L-homocysteate, D-glutamate, ibotenate greater than D-homocysteate, L-aspartate, D-aspartate greater than L-glutamate, kainate and quisqualate. 3. These effects are consistent with the existence of different excitatory amino acid receptors, one type being sensitive to the actions of the antagonists, and activated predominantly by the NMDA group of excitants, with other receptors being relatively insensitive to DalphaAA and DalphaAS and activated predominantly by quisqualate and kainate. On this hypothesis, many amino acids are assumed to have mixed actions on DalphaAA-sensitive and -insensitive receptors. 4. 2-Amino-4-phosphonobutyrate (2APB) and L-glutamic acid diethyl ester (GDEE) produced different patterns of antagonism of excitatory amino acid-induced responses from those observed with DalphaAA and DalphaAS. Neither substance was as potent as DalphaAA or DalphaAS as an excitatory amino acid antagonist. 5. Both DalphaAA and DalphaAS selectively antagonized synaptic excitation of Renshaw cells evoked by dorsal root stimulation without affecting cholinergic excitation of these cells evoked by ventral root stimulation. These latter responses were selectively antagonized by dihydro-beta-erythroidine (DHbetaE). DalphaAA also antagonized synaptic excitation of unidentified dorsal horn neurones of the spinal cord evoked by dorsal root stimulation. Neither GDEE (particularly) nor 2APB were as effective as DalphaAA or DalphaAS as depressants of synaptic excitation. 6. Taken in conjunction with the results of in vitro studies on the specificity of action of Dalpha¿ and related substances, these observations suggest that certain synaptic excitations in the spinal cord are mediated by an excitatory amino acid transmitter, and that this transmitter interacts with receptors which are activated selectively by NMDA, less selectively by other amino acids, including L-aspartate, and probably only slightly by quisqualate, kainate and (exogenous) L-glutamate.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann H., ten Bruggencate G., Pickelmann P., Steinberg R. Effects of glutamate, aspartate, and two-presumed antagonists on feline rubrospinal neurones. Pflugers Arch. 1976 Aug 24;364(3):249–255. doi: 10.1007/BF00581763. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. Glutamate uptake by brain slices and its relation to the depolarization of neurones by acidic amino acids. J Neurobiol. 1972;3(4):295–301. doi: 10.1002/neu.480030403. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Davies J., Dray A., Evans R. H., Francis A. A., Martin M. R., Watkins J. C. Depression of synaptic excitation and of amino acid induced excitatory responses of spinal neurones by D-alpha-aminoadipate, alpha,epsilon-diaminopimelic acid and HA-966. Eur J Pharmacol. 1977 Oct 1;45(3):315–316. doi: 10.1016/0014-2999(77)90017-6. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Davies J., Dray A., Evans R. H., Martin M. R., Watkins J. C. D-alpha-aminoadipate, alpha, epsilon-diominopimelic acid and HA-966 as antagonists of amino acid-induced and synpatic excitation of mammalian spinal neurones in vivo. Brain Res. 1978 Jun 16;148(2):543–548. doi: 10.1016/0006-8993(78)90745-x. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Francis A. A., Martin M. R., Watkins J. C., Davies J., Dray A. D-alpha-Aminoadipate as a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature. 1977 Dec 22;270(5639):743–745. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Headley P. M., Martin M. R., Watkins J. C. Structure-activity relations of excitatory amino acids on frog and rat spinal neurones. Br J Pharmacol. 1976 Nov;58(3):373–382. doi: 10.1111/j.1476-5381.1976.tb07714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol. 1963 Apr;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Davies J. An evaluation of D-alpha-aminoadipate and D-(and DL-)alpha-aminosuberate as selective antagonists of excitatory amino acids in the substantia nigra and mesencephalic reticular formation of the rat. Neuropharmacology. 1979 Feb;18(2):193–199. doi: 10.1016/0028-3908(79)90061-3. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Headley M. H., Watkins J. C. Actions of L- and D-homocysteate in rat CNS: a correlation between low-affinity uptake and the time courses of excitation by microelectrophoretically applied L-glutamate analogues. J Neurochem. 1977 Sep;29(3):579–588. doi: 10.1111/j.1471-4159.1977.tb10707.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., Teb ecis A. K., Watkins J. C. Excitation of mammalian central neurones by acidic amino acids. Brain Res. 1972 Jun 22;41(2):283–301. doi: 10.1016/0006-8993(72)90503-3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A., Game C. J., McCulloch R. M. Antagonism of neuronal excitation by 1-hydroxy-3-aminopyrrolidone-2. Brain Res. 1973 Jan 30;49(2):467–470. doi: 10.1016/0006-8993(73)90444-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Dray A. Effects of D-alpha-aminoadipate on physiologically evoked responses of cat dorsal horn neurones. Experientia. 1979 Mar 15;35(3):353–354. doi: 10.1007/BF01964348. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Microelectrophoretic studies on the depressant action of HA-966 on chemically and synaptically excited neurones in the cat cerebral cortex and cuneate nucleus. Brain Res. 1973 Sep 14;59:311–322. doi: 10.1016/0006-8993(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Dudel J. Aspartate and other inhibitors of excitatory synaptic transmission in crayfish muscle. Pflugers Arch. 1977 May 6;369(1):7–16. doi: 10.1007/BF00580803. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Watkins J. C. Mg2+-like selective antagonism of excitatory amino acid-induced responses by alpha, epsilon-diaminopimelic acid, D-alpha-aminoadipate and HA-966 in isolated spinal cord of frog and immature rat. Brain Res. 1978 Jun 16;148(2):536–542. doi: 10.1016/0006-8993(78)90744-8. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Watkins J. C. Specific antagonism of excitant amino acids in the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1978 Jul 15;50(2):123–129. doi: 10.1016/0014-2999(78)90007-9. [DOI] [PubMed] [Google Scholar]
- Haldeman S., Huffman R. D., Marshall K. C., McLennan H. The antagonism of the glutamate-induced and synaptic excitations of thalamic neurones. Brain Res. 1972 Apr 28;39(2):419–425. doi: 10.1016/0006-8993(72)90445-3. [DOI] [PubMed] [Google Scholar]
- Hall J. G., McLennan H., Wheal H. V. The actions of certain amino acids as neuronal excitants [proceedings]. J Physiol. 1977 Oct;272(1):52P–53P. [PubMed] [Google Scholar]
- Johnston G. A., Curtis D. R., Davies J., McCulloch R. M. Spinal interneurone excitation by conformationally restricted analogues of L-glutamic acid. Nature. 1974 Apr 26;248(5451):804–805. doi: 10.1038/248804a0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Twitchin B. Action of the neurotoxin kainic acid on high affinity uptake of L-glutamic acid in rat brain slices. J Neurochem. 1979 Jan;32(1):121–127. doi: 10.1111/j.1471-4159.1979.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Lodge D., Headley P. M., Curtis D. R. Selective antagonism by D-alpha-aminoadipate of amino acid and synaptic excitation of cat spinal neurons. Brain Res. 1978 Sep 8;152(3):603–608. doi: 10.1016/0006-8993(78)91117-4. [DOI] [PubMed] [Google Scholar]
- McLennan H., Hall J. G. The action of D-alpha-aminoadipate on excitatory amino acid receptors of rat thalamic neurones. Brain Res. 1978 Jun 30;149(2):541–545. doi: 10.1016/0006-8993(78)90501-2. [DOI] [PubMed] [Google Scholar]
- McLennan H., Lodge D. The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Res. 1979 Jun 15;169(1):83–90. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- WATKINS J. C. THE SYNTHESIS OF SOME ACIDIC AMINO ACIDS POSSESSING NEUROPHARMACOLOGICAL ACTIVITY. J Med Pharm Chem. 1962 Nov;91:1187–1199. doi: 10.1021/jm01241a010. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Curtis D. R., Brand S. S. Phosphonic analogues as antagonists of amino acid excitants. J Pharm Pharmacol. 1977 May;29(5):324–324. doi: 10.1111/j.2042-7158.1977.tb11328.x. [DOI] [PubMed] [Google Scholar]
- White W. F., Nadler J. V., Hamberger A., Cotman C. W., Cummins J. T. Glutamate as transmitter of hippocampal perforant path. Nature. 1977 Nov 24;270(5635):356–357. doi: 10.1038/270356a0. [DOI] [PubMed] [Google Scholar]


