Abstract
In the Chx10-null ocular retardation (orJ) mouse, retinal progenitor cell (RPC) proliferation is impaired, and bipolar neurons, a late born cell type, fail to differentiate. It is unclear whether Chx10 is required to maintain proliferation throughout retinogenesis or whether the bipolar cell defect is an indirect effect of growth arrest. We show that Chx10 is dispensable for late-stage RPC proliferation but is essential to promote bipolar cell genesis in place of rods. Ectopic Chx10 expression drove bipolar instead of rod cell differentiation without affecting division. Converting Chx10 to an activator impaired bipolar cell differentiation, implying that repression is important for Chx10 activity. In the Chx10 null orJ retina, only a small fraction of cells expressing mutated Chx10 mRNA were rods, but this fraction increased after p27Kip1 inactivation, which partially rescues proliferation. Most significantly, acute Chx10 knockdown in the postnatal retina promoted rods in place of bipolar neurons without affecting division. Thus, Chx10 directly controls bipolar cell genesis by inhibiting rod differentiation independent of its temporally limited early effect on RPC proliferation.
Keywords: CVC domain, homeobox, homeodomain, short-hairpin RNA
Neurogenesis involves retinal progenitor cell (RPC) expansion, cell-cycle exit, and differentiation of multiple cell types. Several transcription factors, especially basic helix–loop–helix and homeodomain proteins, have been identified that act as intrinsic regulators of this developmental cascade (1–3). The Chx10 homeobox gene is thought to regulate both proliferation and differentiation during retinogenesis, and homozygous-null mutations cause microphthalmia in mice and humans (4, 5). Chx10 orthologues exist in lower vertebrates including Vsx2 in goldfish and Alx1 in zebrafish (6–8). Antisense Alx1 oligonucleotides induced microphthalmia in goldfish similar to Chx10 loss in mice and humans (8). Thus, Chx10 has a conserved role in eye development.
The ocular retardation (orJ) mouse has a nonsense mutation in the homeobox, resulting in a severe proliferation defect in the embryonic retina (4). There are 19-fold fewer cells in the orJ vs. WT postnatal retina (9). Chx10 is expressed in all RPCs (10), but it is unclear whether Chx10 regulates RPC division throughout retinogenesis or whether its loss specifically perturbs early RPC division, which indirectly affects late-stage RPC production.
The mature retina has seven cell types with cell bodies in three layers. The outer nuclear layer (ONL) contains rods and cones; the inner nuclear layer (INL) consists of horizontal, bipolar, amacrine, and Müller cells; and the innermost ganglion cell layer contains both ganglion and amacrine cells. During development, RPCs traverse stages of competence during which they give birth to different cohorts of postmitotic transition cells (11). Ganglion, horizontal, cone, and amacrine cells are born in the embryonic phase of mouse development, whereas bipolar neurons and Müller glia are born postnatally (12). Rods, the most abundant cell type, are born throughout retinogenesis. There is evidence that, as well as driving RPC proliferation, Chx10 may affect differentiation in the postnatal retina. In most postmitotic transition cells, Chx10 expression is down-regulated but is maintained in bipolar cells and some Müller glia (10, 13). Ectopic Chx10 expression in rodent explants promotes INL cells at the expense of photoreceptors, but this in vitro system can only be maintained for limited periods, and half of the Chx10-expressing cells never differentiate (1). Chx10 also inhibits photoreceptor differentiation in dissociated chick cells, but the alternate fate of these cells or effects on proliferation were not measured (14). The role of Chx10 in proliferation and differentiation of the postnatal retina in vivo is unclear. The effect of removing Chx10 at this stage has never been addressed.
The orJ central retina exhibits all cell types except bipolar cells (4). However, this finding is only indirect evidence that Chx10 promotes their differentiation because it is unclear whether their absence reflects a direct role for Chx10 in bipolar differentiation or whether the severe defects in proliferation, which alter expression of many genes (15–17), has an indirect effect on late-stage neurogenesis. These cells are the last retinal neurons born (12); thus, the severe proliferation defect in the orJ retina may deplete RPCs before bipolar cell specification. Müller glia, born at the same time as bipolar cells, still develop in Chx10-null mice (4), suggesting that the bipolar cell deficit is not secondary to proliferation defects. Nevertheless, Müller glia are closely related to RPCs, both in terms of markers and their potential to divide and generate multiple cell types (18, 19). In view of the failure of most of the orJ retina to differentiate at all (4, 9, 20), some RPCs in the central retina may default toward the overlapping glial fate. The idea that Chx10 may directly regulate bipolar cell differentiation is supported by the observation that inactivating the cyclin-dependent kinase inhibitor p27Kip1 partially rescues division in the orJ retina but does not rescue bipolar cell genesis (9). However, cell numbers in the Chx10−/−;p27Kip1−/− retina are still 4-fold lower than WT (9); therefore, this large cell-cycle defect, which likely perturbs many pathways, may indirectly impair late-stage neuronal differentiation. It is difficult to clarify a role for any protein in differentiation if its loss also perturbs proliferation; thus, the role of Chx10 in postnatal retinal development cannot be resolved by using orJ mice alone.
In this article, we use ectopic expression and acute short-hairpin RNA (shRNA)-mediated knockdown to study the role of Chx10 in postnatal retinogenesis. We show that Chx10 does not affect late-stage RPC-proliferation but is essential to promote bipolar cell differentiation and does so at the expense of rods.
Results
Chx10 Drives Bipolar Cell Differentiation at the Expense of Rods Without Altering Proliferation.
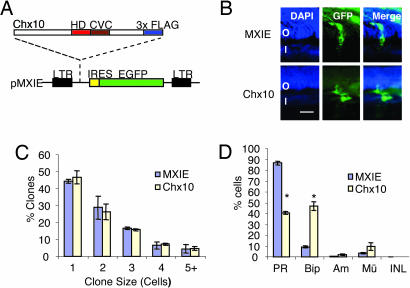
As a first step to elucidate the effect of Chx10 on cell fate, we injected control or Chx10-expressing replication-deficient retrovirus into newborn rat eyes and analyzed the infected clones 21 days later. Virus titer was such that each clone arises from infection of a single progenitor (21). The MXIE retrovirus expresses eGFP from an internal ribosome entry site (Fig. 1A), permitting identification of positive clones. GFP+ cell types were scored by their position and morphology (Fig. 1B and see Fig. 6A, which is published as supporting information on the PNAS web site), a reliable approach for cell type identification (22). Chx10 did not alter clone size (Fig. 1C) but dramatically affected cell-type composition (Fig. 1 B and D and see Fig. 6B). The proportion of photoreceptors fell from 86.6% in control clones to 40.7% in Chx10 clones. This effect was due almost exclusively to an increase in bipolar cells, which rose from 9.2% to 46.7%. A large increase was noted in one, two, or three cell clones consisting solely of bipolar cells, and these neurons were overrepresented in clones with other cell types (Fig. 1B and see Fig. 6B). The ability of Chx10 to induce bipolar cells in place of rods is consistent with the reciprocal expression of Chx10 and photoreceptor markers during retinal development (Fig. 6C).
Fig. 1.
Retroviral expression of Chx10 in neonatal rats promotes bipolar cells at the expense of photoreceptors. (A) Schematic diagram of retroviral vector pMXIE. Flag-tagged Chx10 was subcloned upstream of the internal ribosome entry site-eGFP cassette. (B) Clones expressing the empty vector (MXIE; green) contained mostly photoreceptors (Upper), whereas clones expressing Chx10 (Lower) contained less photoreceptors and more bipolar cells. (Scale bar: 10 μm.) (C) Chx10 expression does not affect clone size (P > 0.05). (D) Chx10 increases the proportion of bipolar to total GFP+ cells >5-fold at the expense of photoreceptors. Error bars represent SD. Asterisks indicate significant difference from control (P ≤ 0.001). O, ONL; I, INL; Am, amacrine cells; BiP, bipolar cells; GCL, ganglion cell layer; Mü, Müller cells; PR, photoreceptor cells.
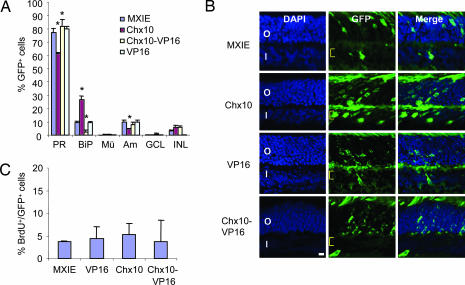
To confirm and extend these data, we introduced Chx10 into newborn mouse retina. MXIE control or MXIE-Chx10 vectors were injected into the retina of newborn mice, and DNA uptake was induced by electroporation (23, 24). Retinas were harvested 21 days later, and GFP+ cell types were scored as above. Similar to the retroviral data in rat retina (Fig. 1), Chx10 reduced photoreceptors and increased bipolar cells (Fig. 2A and B). Chx10 also had a negative effect on amacrine cell genesis (Fig. 2A), which was not seen in rat (Fig. 1D) and is also inconsistent with knockdown experiments described below (see Fig. 5B). To complement the morphology-based analysis, we scored cell-type proportions in this experiment with cell-specific markers (see Supporting Methods, which is published as supporting information on the PNAS web site). The effects on rod and bipolar cell genesis, reported by using morphology (Fig. 2A), were reproduced in this alternative approach (see Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 2.
Electroporation of newborn mouse retina with Chx10 or Chx10-VP16 vectors. (A) Chx10 promotes bipolar cells and reduces photoreceptors, whereas Chx10-VP16 inhibits bipolar cell differentiation. P0 mouse retina was electroporated with the indicated vectors, cell counts were performed at P21, and cell-type distribution as a fraction of transfected (GFP+) cells was plotted. Error bars represent SD. Asterisks indicate significant difference from results with control MXIE vector (P ≤ 0.05). (B) Images of transfected cells used to generate the data in A. Yellow brackets indicate the bipolar cell layer. (Scale bar: 10 μm.) (C) Neither Chx10 nor Chx10-VP16 affect proliferation as measured by BrdU incorporation at P3 (P > 0.05).
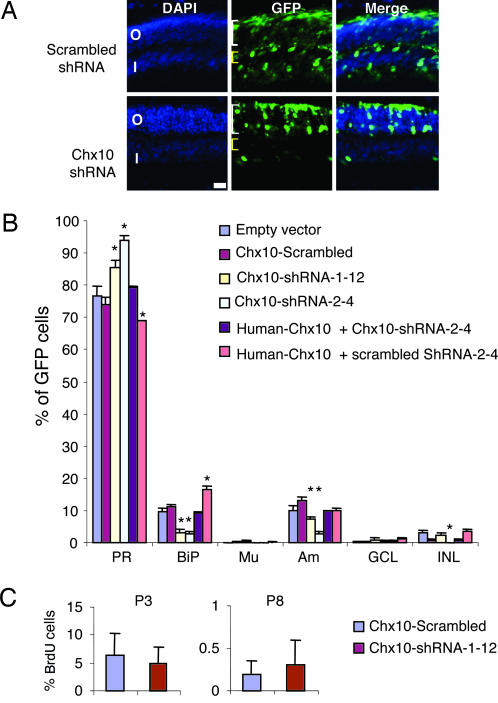
Fig. 5.
ShRNA-mediated acute Chx10 knockdown blocks bipolar cell differentiation and promotes photoreceptor cell differentiation. (A) P0 mouse retina was electroporated with scrambled shRNA (Upper) or Chx10 shRNA (Lower) vectors together with a GFP vector to mark transfected cells (green). Chx10 shRNA reduced the proportion of bipolar cells in the INL (yellow brackets) and increased the proportion of photoreceptors in the ONL (white brackets). (B) Quantification of cell-types in mature retina after electroporation at P0. Both Chx10 shRNA vectors (Chx10-shRNA-1-12, Chx10-shRNA-2-4) significantly reduced the proportion of bipolar cells and increased the proportion of photoreceptors. ShRNA-resistant human Chx10 vector reversed the effects of Chx10 shRNA. Human Chx10 plus a scrambled shRNA vector increased bipolar cell production. Error bars represent SD. Asterisks indicate significant difference from the empty vector control (P ≤ 0.05). (C) Chx10 shRNA has no significant effect on proliferation (P > 0.05). BrdU was injected 2 h before death, and incorporation was measured at P3 and P8 in cells expressing scrambled shRNA or Chx10 shRNA.
Chx10 drives early RPC proliferation, and because bipolar cells are born last, increased division could delay cell-cycle exit and indirectly increase their number. To examine whether Chx10 affects RPC proliferation, retinas were electroporated at postnatal day 0 (P0), and mice received a pulse of BrdU 2 h before death at P3 or P8. No difference was found in the number of dividing cells at either time point (Fig. 2C and data not shown). The number of TUNEL-positive apoptotic cells was also unaffected (see Fig. 8B, which is published as supporting information on the PNAS web site). Thus, Chx10 directly promotes bipolar cells at the expense of rods without affecting cell division or survival.
Chx10-VP16 Activator Interferes with Bipolar Cell Development.
We showed that Chx10 represses transcription in vitro in several contexts (25); thus, Chx10 might inhibit photoreceptor specification at least in part through negative gene regulation. We asked whether converting Chx10 to an activator by fusing it to the VP16 activation domain would interfere with bipolar cell differentiation. The fusion protein was expressed at similar levels as Chx10 and activated transcription of reporter vectors that were repressed by Chx10 (data not shown). In contrast to the ≈2.8 -fold positive effect of Chx10 on bipolar cell differentiation, Chx10-VP16 decreased the fraction of morphologically recognizable bipolar cells by ≈4.3-fold (Fig. 2 A and B and see Fig. 7). This reduction was accompanied by a small increase in photoreceptors (Fig. 2 and see Fig. 7). The VP16 activation domain alone did not affect the distribution of identifiable cell types, although it reduced unidentifiable INL cells relative to Chx10 or Chx10-VP16 but not the control MXIE vector (Fig. 2 A and B and see Fig. 8A). No vector affected cell division (Fig. 2C) or survival (see Fig. 8B). Thus, repression may be important for Chx10 to regulate differentiation.
Chx10 mRNA+ Rods in the orJ Retina.
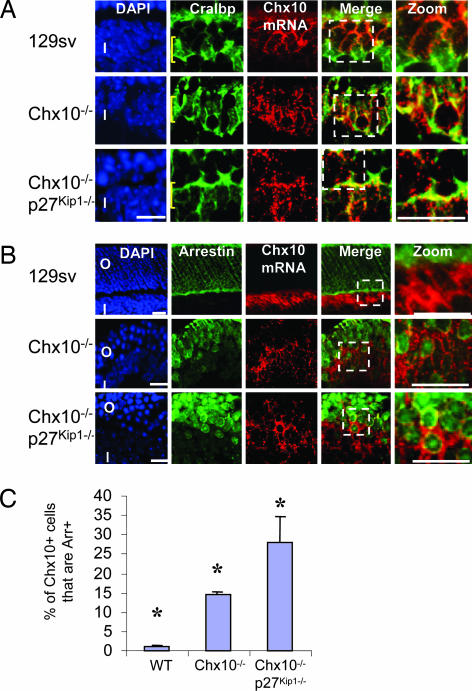
In view of the ectopic expression data, Chx10 loss should drive rod differentiation at the expense of bipolar cells. To address this issue, we first turned to the orJ mouse, which is homozygous for a nonsense mutation in the Chx10 homeobox (4). The truncated Chx10 protein is undetectable (4), but mutated mRNA can be detected by in situ hybridization (Fig. 3A). Thus, we asked whether Chx10 mRNA is present in orJ rods. Surprisingly, most of the Chx10 mRNA+ cells were located in the INL and expressed the Müller glia marker Cralbp (Fig. 3A). Notably however, 14.6% of all orJ Chx10 mRNA+ cells were in the outer retina and coexpressed the photoreceptor marker rod arrestin. Such cells were rare in the WT retina (Fig. 3 B and C).
Fig. 3.
Presence of mutated Chx10 mRNA in photoreceptor and glial-like cells in the orJ retina. (A) In the WT retina (Top), Chx10 mRNA (red) is expressed mainly in bipolar cells in the INL. Most Müller glia (Cralbp protein; green) do not express Chx10. In the Chx10−/− orJ retina, however, mutated Chx10 mRNA is expressed in the majority of Cralbp+ cells (Middle). The proportion of glia expressing Chx10 declines when proliferation is partially rescued in the Chx10−/−;p27Kip1−/− retina (Bottom). Yellow brackets indicate the position of Müller glia cell bodies in the INL as opposed to processes that also label with Cralbp. (B) Expression of Chx10 mRNA (red) in photoreceptors (rod arrestin protein; green) is extremely rare in the WT (Top) but is detected in the orJ retina (Middle) and is even more frequent in the Chx10−/−;p27Kip1−/− retina (Bottom). Images to the far right in A and B show enlarged views of the boxed areas. (C) Quantification of the fraction of cells expressing Chx10 mRNA and rod arrestin protein in the WT, orJ, or Chx10−/−;p27Kip1−/− retina. Error bars represent SD. Asterisks indicate significant difference (P ≤ 0.05) from WT (Chx10−/−) or Chx10−/− (Chx10−/−;p27Kip1−/−) retina. (Scale bars: A and B, 10 μm.) O, ONL; I, INL.
Increased Proportion of Chx10 mRNA+ Rods When Proliferation Is Partially Rescued.
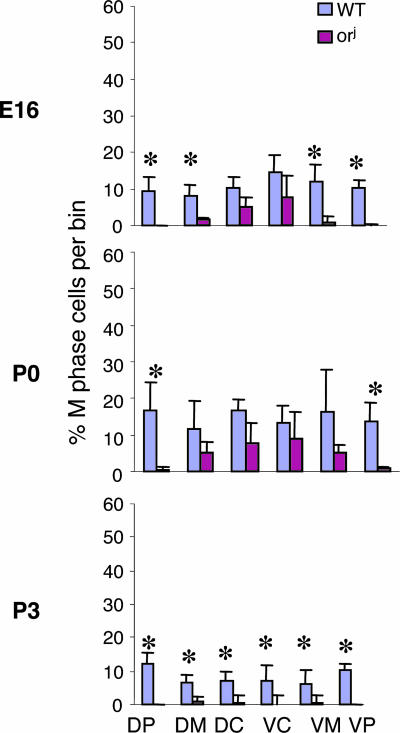
These data support the idea that Chx10 suppresses rods. The large fraction of Chx10 mRNA+ cells that express Cralbp in the orJ retina could imply that Chx10 also suppresses Müller cells, but this possibility contradicts our in vivo data and other's prior in vitro data in which ectopic Chx10 expression did not inhibit Müller cell differentiation (Fig. 1D) (1). It is also inconsistent with the expression of Chx10 in a subset of Müller glia in the WT retina (13). Thus, although the presence of Chx10 mRNA+/Cralbp+ cells may be a direct determination effect on cells that express nonfunctional Chx10, it might also be a secondary consequence of reduced proliferation such that Chx10 is not properly down-regulated during gliogenesis. Prior analyses of proliferation in the orJ retina focused on early time points between embryonic day 11.5 (E11.5) and E14.5, and the central orJ retina, where differentiation eventually occurs, is virtually unaffected at these times (4, 20). We quantified mitotic cells in the orJ and retina at E16, P0, and P3 by using anti-phosphohistone H3 antibodies (Fig. 4). Labeled cells were counted in six bins of equal length from the dorsal periphery to the ventral periphery. Severe inhibition was already evident in the mid and far periphery at E16, similar to earlier time points (4, 20). However, a statistically significant drop in M-phase cells in the central retina was not detected until P3, which is also the peak of bipolar cell genesis (12), and M-phase cells were almost undetectable (Fig. 4). Thus, proliferation is progressively retarded in the central orJ retina, although delayed relative to the periphery.
Fig. 4.
Severe reduction in proliferation in the postnatal orJ central retina. The effect of Chx10 loss on central retinal proliferation was assessed by counting the proportion of PH3-positive mitotic cells at three time points. By P3, proliferation in the central orJ retina had decreased dramatically. Error bars represent SD. Asterisks indicate significant difference between WT and orJ (P ≤ 0.03). DP, dorsal peripheral; DM, dorsal medial; DC, dorsal central; VC, ventral central; VM, ventral medial; VP, ventral peripheral.
The orJ proliferation defect is partially rescued by inactivating the cycle-dependent kinase inhibitor p27Kip1 (9). We asked whether this rescue also increases the number of Chx10 mRNA+ rods in the orJ retina. Indeed, the fraction of double-labeled Chx10 mRNA+/rod arrestin+ cells in the Chx10−/−;p27Kip1−/− retina rose to 27.8%, double that seen in orJ mice (Fig. 3 B and C). These data imply that the physiologically relevant role of Chx10 in the postnatal retina is to block rod differentiation. The simplest explanation for Chx10 mRNA+/Cralbp+ cells in the mature orJ retina is that the impaired RPC proliferation in the postnatal retina reported here perturbs gliogenesis, interfering with down-regulation of Chx10 in differentiating Müller glia.
Acute Chx10 Knockdown Triggers a Bipolar to Rod Switch Without Affecting Proliferation.
To directly test whether Chx10 blocks rod differentiation, independent of proliferation effects, we asked whether acute Chx10 knockdown in the postnatal retina would increase rods at the expense of bipolar cells. Several shRNAs were designed to target Chx10 mRNA (see Fig. 9A, which is published as supporting information on the PNAS web site). To test their efficacy, we exploited the bicistronic pMXIE-Chx10 plasmid in which Chx10 and GFP are expressed from the same message (Fig. 1A). Any shRNA that down-regulates Chx10 message would simultaneously reduce GFP expression. Phoenix-Eco cells were transfected with pMXIE-Chx10 or control pMXIE plasmid, together with one of three shRNA vectors that target Chx10 or a scrambled control. A dsRed vector was included to assess transfection efficiency. Quantification of GFP+/dsRed+ cells revealed that two shRNA vectors 1-12 and 2-4 reduced GFP+ (and hence Chx10+) cells (see Fig. 9 B and C). Scrambled control shRNA had no effect (see Fig. 9 B and C).
To address the effect of Chx10 knockdown in vivo, a newborn mouse retina was electroporated with a 2:1 ratio of shRNA/eGFP (pMXIE) vector. GFP+ cell types were scored in the mature retina as above. Immunostaining confirmed the absence of Chx10 protein in GFP+ cells expressing Chx10 shRNA (see Fig. 9D). Either of the Chx10 shRNAs (1-12 or 2-4) dramatically reduced the fraction of bipolar cells, but scrambled shRNA had no effect (Fig. 5A and B). The negative effect of Chx10 shRNA on bipolar cell genesis was matched by a comparable increase in rods (Fig. 5B). Consistent with a general function for Chx10 in promoting INL cell types (1), shRNA also inhibited amacrine cell genesis, but whereas both shRNAs were equally potent in blocking bipolar cell differentiation, one shRNA was less efficient in inhibiting amacrine cell development (Fig. 5B). As noted earlier, the effects of Chx10 modulation on amacrine cells are inconsistent, but the interchange between bipolar and rod cells is reproducible in different species or with different technical approaches (Figs. 1D, 2A, and 5B).
The above effects of Chx10 shRNA were due to down-regulation of Chx10 and not an off-target mRNA because a vector expressing human Chx10, resistant to mouse shRNA (data not shown), rescued bipolar cell loss induced by mouse Chx10 shRNA (Fig. 5B). Indeed, the proportion of bipolar cells when mouse Chx10 was knocked down and human Chx10 ectopically expressed was very similar to that seen in the control (Fig. 5B). When human Chx10 was cotransfected with a scrambled shRNA, the combination of endogenous mouse Chx10 and ectopic human protein increased the fraction of bipolar neurons relative to the control-transfected sample (Fig. 5B). In summary, acute Chx10 knockdown inhibited bipolar cell generation and promoted rod differentiation (Fig. 5), and, in three separate scenarios (Figs. 1, 2, and 5), Chx10 overexpression had the converse effect.
To assess the effect of acute Chx10 knockdown on division, mice electroporated at P0 with scrambled or Chx10 shRNA vectors were pulsed with BrdU 2 h before death, and retinal sections were labeled with anti-BrdU antibody. GFP+/BrdU+ cell counts were not altered either 3 or 8 days posttransfection (Fig. 5C). Thus, unlike the dramatic effect of Chx10 loss on embryonic RPC proliferation, division is not altered after acute Chx10 knockdown in postnatal RPCs. This result is consistent with the findings that Chx10 retrovirus does not affect clone size in the postnatal rat retina (Fig. 1C) and that Chx10 electroporation in mouse retina does not affect BrdU incorporation (Fig. 2C). TUNEL analysis revealed that the fraction of apoptotic GFP+ cells was small both in retinas electroporated with scrambled or Chx10 shRNA (<1%) and was not different at either P3 or P8 (data not shown). Thus, acute Chx10 knockdown switches the postnatal differentiation program without affecting cell division or death.
Discussion
Chx10 Function in Late-Stage Retinal Development.
The Chx10-deficient orJ retina has a profound defect in proliferation and lacks bipolar cells (4). Division is reduced in the peripheral orJ retina at E11.5 and virtually halted by E14.5 (4, 20). The central retina is less affected, but data here show that, by P3, division is severely reduced. The early defect in RPC expansion probably reflects a requirement for Chx10 to regulate one or more genes that directly or indirectly affect proliferation (9, 13, 15, 17). Deregulation of these genes early in retinogenesis probably has enormous indirect consequences on late-stage development; thus, the orJ model cannot reveal whether Chx10 plays a role either in proliferation of late-stage RPCs or in driving bipolar cell differentiation. Our data indicate that late-stage RPC proliferation is Chx10-independent because neither ectopic expression nor knockdown of Chx10 altered the expansion of postnatal RPCs. In stark contrast, Chx10 expression promoted bipolar cell differentiation at the expense of rods, whereas acute Chx10 knockdown had the reciprocal effect. Thus, independent of early effects on RPC proliferation, Chx10 is critical to promote and inhibit bipolar and rod differentiation, respectively.
The effects of acute (shRNA) vs. long-term (orJ) Chx10 loss on late-stage retinal differentiation show some overlap but also highlight the difficulty of interpreting apparent fate changes in the context of a proliferation defect. The presence of mutated Chx10 mRNA+ rods in the orJ retina mirrors the bipolar to photoreceptor switch after acute Chx10 knockdown. However, most Chx10 mRNA+ cells in the orJ retina were in the INL and expressed the glial marker Cralbp. Analysis of the Chx10/p27Kip1 double-null retina suggested that these cells arise as an indirect consequence of the severe proliferation defect, which probably impairs gliogenesis causing sustained Chx10 expression in Cralbp+ cells. The alternative, that Chx10 might block gliogenesis, is inconsistent with our in vivo studies and other's in vitro ectopic expression studies in which Chx10 did not inhibit Müller cell differentiation (Fig. 1D) (1), with the presence of Chx10 in a small subset of normal Müller glia (13), and, most importantly, with our shRNA data showing that acute Chx10 knockdown switches bipolar cells to photoreceptors.
Other defects in the orJ retina may also be an indirect consequence of early and progressive impairment of RPC proliferation. For example, photoreceptor genes such as Crx exhibit delayed expression in the orJ retina, and the final proportion of rods is low (26). Our acute knockdown results show that rod production is increased when Chx10 is down-regulated in the postnatal retina and proliferation is unaffected.
How Does Chx10 Suppress Photoreceptor Development?
The observation that Chx10 inhibits rods, rather than other cell types, generates new models as to how this protein might act. Chx10 can repress transcription (25); thus, it may inhibit the expression of genes required for rod specification. Fusing an activation domain to Chx10 perturbs its ability to support bipolar cell differentiation, implying that repression may be a component of Chx10 function in vivo. Possible target genes might include the basic helix–loop–helix protein Nrl or the paired-like homeodomain protein Otx2, which are essential for rod specification in mice (27, 28). However, Otx2 message is present in the outer region of the mouse INL at P6, the location of developing bipolar cells (28), and the protein is present in adult bipolar nuclei (29). Moreover, the Otx2 target gene Crx, which is required for photoreceptor maturation, is also expressed in bipolar cells (30). Therefore, Chx10 does not appear to suppress Otx2 expression or activity in these neurons. Intriguingly, in Xenopus retina, the Otx2 homologue Xotx2 is only expressed in bipolar cells and promotes their differentiation at the expense of rods (31). Xotx5b, a related homeodomain protein, is present in both frog photoreceptor and bipolar cells and promotes photoreceptor differentiation, and Xotx2 overrides this activity to promote bipolar cell differentiation (31). Thus, even though ectopic Chx10 does not appear to affect bipolar cell genesis in frogs, these data raise the possibility that Chx10 may promote bipolar cell differentiation in mammals by regulating the expression of Otx relatives. Other distinct possibilities are that Chx10 acts downstream of Otx2 to regulate expression of another transcription factor essential for rod genesis, such as Nrl as mentioned above, or that, because paired-like homeodomain proteins can heterodimerize (32), Chx10 modulates Otx2 and/or Crx target specificity. Although the fate determinants that Chx10 regulates are unknown, we showed that it binds and represses certain late-stage genes involved in photoreceptor differentiation (33). Thus, Chx10 appears to inhibit both photoreceptor specification and subsequent maturation.
Chx10 can also activate transcription in specific contexts (25, 34, 35). Thus, as well as blocking expression of rod promoting factors, Chx10 might also act by inducing inhibitors that interfere with rod development. Elucidating the details will require identification of the full complement of Chx10 in vivo targets.
Chx10 and RPC Proliferation.
It is intriguing that Chx10 has such a fundamental role in RPC proliferation in the prenatal retina yet becomes entirely dispensable for this process in the postnatal retina. This striking difference attests to the changing molecular milieu in RPCs, also reflected in the evolving competency to generate various cell types (11). Perhaps, the molecular context alters the target specificity of Chx10. Alternatively, the regulatory consequences of Chx10 binding may be altered by changes in other proteins that either bind Chx10 or Chx10 targets. Finally, the ability of Chx10 targets to affect proliferation may change as RPCs mature.
Our findings highlight the difficulties in interpreting differentiation defects in the orJ mouse in which there is an early and progressive defect in proliferation. Acute ectopic expression and knockdown studies reveal that Chx10 is only required for RPC proliferation during the early retinal development and clarify an essential later role in promoting bipolar cell differentiation and inhibiting rod development.
Methods
Mouse Strains and Genotyping.
All mice were treated in accordance with institutional and national guidelines. The orJ and p27Kip1 mutant strains were obtained from The Jackson Laboratory and genotyped by using the provided protocols.
Retroviral Injections.
Newborn CD rat pups (P0) were anesthetized on ice. Retrovirus (2 μl) was injected into the subretinal space through a small corneal incision. At P21, rats were killed by cervical dislocation, and the eyes were removed. The cornea was nicked to allow fixative penetration, and the eyes were immersed in 4% paraformaldehyde (pH 7.2) for 1 h at room temperature, then equilibrated in 30% sucrose, and frozen in embedding medium for cryosectioning. Sections (20 μm) were used for immunostaining. At least three retinas from three different litters were used for clonal analysis.
Electroporation.
Newborn mice (P0) received subretinal DNA injections (1–2 μl) as above and were electroporated by placing tweezer electrodes on both eyes and delivering eight pulses of 80 V through a CUY21 pulse generator (NEPA GENE, Chiba, Japan; see ref. 24).
Statistical Analysis.
We used Student's t test for two-group analysis or ANOVA followed by ad hoc Tukey's analysis for multiple-groups.
Plasmids, Immunofluorescence, in Situ Hybridization, and Cell-Type Analysis.
See Supporting Methods.
Supplementary Material
Acknowledgments
We thank Z. Xu for help with statistics; M. Dyer and C. Cepko for illustrating subretinal injections and for advice on clonal analysis; R. Kageyama for suggesting in situ assays; D. van der Kooy for pMXIE vector with permission from G. Nolan; P. Monnier for electroporation equipment; T. Collins for help with imaging; and V. Wallace, T. Inoue, and D. van der Kooy for comments. M.P. is a recipient of a Vision Science Research Program (VSRP) Award from the University of Toronto. K.M.D. is a recipient of a VSRP Award from the University of Toronto and an E. A. Baker Foundation and the Canadian National Institute for the Blind/Canadian Institute for Health Research (CIHR) Partnership Doctoral Research Fellowship. Funding for this project was provided by the CIHR.
Abbreviations
- RPC
retinal progenitor cell
- orJ
ocular retardation
- ONL
outer nuclear layer
- INL
inner nuclear layer
- shRNA
short-hairpin RNA
- Pn
postnatal day n
- En
embryonic day n.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hatakeyama J., Tomita K., Inoue T., Kageyama R. Development (Cambridge, U.K.) 2001;128:1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- 2.Wang J. C., Harris W. A. Dev. Biol. 2005;285:101–115. doi: 10.1016/j.ydbio.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Hatakeyama J., Kageyama R. Semin. Cell. Dev. Biol. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Burmeister M., Novak J., Liang M. Y., Basu S., Ploder L., Hawes N. L., Vidgen D., Hoover F., Goldman D., Kalnins V. I., et al. Nat. Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- 5.Ferda Percin E., Ploder L. A., Yu J. J., Arici K., Horsford J. D., Rutherford A., Bapat B., Cox D. W., Duncan A. M., Kalnins V. I., et al. Nat. Genet. 2000;25:397–401. doi: 10.1038/78071. [DOI] [PubMed] [Google Scholar]
- 6.Levine E. M., Passini M., Hitchcock P. F., Glasgow E., Schechter N. J. Comp. Neurol. 1997;387:439–448. [PubMed] [Google Scholar]
- 7.Passini M. A., Levine E. M., Canger A. K., Raymond P. A., Schechter N. J. Comp. Neurol. 1997;388:495–505. doi: 10.1002/(sici)1096-9861(19971124)388:3<495::aid-cne11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Barabino S. M., Spada F., Cotelli F., Boncinelli E. Mech. Dev. 1997;63:133–143. doi: 10.1016/s0925-4773(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 9.Green E. S., Stubbs J. L., Levine E. M. Development (Cambridge, U.K.) 2003;130:539–552. doi: 10.1242/dev.00275. [DOI] [PubMed] [Google Scholar]
- 10.Liu I. S. C., Chen J., Ploder L., Vidgen D., van der Kooy D., Kalnins V. I., McInnes R. R. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 11.Cepko C. L., Austin C. P., Yang X., Alexiades M., Ezzeddine D. Proc. Natl. Acad. Sci. USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young R. W. Anat. Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 13.Rowan S., Cepko C. L. Dev. Biol. 2004;271:388–402. doi: 10.1016/j.ydbio.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Toy J., Norton J. S., Jibodh S. R., Adler R. Invest. Ophthalmol. Visual Sci. 2002;43:3522–3529. [PubMed] [Google Scholar]
- 15.Chow L., Levine E. M., Reh T. A. Mech. Dev. 1998;77:149–164. doi: 10.1016/s0925-4773(98)00135-x. [DOI] [PubMed] [Google Scholar]
- 16.Rowan S., Chen C. M., Young T. L., Fisher D. E., Cepko C. L. Development (Cambridge, U.K.) 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- 17.Horsford D. J., Nguyen M. T., Sellar G. C., Kothary R., Arnheiter H., McInnes R. R. Development (Cambridge, U.K.) 2005;132:177–187. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- 18.Fischer A. J., Reh T. A. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 19.Blackshaw S., Harpavat S., Trimarchi J., Cai L., Huang H., Kuo W. P., Weber G., Lee K., Fraioli R. E., Cho S. H., et al. PLoS. Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone-Larson C., Basu S., Radel J. D., Liang M., Perozek T., Kapousta-Bruneau N., Green D. G., Burmeister M., Hankin M. H. J. Neurobiol. 2000;42:232–247. doi: 10.1002/(sici)1097-4695(20000205)42:2<232::aid-neu7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Cepko C. L., Ryder E., Austin C., Golden J., Fields-Berry S., Lin J. Methods. 1998;14:393–406. doi: 10.1006/meth.1998.0594. [DOI] [PubMed] [Google Scholar]
- 22.Turner D. L., Cepko C. L. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 23.Dezawa M., Takano M., Negishi H., Mo X., Oshitari T., Sawada H. Micron. 2002;33:1–6. doi: 10.1016/s0968-4328(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda T., Cepko C. L. Proc. Natl. Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorval K. M., Bobechko B. P., Ahmad K. F., Bremner R. J. Biol. Chem. 2005;280:10100–10108. doi: 10.1074/jbc.M412676200. [DOI] [PubMed] [Google Scholar]
- 26.Rutherford A. D., Dhomen N., Smith H. K., Sowden J. C. Invest. Ophthalmol. Vision Sci. 2004;45:375–384. doi: 10.1167/iovs.03-0332. [DOI] [PubMed] [Google Scholar]
- 27.Mears A. J., Kondo M., Swain P. K., Takada Y., Bush R. A., Saunders T. L., Sieving P. A., Swaroop A. Nat. Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 28.Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T. Nat. Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 29.Baas D., Bumsted K. M., Martinez J. A., Vaccarino F. M., Wikler K. C., Barnstable C. J. Brain Res. Mol. Brain Res. 2000;78:26–37. doi: 10.1016/s0169-328x(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 30.Bibb L. C., Holt J. K., Tarttelin E. E., Hodges M. D., Gregory-Evans K., Rutherford A., Lucas R. J., Sowden J. C., Gregory-Evans C. Y. Hum. Mol. Genet. 2001;10:1571–1579. doi: 10.1093/hmg/10.15.1571. [DOI] [PubMed] [Google Scholar]
- 31.Viczian A. S., Vignali R., Zuber M. E., Barsacchi G., Harris W. A. Development (Cambridge, U.K.) 2003;130:1281–1294. doi: 10.1242/dev.00343. [DOI] [PubMed] [Google Scholar]
- 32.Wilson D., Sheng G., Lecuit T., Dostatni N., Desplan C. Genes Dev. 1993;7:2120–2134. doi: 10.1101/gad.7.11.2120. [DOI] [PubMed] [Google Scholar]
- 33.Dorval K. M., Bobechko B. P., Fujieda H., Chen S., Zack D. J., Bremner R. J. Biol. Chem. 2005;281:744–751. doi: 10.1074/jbc.M509470200. [DOI] [PubMed] [Google Scholar]
- 34.Mikkola I., Bruun J. A., Holm T., Johansen T. J. Biol. Chem. 2001;276:4109–4118. doi: 10.1074/jbc.M008882200. [DOI] [PubMed] [Google Scholar]
- 35.Bruun J. A., Thomassen E. I., Kristiansen K., Tylden G., Holm T., Mikkola I., Bjorkoy G., Johansen T. Nucleic Acids Res. 2005;33:2661–2675. doi: 10.1093/nar/gki562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.