Abstract
Immunization with irradiated autologous T cells (T cell vaccination) is shown to induce regulatory T cell responses that are poorly understood. In this study, CD4+ regulatory T cell lines were generated from patients with multiple sclerosis that received immunization with irradiated autologous myelin basic protein-reactive T cells. The resulting CD4+ regulatory T cell lines had marked inhibition on autologous myelin basic protein-reactive T cells and displayed two distinctive patterns distinguishable by the expression of transcription factor Foxp3 and cytokine profile. The majority of the T cell lines had high Foxp3 expression and secreted both IFN-γ and IL-10 as compared with the other pattern characteristic of low Foxp3 expression and predominant production of IL-10 but not IFN-γ. CD4+ regulatory T cell lines of both patterns expressed CD25 and reacted with activated autologous T cells but not resting T cells, irrespective of antigen specificity of the target T cells. It was evident that they recognized preferentially a synthetic peptide corresponding to residues 61–73 of the IL-2 receptor α chain. T cell vaccination correlated with increased Foxp3 expression and T cell reactivity to peptide 61–73. The findings have important implications in the understanding of the role of CD4+ regulatory T cell response induced by T cell vaccination.
Keywords: Foxp3, myelin basic protein
Immunization with inactivated autoreactive T cells (T cell vaccination) has been demonstrated to induce regulatory immune responses in autoimmune conditions of both human and experimental animal models, which are attributed to the suppression of disease activities (1–3). At least two types of regulatory T cell responses have been identified, which include antiidiotypic and so-called antiergotypic T cell responses (2–7). Recent clinical trials in patients with multiple sclerosis (MS) have shown that antiidiotypic T cells induced by T cell vaccination represent CD8+ cytolytic T cells capable of killing target T cells used for immunization through interaction with target T cell receptor components in the context of MHC class I molecules (3, 6, 8). CD8+ antiidiotypic T cell response is thought to contribute directly to depletion of circulating autoreactive T cells (3, 5, 9). It was also evident in a number of studies that in addition to CD8+ antiidiotypic T cells, T cell vaccination induces CD4+ regulatory T cell responses in both experimental autoimmune encephalomyelitis and human MS (7, 10). CD4+ regulatory T cell responses may represent an important immune regulatory component relevant to clinical effects of T cell vaccination (7, 9, 11). However, the nature and functional properties of these CD4+ regulatory T cells are poorly understood. It has been suggested that CD4+ regulatory T cell responses may be induced through interaction with certain T cell markers loosely and collectively called “ergotopes” (7, 11, 12). There have been some indications that T cell activation molecules such as IL-2 receptor and heat-shock protein 60 may be among candidate ergotopes (11–14). It has been a topic of great interest to characterize the nature and functional properties of human CD4+ regulatory T cell responses induced by T cell vaccination in clinical trials because the findings would have direct implication in the understanding of the potential therapeutic role of T cell vaccination in human autoimmune conditions.
This study was undertaken to characterize CD4+ regulatory T cell responses in patients with MS that received immunization through multiple s.c. injections with irradiated autologous myelin basic protein (MBP)-reactive T cell lines in a previous clinical trial (15). Substantial CD4+ regulatory T cell responses were observed in all MS patients after three immunizations, which correlated with clinical improvement in some of the patients, with respect to disability score, rate of relapse, and brain lesions by magnetic resonance imaging (15). A panel of CD4+ regulatory T cell lines was raised against autologous MBP-reactive T cells originally used for immunization. We first determined whether CD4+ regulatory T cell responses induced by T cell vaccination represented heterogeneous populations of distinctive functional and phenotypic patterns. Experiments were designed to define whether a proportion of the resulting CD4+ regulatory T cell lines expanded from the CD4+CD25+Foxp3+ regulatory T cell pool, a hypothesis proposed in this study. Attempts were made to further address whether human CD4+ regulatory T cells induced by T cell vaccination shared the features of antiergotypic T cells seen in experimental animal models in recognition of candidate ergotopes. The findings described here support the important role of CD4+ regulatory T cell responses in T cell vaccination, which may have therapeutic relevance in MS.
Results
CD4+ Regulatory T Cell Responses Induced by T Cell Vaccination in Patients with MS.
Blood specimens were obtained from MS patients that received multiple s.c. injections of irradiated autologous MBP-reactive T cells in a previous clinical trial of T cell vaccination (15). Peripheral blood mononuclear cells (PBMCs) derived from MS patients before and after immunization were cocultured with irradiated autologous MBP-reactive T cells previously used for immunization to generate regulatory T cell lines. Preliminary characterization of the resulting T cell lines included phenotypic analysis for CD4 and CD8 by flow cytometry and the inhibitory activity on the proliferation of autologous immunizing MBP-reactive T cells. T cell lines were defined as CD4+ regulatory T cell lines when they expressed exclusively the CD4 phenotype (>90% staining) and exhibited at least 40% specific inhibition rate on autologous MBP-reactive T cells. The analyses revealed that CD4+ regulatory T cell lines could be generated at a range of 8–18 T cell lines per patient from postimmunization specimens of ten million PBMCs, whereas, in most cases, they were not detectable in prevaccination PBMCs of the same patients under similar experimental conditions. A panel of 30 stable CD4+ regulatory T cell lines derived from three patients was further characterized here. These T cell lines were shown to consistently inhibit the proliferation of autologous MBP-reactive T cells at inhibition rates of between 62% and 98% (median 78.5%). However, the inhibition was not antigen specific because they had similar inhibition rates, when tested in parallel experiments, for autologous nonspecific T cell targets raised by antibodies to CD3 and CD28 (Fig. 6, which is published as supporting information on the PNAS web site).
Phenotypes and Transcription Factor Foxp3 Expression of CD4+ Regulatory T Cell Lines.
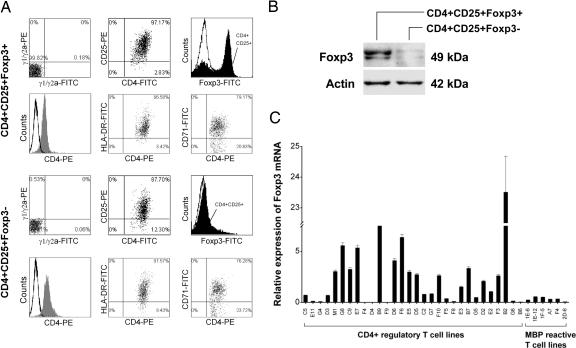
The resulting CD4+ regulatory T cell lines were further characterized for surface staining of CD25 by using flow cytometry and the expression of transcription factor Foxp3 by real-time PCR and immunoblot. All T cell lines examined expressed CD25 at various degrees (between 47.1 and 95.1%; median, 82.14%). Fig. 1 shows two representative patterns of the T cell lines, which was distinguishable by the expression levels of Foxp3. Those characterized as CD4+CD25+Foxp3+ T cell lines had consistently high expression levels of Foxp3, whereas other T cell lines were characteristic of low expression of Foxp3 (CD4+CD25+Foxp3−) (Fig. 1 A–C). Furthermore, T cell lines of the CD4+CD25+Foxp3+ pattern differed significantly in cytokine profile from those of CD4+CD25+Foxp3−. The former produced both IL-10 and IFN-γ in large amounts, whereas the latter secreted predominantly IL-10 but little or no IFN-γ, even though a few exceptions were noted (Fig. 7, which is published as supporting information on the PNAS web site). However, the expression patterns did not seem to correlate with the inhibitory activities because all CD4+ regulatory T cell lines examined exhibited high percentage of the inhibitory activity against the proliferation of autologous MBP-reactive T cells. The patterns were not related to activation state of the T cells because the expression of T cell activation makers was similar between the T cell lines of the two expression patterns. Furthermore, the inhibitory activity of CD4+CD25+Foxp3− regulatory T cell lines was related to their ability to secrete high concentrations of IL-10 because the inhibition could be blocked by antibody to human IL-10 (Fig. 8, which is published as supporting information on the PNAS web site).
Fig. 1.
Expression of Foxp3 mRNA in CD4+ regulatory T cell lines derived from MS patients immunized with irradiated autologous MBP-reactive T cells. (A) Two representative patterns of CD4+ regulatory T cell lines by flow cytometry. Representative T cell lines (lines B9 and F8) were analyzed for the expression of CD4 paired with that of CD25, HLA-DR, CD71, and Foxp3. Open curves in the histograms represent isotype control. Staining with specific antibody to CD4 or Foxp3 is indicated by gray or solid curves, respectively. (B) Immunoblot analysis of the same T cell lines for protein expression of transcription factor Foxp3. (C) Foxp3 mRNA expression by real-time PCR in all 30 CD4+ regulatory T cell lines and six reference MBP-reactive T cell clones derived from the same patients.
Foxp3 Expression and Inhibitory Function of the CD4+CD25+ T Cell Pool in Relation to T Cell Vaccination.
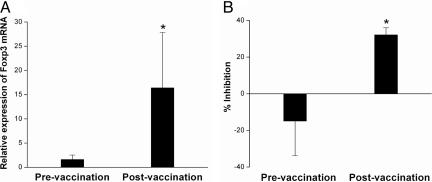
The observation that the majority of the CD4+ regulatory T cells expressed high levels of Foxp3 prompted us to evaluate whether repeated immunization with irradiated autologous MBP-reactive T cells would alter the CD4+CD25+ regulatory T cell pool in patients with MS. To this end, CD4+CD25+ T cell preparations were purified from preimmunization and postimmunization PBMCs and examined for changes in the expression of Foxp3 and inhibitory activity. As seen in Fig. 2A, Foxp3 expression was significantly elevated in CD4+CD25+ T cell populations from baseline value of 1.61±0.92 to postimmunization value of 16.4±11.4 (P < 0.05) in 20 MS patients examined. Consistent with the elevated Foxp3 expression was the increased inhibitory activity of purified CD4+CD25+ T cells derived from postimmunization PBMCs compared with the baseline activity in preimmunization samples (n = 20, P< 0.05) (Fig. 2B). The results supported the possibility that T cell vaccination induced regulatory mechanism leading to the expansion of the CD4+CD25+ regulatory T cell pool in MS patients. Because there is a deficit in the function and number of CD4+CD25+ regulatory T cells in MS (16, 17), such a regulatory mechanism induced by T cell vaccination may be of therapeutic importance.
Fig. 2.
Expression of Foxp3 in paired PBMCs obtained from MS patients before and after T cell vaccination. Purified CD4+CD25+ T cells were obtained from MS patients (n = 20) before and after T cell vaccination. The resulting T cells were subject to real-time PCR analysis for mRNA expression (A) and inhibition rate on the proliferation of autologous T cells stimulated by anti-CD3 antibody (B). Percent inhibition is calculated as [1 − (experimental cpm per control cpm)] × 100%. ∗, statistical differences between the groups (P < 0.05).
Reactivity Patterns and the Recognition of IL-2 Receptor-Derived Peptides by CD4+ Regulatory T Cell Lines.
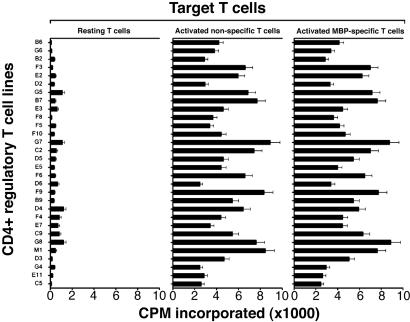
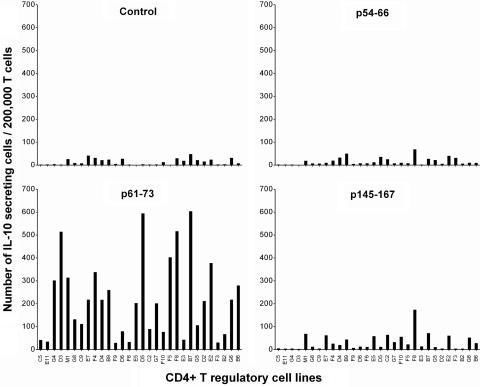
CD4+ regulatory T cell lines were further characterized for their reactivity patterns by using resting T cells, activated MBP-reactive T cells, and activated nonspecific T cells as autologous target cells. As illustrated in Fig. 3, all T cell lines tested appeared to react with autologous activated T cells but not resting T cells. The reactivity appeared irrespective of antigen specificity of the target T cells, suggesting that they recognized marker(s) associated with T cell activation and not restricted to reactivity to MBP. The observed reactivity of CD4+ regulatory T cell lines was restricted to MHC class II molecules because a monoclonal antibody specific for MHC class II could inhibit variably the T cell reactivity (inhibition rate between 40% and 86%). The findings and the recent reports by Mimran and Mor and their colleagues (refs. 11 and 13, respectively) led us to further characterize whether human CD4+ regulatory T cell lines induced by T cell vaccination recognized epitopes contained in the IL-2 receptor α chain. For this purpose, we selected three peptides of IL-2 receptor α chain that had high binding affinity to HLA DRB1*1501 expressed in all three patients from whom CD4+ regulatory T cell lines were generated or DRB1*0401 found in one patient. As shown in Fig. 4, these independent T cell lines appeared to preferentially recognize the peptide corresponding to residues 61–73 of IL-2 receptor α chain as compared with other selected and control peptides. CD4+ regulatory T cell lines were found to react to peptide 61–73 in the context of DRB1*1501 as the reactivity occurred only when the peptide was presented by fibroblast cells transfected with DRB1*1501. In the ten selected T cell lines tested, mean cpm in the presence of transfected fibroblast cells exceeded control cpm (untransfected fibroblast cells and medium control) by at least 3.5 times in proliferation assays.
Fig. 3.
Reactivity of CD4+ regulatory T cell lines to autologous T cell targets. CD4+ regulatory T cell lines were examined for reactivity to resting T cells, activated MBP-reactive T cells used for vaccination, and activated T cells raised by anti-CD3/CD28 mAbs. All target T cells were of the autologous origin and irradiated before use. T cell reactivity to target cells was determined in proliferation assays by measuring [3H]-thymidine incorporation.
Fig. 4.
Reactivity of CD4+ regulatory T cells to peptides derived from IL-2R α chain. The reactivity of CD4+ regulatory T cells to three selected peptides derived from IL-2 receptor α chain was measured by ELISPOT. An irrelevant T cell antigen receptor peptide was used as a control. CD4+ regulatory T cell lines were cultured at 200,000 cells per well in triplicate in the presence of irradiated antigen-presenting cells and the indicated synthetic peptides at a concentration of 20 μg/ml in precoated ELISPOT plates. The results were presented as IL-10 secreting T cells per 200,000 CD4+ regulatory T cells. Among the T cell lines examined, E11, G4, F4, D4, F9, F5, F8, G5, G6, and B6 are considered as CD4+CD25+Foxp3− T cell lines.
T Cell Reactivity to Peptide 61–73 in Relation to T Cell Vaccination.
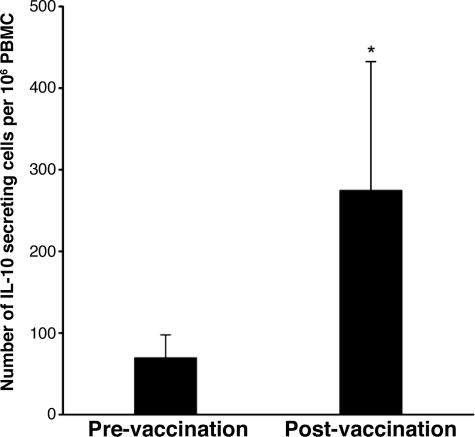
We then addressed whether T cells recognizing peptide 61–73 of IL-2 receptor α chain were induced by repeated immunization with autologous activated MBP-reactive T cells in patients with MS. PBMC specimens obtained before and after T cell vaccination were analyzed for reactivity to the peptide by using the enzyme-linked immunospot (ELISPOT) method. As illustrated in Fig. 5, significantly increased T cell reactivity to peptide 61–73 was found in postimmunization PBMCs compared with preimmunization PBMCs, suggesting priming of CD4+ regulatory T cells secreting IL-10 by T cell vaccination. Furthermore, a panel of eight short-term CD4+ T cell lines was raised against peptide 61–73 from postimmunization PBMC specimens. These CD4+ T cell lines expressed CD25 (mean, 78% by double staining) and high levels of Foxp3 (Table 1, which is published as supporting information on the PNAS web site). They exhibited significant inhibitory properties on the proliferation of autologous MBP-reactive T cells and secreted both IL-10 and IFN-γ. These CD4+ regulatory T cell lines raised against peptide 61–73 were of resemblance to those that belonged to the CD4+CD25+Foxp3+ pattern and were generated against irradiated autologous MBP-reactive T cells used for T cell vaccination.
Fig. 5.
The frequency of CD4+ regulatory T cells in response to peptide 61–73 of IL-2R α chain in patients before and after T cell vaccination. The frequency of T cells reactive to peptide 61–73 was measured in PBMCs obtained from MS patients (n = 20) before and after T cell vaccination. PBMCs were cultured in triplicate at 200,000 cells per well in the presence of peptide 61–73 at a concentration of 20 μg/ml in precoated ELISPOT plates. The data are presented as IL-10 secreting T cells in 1 million PBMCs. ∗, statistical difference between the groups (P < 0.05).
Discussion
CD4+ regulatory T cells represent an important component of immune responses induced by T cell vaccination and may play an essential role in therapeutic effects of T cell vaccination seen in experimental autoimmune encephalomyelitis and other autoimmune models and in preliminary clinical trials of MS. Studies published so far indicate that CD4+ regulatory T cells are among the predominant T cell populations that respond to repeated immunization with irradiated autologous T cells selected for autoantigen. However, the functional properties and regulatory mechanism of CD4+ regulatory T cells, especially in humans, are poorly understood. In experimental animal models, there is some evidence suggesting that at least a portion of CD4+ T cells induced by T cell vaccination represent antiergotypic T cells and recognize candidate ergotopes, such as IL-2 receptor α chain and heat-shock protein 60 (11–13). In this study, we demonstrated that CD4+ regulatory T cells induced in MS patients by T cell vaccination are largely composed of T cell populations of two distinct patterns distinguishable by the expression of Foxp3 and cytokine profile. The majority of CD4+ regulatory T cell lines obtained expresses high levels of Foxp3 and produces both IL-10 and IFN-γ when compared with those characteristic of low expression of Foxp3 and selective IL-10 production. Although CD4+ regulatory T cells described here all share the same functional feature in the inhibition of activated T cells, they may exert inhibitory function through different mechanisms. Regulatory T cells of the CD4+CD25+Foxp3+ pattern bear a remarkable resemblance to naturally occurring CD4+CD25+ regulatory T cells and may directly expand from the existing CD4+CD25+ regulatory T cell pool by T cell vaccination. This possibility is supported by additional evidence here that there was significantly elevated expression of Foxp3 and inhibitory function in CD4+CD25+ T cells derived from postimmunization PBMCs compared with baseline specimens. The observations suggest that T cell vaccination induces up-regulation and expansion of the CD4+CD25+ regulatory T cell pool that is found deficient in MS patients (16, 17). The other regulatory T cell population characterized here as CD4+CD25+Foxp3− T cells may inhibit activated T cells through the high production of inhibitory cytokines (i.e., IL-10) that are not antagonized by IFN-γ. This possibility is supported by additional evidence described here that the inhibitory activity of CD4+CD25+Foxp3− regulatory T cells was blocked by antibody to IL-10. It should be noted that the same patterns of CD4+ regulatory T cells were found in a recent T cell vaccination trial in rheumatoid arthritis patients in which selected synovial T cells were irradiated and used for immunization (G. Chen, N. Li, Y. C. Q. Zang, D. Zhang, D. He, G. Feng, L. Ni, R. Xu, L. Wang, B. Shen, and J. Z. Z., unpublished work).
In this study, we present important evidence indicating that CD4+ regulatory T cells, mainly the CD4+CD25+Foxp3+ population, induced by T cell vaccination preferentially recognize an epitope corresponding to residues 61–73 of the IL-2 receptor α chain. The result is consistent with the observed reactivity of antiergotypic T cells with IL-2 receptor α chain in rodents (11). Furthermore, this unique property of peptide 61–73 becomes more evident as it is able to elicit CD4+ regulatory T cell responses in postimmunization specimens. It is conceivable that CD4+ regulatory T cells recognizing an epitope in peptide 61–73 are sensitized by repeated immunization with autologous activated MBP-reactive T cells. This epitope may play an instrumental role in the induction of CD4+ regulatory T cell responses in T cell vaccination. Similar to CD4+ regulatory T cells induced by T cell vaccination, T cells induced in vitro by stimulation with peptide 61–73 of IL-2 receptor α chain largely share the same features of CD4+CD25+Foxp3+ T cell population described in this study. Interestingly, similar CD4+ regulatory T cell response can be induced by T cell receptor (TCR) peptides in humans and rodents (18, 19). The relevance of the identified peptide of IL-2 receptor α chain and its effectiveness in the induction of CD4+ regulatory T cell responses, when used alone or in combination with selected TCR peptides, warrant further investigation to explore its potential therapeutic implications.
The detailed characterization of CD4+ regulatory T cell responses described here has direct relevance to the understanding of their role in the immunological and perhaps therapeutic effects of T cell vaccination. It is likely that CD8+ cytotoxic antiidiotypic T cell response is mainly responsible for marked and rather rapid depletion of circulating autoreactive T cells as seen in several independent MS clinical trials (3–5, 15, 20–22). The mechanism of this CD8+ antiidiotypic T cell regulation is relatively well characterized and is found to involve components of target T cell receptor in the context of MHC class I molecules (3–6). However, it remains to be determined whether depletion of circulating autoreactive T cells alone would be sufficient to induce or account for clinical improvements seen in some of MS patients treated with T cell vaccination (3, 5, 15, 20–23). In this regard, it is insightful that Cohen and colleagues (7, 12) and other investigators showed in experimental autoimmune encephalomyelitis experiments that only activated, but not resting, encephalitogenic T cells could induce full protection through T cell vaccination. The observation suggests that CD4+ regulatory T cells induced by ergotopes expressed on activated T cells play an important role in the induction of other regulatory functions. These regulatory functions may not directly result in rapid depletion of circulating autoreactive T cells but are critical to maintaining homeostasis of the immune system through cytokine regulation and CD4+CD25+ regulatory T cells. This relatively broad spectrum of regulation irrespective of antigen specificity, when combined with specific depletion of autoreactive T cells by CD8+ cytotoxic antiidiotypic T cells, becomes highly effective. The combined regulatory mechanisms confer full protection seen in experimental autoimmune encephalomyelitis and may produce treatment effects for autoimmune conditions such as MS. The combined regulatory mechanisms are particularly important in MS in which aberrant autoimmunity is not simply limited to myelin autoreactive T cells but also involves a cytokine milieu seemingly skewed to a proinflammatory characteristic and a functional defect of CD4+CD25+ regulatory T cell response that normally keeps autoreactive and inflammatory T cells in check.
Materials and Methods
Specimens and Reagents.
Blood specimens were obtained from MS patients involved in a previous T cell vaccination clinical trial in which patients received three s.c. injections of irradiated autologous MBP-reactive T cell lines (30–40 million T cells per injection) at a 2-month interval (15). Blood specimens were collected at baseline (preimmunization) and after the last immunization (postimmunization). PBMCs were prepared by Ficoll separation, cryopreserved, and thawed before analysis. The protocol was approved by the Institutional Human Subjects Committee (Baylor College of Medicine, Houston).
tepitope is a Windows application that allows the identification of HLA ligand binding epitopes (24–26). Three peptides of human IL-2 receptor α chain corresponding to the predicted HLA DRB1*1501 or DRB1*0401 binding sequences (1% threshold) were synthesized by using the Mayfield method and were purified. The purity of the peptides was >90%. The amino acid sequences of the peptides were GFRRIKSGSLYML (IL-2R-α residues 54–66), GSLYMLCTGNSSH (IL-2R-α residues 61–73), and GQMVYYQCVQGYR (IL-2R-α residues 145–167). A reference peptide of unrelated T cell antigen receptor CDR3 sequence (SSLGRAGLTYEQYFG) was used as a control.
Generation of CD4+ Regulatory T Cell Lines Against Irradiated Autologous MBP-Reactive T Cells or IL-2 Receptor Peptides and Inhibition Assays.
To generate CD4+ regulatory T cell lines responding to immunizing MBP-reactive T cells, PBMCs were cultured at 5 × 104 per well with irradiated immunizing T cells (5 × 104 per well) used as stimulator. After 7 days, the cultures were restimulated with the same irradiated T cell clones and supplemented with rIL-2. At day 14, 50% of cells of each well were irradiated at 2,000 rads (to prevent their own proliferation) and transferred in duplicate to wells containing immunizing MBP-reactive T cells in the presence of PBMCs pulsed with MBP as a source of antigen-presenting cells. Inhibitory effect of each culture on the proliferation of MBP-reactive T cells was measured subsequently in proliferation ([3H]-thymidine uptake) assays. Percent inhibition was measured as [1 − (proliferation in the presence of irradiated regulatory T cells/proliferation in the absence of the regulatory T cells)] × 100%. Cultures exerting at least 40% inhibition were considered as regulatory T cell lines. The obtained regulatory T cell lines were expanded and further characterized for surface expression of the CD4, CD8, and CD25 phenotypes by flow cytometry. Only the CD4+ regulatory T cell lines were selected and further expanded for characterization.
To generate short-term CD4+ T cell lines against IL-2 receptor peptide, a slightly modified protocol was used. Postimmunization PBMCs were plated out at 200,000 cells per well (a total of 60 wells per patient) in the presence of the peptide at a final concentration of 20 μg/ml and cultured for 7 days. The cultures were tested for specific reactivity to the peptides and considered as positive when stimulation index (mean experimental cpm per background cpm) exceeded 3.0. Selected CD4+ T cell lines were expanded by restimulation with peptide 61–73 and subject to detailed characterization. To examine MHC restriction, mouse fibroblast cells transfected with human HLA DRB1*1501 (courtesy of E K. Wucherpfennig, Dana–Faber Cancer Institute, Boston) were incubated with peptide 61–73 (50 μg/ml) and washed to remove free peptide before irradiation. Transfected fibroblast cells (105 cells per well) were cocultured with T cell lines (104 cells per well) for 3 days. Untransfected fibroblast cells were used as a control. Cell proliferation was measured subsequently by [3H]-thymidine uptake.
Flow Cytometric Analysis.
Cells (0.5–1 × 106) were resuspended in PBS containing 1% BSA (Irvine) and 0.1% sodium azide (Sigma-Aldrich). For surface expression of CD4, CD25, HLA-DR, and CD71, cells were incubated with FITC- or phycoerythrin-conjugated antibodies to the indicated markers (BD Biosciences) or their isotype control antibodies for 30 min on ice for double-color staining. For intracellular staining of Foxp3, cells were permeablized with CytoFix/CytoPerm solution (BD Biosciences, San Jose, CA). Resulting cells were stained with mouse anti-human Foxp3 mAb (Courtesy of A. Banham, University of Oxford, Oxford), followed by washing and staining with a conjugated secondary goat anti-mouse antibody. Stained cells were fixed with 1% paraformaldehyde and analyzed by a FACSCalibur (BD Biosciences).
Foxp3 mRNA Expression by Real-Time PCR.
Quantitative real-time RT-PCR was performed on a PRISM 7000 sequence detection system (Applied Biosystems). Hypoxanthine phosphoribosyltransferase (HPRT) was used as a reference for sample normalization. Total RNA isolated from PBMCs or CD4+ T cell lines was reverse-transcribed into cDNA by using random hexamer. Human Foxp3 primers (forward, 5′-CAC CTG GCT GGG AAA ATG G-3′; reverse, 5′-GGA GCC CTT GTC GGA TGA T-3′) and TaqMan MGB probe (5′-6FAM-ACT GAC CAA GGC TTC AT-3′) (FAM, 6-carboxy-fluorescein) sequences were designed by using primer express application program (Applied Biosystems). Mouse Foxp3 primers/probe and all HPRT primers/probe were purchased as forms of assay-on-demand (Applied Biosystems). The amplification protocol used was described as follows: 1 μl of synthesized cDNA product was subsequently added into PCR mix containing 25 μl of TaqMan 2x PCR master mix (Applied Biosystems), 30 pmol human Foxp3 primer with 10 pmol probe or 2.5 μl of mouse Foxp3 primer/probe set, 2.5 μl HPRT primer/probe set (assay-on-demand, Applied Biosystems), and distilled water was added to make a total reaction volume of 50 μl. The PCR was programmed as an initial incubation for 10 min at 95°C followed by 40 thermal cycles of 15 s at 95°C and 1 min at 60°C. Relative quantification of gene expression was calculated by using a ΔCT method based on signal intensity of the PCRs according to the following formula: 2−ΔCT = [2−(sample Ct − normalizer Ct)] (Ct = threshold cycle of real-time PCR). All reactions were performed in triplicate and results were confirmed by at least one additional independent run.
Immunoblot Analysis.
CD4+ T cell lines were directly lysed in Laemmli sample buffer (Bio-Rad) and separated by 10% SDS/PAGE. Immunoblot analysis was performed by initial transfer of proteins onto nitrocellulose filters by using Mini TransBlot (Bio-Rad) and followed by a blocking step by using Tris-buffered saline with 0.1% Tween 20 plus 5% freeze-dried milk for 4 h. After washing, the filters were incubated with an antibody to human Foxp3 (courtesy of A. Rudensky, University of Washington, Seattle) or a control antibody (specific for human β-actin, Santa Cruz Biotechnology), respectively, overnight at 4°C under the same experimental conditions. After washing and subsequent incubation with a conjugated goat anti-mouse antibody (Santa Cruz Biotechnology) for 1 h at room temperature, filters were developed with enhanced chemiluminescence kit (Amersham Pharmacia Biosciences) according to the manufacture’s instructions.
Measurement of Cytokine Production.
Supernatants were collected from cell culture and diluted for measurement of cytokine concentration by ELISA. Antibody pairs and standards for IL-10 and IFN-γ were all purchased from BD Biosciences. Briefly, microtiter plates precoated with capturing mAbs were blocked with 2% BSA/PBS. After washing, samples and controls were added at 50 μl per well and incubated for 2 h with a biotinylated detecting antibody (50 μl per well) in 2% BSA/PBS/Tween-20. Plates were washed and incubated for 30 min with streptavidin-conjugated horseradish peroxidase. Next, 100 μl of 0.0125% tetramethylbenzidine and 0.008% H2O2 in citrate buffer was used as substrate. A standard curve was performed for each plate and used to calculate the absolute concentrations of cytokines.
Isolation of CD4+CD25+ Regulatory T Cells.
Isolation of CD4+CD25+ T cells was performed by using a human regulatory T cell isolation kit (Miltenyi Biotec, Auburn, CA) according to manufacturer’s instructions. Briefly, CD4+ T cells were isolated through negative selection by removing all other cell types after a 10-min incubation with a mixture of biotin-conjugated antibodies (10 μl per 107 cells). This step was followed by incubation of T cells with magnetic beads conjugated with anti-biotin antibody for 15 min at 4°C. The labeled cells were subsequently depleted by separation over a MACS column (Miltenyi Biotec). Preisolated CD4+ T cells were incubated with 10 μl of magnetic beads conjugated with anti-CD25 antibody (for 107 cells) for 15 min at 4°C. The magnetically labeled CD4+CD25+ T cells were separated from unlabeled CD4+CD25− T cells by using a MACS column. The purity of the resulting T cell populations was confirmed to be >97% by flow cytometry.
To examine the inhibition rate of purified CD4+CD25+ T cells obtained from MS patients before and after T cell vaccination, the resulting CD4+CD25+ T cell preparations (inhibitor) were cocultured (5 × 103 per well) at 1:1 ratio with CD4+CD25− T cells (responder) in the presence or absence of 2 μg/ml of anti-CD3 and anti-CD28 mAb. All cells were cultured in a final volume of 200 μl in the presence of 105 irradiated antigen-presenting cells (APCs). At day 7, each well was pulsed with 1 μCi (1 Ci = 37 GBq) [3H]-thymidine during the last 6 h of culture. Cells were subsequently harvested by using an automated cell harvester, and [3H]-thymidine incorporation was measured in a β-plate counter. The results were calculated as percent inhibition as follows: [1 − (experimental cpm per control cpm)] × 100%. To assess the role of IL-10 in the inhibitory activity of CD4+CD25+Foxp3− regulatory T cells, regulatory T cells were cocultured with autologous MBP reactive T cells in the presence of APC and the corresponding MBP peptide. The ratio of regulatory T cell to MBP-reactive T cell was 1 (104 cells in total per well). Mouse monoclonal antibody to human IL-10 was added at the indicated serial concentrations. An isotype-matched antibody was used as a control. Percent inhibition was measured as described above.
T Cell Frequency Analysis by ELISPOT.
PBMC or CD4+ T cell lines were plated out at 200,000 cells per well in triplicate in the presence of peptides (20 μg/ml) in nitrocellulose-coated microtiter plates pretreated with antibody to human IL-10 (BD Pharmingen). Next, 105 PBMCs predepleted for T cells were used as antigen-presenting cells. RPMI medium 1640 containing 5% human AB serum was used. Plates were incubated for 24 h at 37°C, and cells were subsequently discarded. A biotin-labeled secondary mAb was added, followed by extensive washes and addition of streptavidin-alkaline phosphatase and substrate to develop optimal color reaction. Positively stained spots were quantified by using Immunospot (CTL Technology, Cleveland) equipped with a high resolution lens camera and analytical software. Mean spots per well were calculated, and net counts were established after subtraction of background. The frequency was expressed as specific spot-forming cells in the indicated numbers of PBMCs or T cells.
Statistical Analysis.
Difference between the groups were calculated by using the Student’s t test for normally distributed variables and nonparametric Mann–Whitney test for nonnormally distributed variables. A P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01-NS48860; Chinese Academy of Sciences Project KSCX2-SW-212; Chinese Ministry of Science and Technology 863 Project 2002AA216121; Chinese National Natural Science Foundation Projects 30430650 and 30571731; and Shanghai Municipality Projects 04DZ14902, 04DZ19202, 04JC14040, 03XD14015, and T0206.
Abbreviations
- ELISPOT
enzyme-linked immunospot
- MBP
myelin basic protein
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cell.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ben-Nun A., Wekerle H., Cohen I. R. Nature. 1981;292:60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- 2.Lider O., Beraud E., Reshef T., Friedman A., Cohen I. R. J. Autoimmun. 1989;2:87–99. doi: 10.1016/0896-8411(89)90110-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J. Z., Medaer R., Stinissen P., Hafler D., Raus J. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J. Z., Vandevyver C., Stinissen P., Raus J. J. Immunol. 1995;155:5868–5877. [PubMed] [Google Scholar]
- 5.Correale J., Lund B., McMillan M., Ko D. Y., McCarthy K., Weiner L. P. J. Neuroimmunol. 2000;107:130–139. doi: 10.1016/s0165-5728(00)00235-6. [DOI] [PubMed] [Google Scholar]
- 6.Zang Y. C. Q., Hong J., Rivera V. M., Killian J., Zhang J. Z. J. Immunol. 2000;164:4011–4017. doi: 10.4049/jimmunol.164.8.4011. [DOI] [PubMed] [Google Scholar]
- 7.Lohse A. W., Mor F., Karin N., Cohen I. R. Science. 1989;244:820–822. doi: 10.1126/science.2471264. [DOI] [PubMed] [Google Scholar]
- 8.Zang Y. C. Q., Hong J., Rivera V. M., Killian J., Zhang J. Z. Int. Immunol. 2003;15:1073–1080. doi: 10.1093/intimm/dxg105. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J. Z. Expert Rev. Vaccines. 2002;1:285–292. doi: 10.1586/14760584.1.3.285. [DOI] [PubMed] [Google Scholar]
- 10.Zang Y. C. Q., Hong J., Tejada-Simon M. V., Li S., Rivera V. M., Killian J. M., Zhang J. Z. Eur. J. Immunol. 2000;30:908–913. doi: 10.1002/1521-4141(200003)30:3<908::AID-IMMU908>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Mimran A., Mor F., Carmi P., Quintana F. J., Rotter V., Cohen I. R. J. Clin. Invest. 2004;113:924–932. doi: 10.1172/JCI17772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen I. R., Quintana F. J., Mimran A. J. Clin. Invest. 2004;114:1227–1232. doi: 10.1172/JCI23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mor F., Reizis B., Cohen I. R., Steinman L. J. Immunol. 1996;157:4855–4861. [PubMed] [Google Scholar]
- 14.Quintana F. J., Carmi P., Mor F., Cohen I. R. J. Immunol. 2003;171:3533–3541. doi: 10.4049/jimmunol.171.7.3533. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J. Z., Rivera V. M., Tejada-Simon M. V., Yang D., Hong J., Li S., Haykal H., Killian J., Zang Y. C. Q. J. Neurol. 2002;249:212–218. doi: 10.1007/pl00007867. [DOI] [PubMed] [Google Scholar]
- 16.Viglietta V., Baecher-Allan C., Weiner H. L., Hafler D. A. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huan J., Culbertson N., Spencer L., Bartholomew R., Burrows G. G., Chou Y. K., Bourdette D., Ziegler S. F., Offner H., Vandenbark A. A. J. Neurosci. Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 18.Buenafe A. C., Tsaknaridis L., Spencer L., Hicks K. S., McMahan R. H., Watson L., Culbertson N. E., Latocha D., Wegmann K., Finn T., et al. J. Neurosci. Res. 2004;76:129–140. doi: 10.1002/jnr.20066. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V. J. Clin. Invest. 2004;114:1222–1226. doi: 10.1172/JCI23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Aa A., Hellings N., Medaer R., Gelin G., Palmers Y., Raus J., Stinissen P. Clin. Exp. Immunol. 2003;131:155–168. doi: 10.1046/j.1365-2249.2003.02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medaer R., Stinissen P., Truyen L., Raus J., Zhang J. Z. Lancet. 1995;346:807–808. doi: 10.1016/s0140-6736(95)91622-9. [DOI] [PubMed] [Google Scholar]
- 22.Achiron A., Lavie G., Kishner I., Stern Y., Sarova-Pinhas I., Ben-Aharon T., Barak Y., Raz H., Lavie M., Barliya T., et al. Clin. Immunol. 2004;113:155–160. doi: 10.1016/j.clim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V., Sercarz E., Zhang J. Z., Cohen I. R. Trends Immunol. 2001;22:539–540. doi: 10.1016/s1471-4906(01)02020-8. [DOI] [PubMed] [Google Scholar]
- 24.Manici S., Sturniolo T., Imro M. A., Hammer J., Sinigaglia F., Noppen C., Spagnoli G., Mazzi B., Bellone M., Dellabona P., Protti M. P. J. Exp. Med. 1999;189:871–876. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelhard V. H. Annu. Rev. Immunol. 1994;12:181–207. doi: 10.1146/annurev.iy.12.040194.001145. [DOI] [PubMed] [Google Scholar]
- 26.Schroers R., Huang X. F., Hammer J., Zhang J. Z., Chen S. Y. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.