Abstract
Vaccination against amyloid β-peptide (Aβ) has been shown to be successful in reducing Aβ burden and neurotoxicity in mouse models of Alzheimer's disease (AD). However, although Aβ immunization did not show T cell infiltrates in the brain of these mice, an Aβ vaccination trial resulted in meningoencephalitis in 6% of patients with AD. Here, we explore the characteristics and specificity of Aβ-induced, T cell-mediated encephalitis in a mouse model of the disease. We demonstrate that a strong Aβ-specific T cell response is critically dependent on the immunizing T cell epitope and that epitopes differ depending on MHC genetic background. Moreover, we show that a single immunization with the dominant T cell epitope Aβ10–24 induced transient meningoencephalitis only in amyloid precursor protein (APP)-transgenic (Tg) mice expressing limited amounts of IFN-γ under an myelin basic protein (MBP) promoter. Furthermore, immune infiltrates were targeted primarily to sites of Aβ plaques in the brain and were associated with clearance of Aβ. Immune infiltrates were not targeted to the spinal cord, consistent with what was observed in AD patients vaccinated with Aβ. Using primary cultures of microglia, we show that IFN-γ enhanced clearance of Aβ, microglia, and T cell motility, and microglia-T cell immunological synapse formation. Our study demonstrates that limited expression of IFN-γ in the brain, as observed during normal brain aging, is essential to promote T cell-mediated immune infiltrates after Aβ immunization and provides a model to investigate both the beneficial and detrimental effects of Aβ-specific T cells.
Keywords: amyloid βpeptide, encephalitis, vaccination
Alzheimer's disease (AD) is characterized by aging-associated deterioration of learning and memory functions of the brain. Affected regions of the brain exhibit accumulation and deposition of amyloid β-peptide (Aβ) and frequently the appearance of neurofibrillary tangles (1). Cleavage of amyloid precursor protein (APP) can yield either Aβ1–40 or Aβ1–42 (2). The amounts and the ratio between the two forms and their deposition in the brain are affected by mutations in the APP and presenilin genes or the presence of the ApoE4 allele (3, 4). Immunolabeling of extracellular Aβ in the brain reveals neuritic and diffuse plaques. The former are colocalized with activated microglia and astrocytes as well as degenerating neurons, whereas the latter do not clearly associate with glial activation or neurotoxicity (5). Recent findings also demonstrate a role for Aβ synaptotoxicity independent of plaques, possibly mediated by soluble Aβ oligomers at intra- and extracellular compartments (6–9).
Parenteral immunization of APP transgenic (Tg) mice with synthetic Aβ in adjuvant can markedly decrease the number and density of Aβ deposits in the brain, with concomitant improvement in neuritic dystrophy and gliosis (10, 11). Positive effects have also been found after repetitive mucosal (intranasal) administration of the Aβ peptide to Tg mice (12, 13). Passive transfer of Aβ antibodies has shown similar beneficial neuropathological effects (14–16); however, brain hemorrhage appears as a possible side effect of this approach if tested in mice with cerebral amyloid angiopathy (7).
The finding that active vaccination with Aβ had profound Aβ-lowering effects in an animal model of AD led to a clinical trial in which an Aβ1–42 synthetic peptide was administered parenterally with adjuvant to patients with mild to moderate AD. Although a phase I safety study in a small number of patients did not reveal significant side effects, a subsequent phase II trial was discontinued shortly after its initiation, when ≈6% of the treated patients developed meningoencephalitis (17). Nonetheless, a cohort of patients with AD vaccinated with Aβ have shown promising results, demonstrating slower decline of cognitive functions over a 1-year period, which was evident also in patients who experienced transient encephalitis (18). Postmortem analysis of brain sections revealed decreased Aβ plaques in neocortex regions associated with activated microglia and T cell infiltrates in the CNS, as compared with unimmunized patients with AD (19).
The meningoencephalitis observed after Aβ vaccination of patients with AD is postulated to be the result of activation of Aβ-reactive T cells in the periphery and their migration to Aβ plaques in the brain. Understanding the factors that are required to induce Aβ encephalitis are crucial for the development of Aβ vaccination approaches in AD. The present study addresses the conditions under which Aβ vaccination elicits T cell responses directed to Aβ plaques in the CNS, responses that result in temporary encephalitis and clearance of pathogenic forms of Aβ.
Results
Aβ Immunogenicity Is Determined by Epitope Specificity.
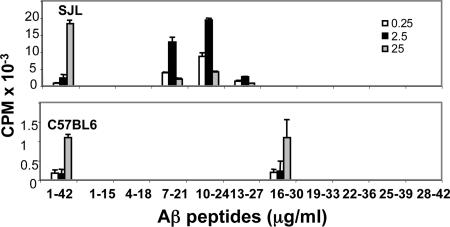
To analyze genetic control of Aβ immunogenicity, we immunized C57BL/6 and SJL mice (H2b and H2s MHC class II haplotypes, respectively) with human Aβ1–42 in complete Freund's adjuvant (CFA) and assessed proliferative responses in popliteal draining lymph nodes (LNs). Aβ-specific T cell proliferation was significantly higher in LNs from SJL than from C57BL/6 mice: 18,376 cpm versus 1,100 cpm, respectively (Fig. 1). To determine the specific Aβ T cell epitopes in each of the strains, T cell proliferation was measured by using 10 overlapping peptides of Aβ1–42. Peptides 7–21, 10–24, and 13–27 induced T cell proliferation in the SJL-derived lymphocyte cultures (Fig. 1), whereas only peptide 16–30 induced proliferation in C57BL/6 mice (Fig. 1). The highest proliferative response was obtained by using 2.5 and 25 μg/ml of Aβ peptide in SJL and C57BL/6 mice, respectively (Fig. 1). Responses to peptides 10–24 in SJL and 16–30 in C57BL/6 mice were equivalent to those induced by Aβ1–42 (Fig. 1). The low T cell reactivity in C57BL/6 mice results from a low-affinity T cell epitope that is presented by the specific I-Ab MHC class II allele (Fig. 5A, which is published as supporting information on the PNAS web site).
Fig. 1.
Aβ10–24 is a highly immunogenic T cell epitope in SJL and NOD mice compared with Aβ16–30 in C57BL/6. Mice were immunized parenterally once with human Aβ1–42 and, after 10 days, lymph nodes were excised from SJL and C57BL/6 mice and analyzed for T cell proliferation induced by Aβ1–42 and 10 overlapping Aβ peptides (each of 15 residues) as described in Materials and Methods.
Because residues 5, 10, and 13 of Aβ are different in rodent as compared with human (see Fig. 5), we sought to determine whether the high T cell responses obtained in SJL mice immunized with human Aβ1–42 were also specific to the self (rodent) Aβ peptide. Thus, SJL mice were immunized with rodent Aβ1–42, and T cell responses to rodent and human Aβ peptides were tested in vitro. Similar T cell proliferation was obtained when human or rodent Aβ7–21 and human or rodent Aβ10–24 were used as the stimulating peptide (Fig. 5B). In addition, there was gradual increase in T cell proliferation using Aβ7–21, Aβ8–22, Aβ9–23, and Aβ10–24 peptides as antigens (Fig. 5B), suggesting that the full-length Aβ T cell epitope in SJL mice is located between residues 10 and 24 and that immunization with either human or rodent Aβ1–42 evokes T cell responses to this peptide. We then measured recall T cell responses to human Aβ1–42 in vitro after immunization of SJL mice with human Aβ10–24. Lymph node-derived T cells from SJL mice immunized with Aβ10–24 proliferated in vitro when stimulated with human Aβ1–42 but not Aβ16–30 (Fig. 5C). Overall, these results demonstrate that different Aβ-specific CD4 T cell epitopes presented by different MHC class II alleles have a significant impact on Aβ immunogenicity.
Immunization with the T Cell Epitope Aβ10–24 Results in Transient Encephalitis in APP/IFN-γ Double Tg Mice.
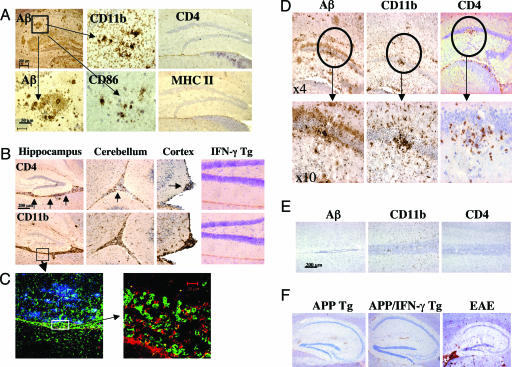
As shown in Fig. 1, the I-As but not I-AbMHC class II haplotype was essential to mount a significant Aβ-specific T cell response. To determine whether carrying the I-As allele was sufficient to induce T cell activation and migration of T cells to the CNS of APP-Tg mice, B6SJLF1 APP-Tg mice (line J20, see Materials and Methods) were immunized with Aβ10–24 (because APP-Tg mice carry the human gene; for the rest of the study, we have used only peptides homologous to human Aβ for immunization) in CFA followed by i.v. injection of pertussis toxin (PT) at the time of immunization and 48 h later. Aβ-specific T cell responses were as high in APP-Tg B6SJLF1 as in immunized SJL mice (data not shown). Infiltrates of CD4 cells were not observed in meningeal tissues or anywhere else in the brain of APP-Tg mice (Fig. 2A and Table 1, which is published as supporting information on the PNAS web site) or non-Tg controls (data not shown). In addition, no MHC class II expression was observed (Fig. 2A and Fig. 6A, which is published as supporting information on the PNAS web site). Immunostaining of brain sections from immunized APP-Tg mice showed a similar pattern to that observed in unimmunized APP-Tg mice, i.e., activated microglia at sites of compact Aβ plaques colocalized with expression of the T cell costimulatory molecule CD86 (Fig. 2A).
Fig. 2.
Immunization with the T cell epitope Aβ10–24 induces meningoencephalitis in APP/IFN-γ double Tg mice. (A) APP-Tg mice (9 months old, B6SJLF1 background) were immunized with Aβ10–24 and injected twice with PT (see Materials and Methods). Mice were killed 2 weeks later, and brain sections were analyzed by immunohistochemistry for Aβ, CD4, and MHC class II. Hematoxylin was used for counterstaining (shown in purple). High-power images show staining for Aβ, CD11b, and CD86 costimulation molecule, taken from the box shown in the Aβ Upper panel. (B–E) APP/IFN-γ Tg or single IFN-γ Tg mice were immunized with Aβ10–24 and injected with PT. At the time indicated, brain and spinal cord tissues were examined for immune infiltrates as described in Materials and Methods. (B) CD4 and CD11b immunostaining in hippocampus, cerebellum, and cortex regions of the CNS 12 days after immunization (see arrows for stained area). (C) High-power images of the meningeal area (taken from the box in B Lower) immunolabeled with antibodies to Aβ (blue), CD11b (green), and CD4 (red) and analyzed by a confocal microscope as described in Materials and Methods. (D) Aβ, CD4, and CD11b immunostaining in the hippocampus of APP/IFN-γ Tg mouse 20 days after immunization. (Lower) Higher-power versions of the circled area shown in the Upper panels. (E) Aβ, CD4, and CD11b immunostaining of spinal cord sections of APP/IFN-γ Tg mice 12 days after immunization with Aβ10–24. (F) Brain sections from nonimmunized APP and APP/IFN-γ Tg mice were immunostained with antibodies to fibrinogen. Brain sections from a mouse brain with EAE were used for a positive control staining.
We then examined the role of microglial activation on the ability of T cells to migrate to the brain and accumulate at sites of Aβ plaques. Although some microglial activation was observed in APP-Tg mice, it was not sufficient to support T cell infiltration. Because IFN-γ is known to up-regulate genes required for antigen processing and presentation (20), we locally activated microglia in the CNS by crossing SJL mice expressing IFN-γ in the CNS under a myelin basic protein (MBP) promoter (21) with APP-Tg mice. Immunization of APP/IFN-γ double Tg mice (9 months old) with Aβ10–24 resulted in a marked meningoencephalitis as early as 12 days after immunization, shown by immunolabeled CD4+ T cells and CD11b+ macrophages, primarily in the hippocampus, but also in the cortex and the cerebellum (Fig. 2B, see arrows). Three-color staining of sections of this time point showed Aβ deposits (blue) in the hippocampal region and accumulating macrophages (green) and T cells (red) in the adjacent meningeal tissues (Fig. 2C). Activated microglia and macrophages migrating from the meninges were colocalized with accumulated Aβ plaques (Fig. 2C). In IFN-γ single-Tg mice, a small number of infiltrating cells were observed only in the meninges (Table 1). In contrast to APP/IFN-γ Tg mice, these cells accumulated in meningeal space and did not migrate to the parenchymal tissue.
APP/IFN-γ Tg mice that were immunized with Aβ10–24 were also analyzed by immunohistochemistry 20, 30, and 60 days after immunization for immune infiltrates associated with Aβ plaques. In contrast to day 12 postimmunization, when CD4 T cells and CD11b macrophages were located primarily in meningeal tissues, on day 20, CD4 and CD11b cells were located at sites of Aβ plaques in the hippocampus, and fewer were observed in the meninges (Fig. 2D). These cells migrated primarily to compact Aβ plaques (Fig. 2D; circled area is magnified in the Lower panels), sites that were occupied by activated microglia before immunization. On day 30, reduced numbers of CD4 and CD11b infiltrates were detected in meningeal tissues of the brain, as well as at sites of Aβ plaques (Table 1). No infiltrates were observed at day 60 postimmunization (Table 1). Of note, immune infiltrates or Aβ were not observed in the spinal cord of these mice at any time (Fig. 2E). As shown in Table 1, CD4 and CD11b cell infiltrates were observed in brain sections of APP/IFN-γ double Tg mice immunized with Aβ10–24 but not in APP/IFN-γ Tg mice immunized with Aβ1–15 or BSA or in APP single-Tg mice having the same genetic background. Immune infiltrates also were not observed in Aβ10–24/CFA immunized APP/IFN-γ Tg mice that were not injected with PT (n = 3, data not shown). Overall, we demonstrate that Aβ10–24 immunization can induce temporary meningoencephalitis primarily targeted to sites of Aβ burden provided that IFN-γ is expressed in the brain. IFN-γ induced an immune milieu in the brain of APP/IFN-γ Tg mice essential to support a dialogue with the immune cells in the CNS and did not significantly reduce the integrity of the blood–brain barrier as compared with APP-Tg mice (Fig. 2F). Immunostaining of brain sections taken from a mouse with experimental autoimmune encephalomyelitis (EAE) is shown as a positive control (Fig. 2F).
Aβ10–24-Induced Encephalitis Is Mediated by Aβ-Specific T Helper (Th) 1 Cells.
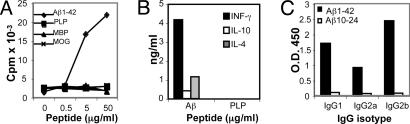
Peripheral immune responses to Aβ were characterized in vitro on days 14, 20, and 30 after Aβ immunization. Proliferation of spleen-derived T cells was induced by Aβ but not by myelin peptides derived from proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG), and MBP, all known to induce EAE in these mouse strains (Fig. 3A). High amounts of IFN-γ and low amounts of IL-4 and IL-10 were secreted by these Aβ-specific T cells, indicating that primarily a Th1 but not Th2 type of immune response was elicited (Fig. 3B). Serum isolated from Aβ10–24-immunized APP-Tg mice had no Aβ-binding antibodies, in contrast to Aβ1–42-immunized mice, in whom high serum titers of all three isotypes IgG1, IgG2a, and IgG2b were detected (Fig. 3C). Taken together, these data demonstrate that, upon immunization with the T cell epitope Aβ10–24, Aβ-reactive T cells migrate specifically to brain regions where Aβ is accumulated and trigger a proinflammatory response that lasts for ≈30 days. Because Aβ10–24 lacks the sites in which the dominant B cell epitopes are located, the immune response did not include production of Aβ antibodies after immunization.
Fig. 3.
Aβ10–24 immunization induced Aβ-specific Th1-type T cell proliferation and no Aβ antibodies in APP/IFN-γ Tg mice. APP/IFN-γ Tg mice were immunized with Aβ10–24, and spleen-derived T cells were analyzed in vitro for T cell proliferation, cytokine production, and Aβ antibodies on days 12, 20, and 30 after immunization as described in Materials and Methods. Representative results are shown for day 30 after immunization. (A) T cell proliferation induced by Aβ, PLP139–151, and MOG35–55 peptides and whole mouse MBP. (B) Supernatants collected from spleen-derived cultures incubated with 50 μg/ml Aβ or PLP were tested for IFN-γ, IL-10, and IL-4 production by sandwich ELISA assay. (C) Sera from APP/IFN-γ Tg or B6SJLF1 mice immunized with Aβ10–24 or human Aβ1–42, respectively, were analyzed for IgG1, IgG2a (allotypes a and b), and IgG2b Aβ antibodies. The data shown are representative results of five different experiments.
Clearance of Aβ in the Hippocampus Is Enhanced by Activated Microglia/Macrophages.
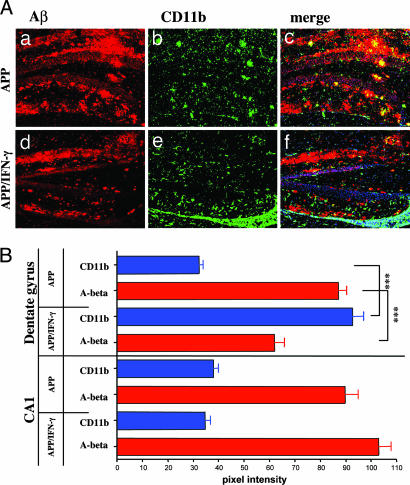
As shown in Figs. 2 and 3, a single immunization with Aβ10–24 resulted in a T cell-mediated encephalitis in APP/IFN-γ double Tg mice in the absence of Aβ antibodies. To determine whether this encephalitis was associated with enhanced clearance of Aβ, brain sections were analyzed for microglial activation colocalized with Aβ in the hippocampus. Nuclei in the brain were stained with the dimeric cyanine nucleic acid dye TOTO-3 iodide, and analysis was localized to the inflammation site below the dentate gyrus and the hippocampus CA1 region, which was relatively devoid of inflammation (Fig. 4). Brain sections of APP-Tg mice showed a few activated microglia (green) primarily at sites of neuritic plaques but not diffuse plaques (Fig. 4 Aa–Ac). In contrast, submeningeal tissues in the hippocampus of APP/IFN-γ mice immunized with Aβ10–24 had many highly activated microglia/macrophages that colocalized with Aβ deposits (Fig. 4 Ad–Af and Fig. 7A,which is published as supporting information on the PNAS web site). These deposits contained significantly decreased amounts of Aβ. Intensity analysis of Aβ and CD11b fluorescence staining in representative areas of the CA1 and the dentate gyrus is shown in Fig. 4B. These data demonstrate significant increased intensity of the CD11b staining and reduction in the intensity of Aβ staining at the site of inflammation in the area of the dentate gyrus of APP/IFN-γ Tg as compared with APP-Tg mice (Figs. 4B and 7B). No significant differences were observed in the CA1 regions between APP/IFN-γ and APP-Tg mice (Fig. 4B). The mean intensity of Aβ staining, at the frame shown in Fig. 4Aa, was calculated from five sections of the hippocampus in each mouse (see Materials and Methods). To further investigate the reduction of Aβ in brains of Aβ10–24-vaccinated APP/IFN-γ Tg mice, we measured the amounts of Aβ in brain extracts by ELISA. Amounts of Aβ1–40 (ng/g wet tissue) were 67 ± 28 (n = 4) and 831 ± 161 (n = 3) in immunized vs. control APP/IFN-γ Tg mice (P = 0.043), respectively. Amounts of Aβ1–42 (ng/g wet tissue) were 658 ± 176 and 2,460 ± 18 in immunized vs. control APP/IFN-γ Tg mice (P = 0.0021), respectively. Thus, immune infiltrates in the brain associated with microglia/macrophage activation can result in clearance of Aβ, and this clearance seems to be more efficient for Aβ1–40 than for Aβ1–42. Because Aβ10–24 immunization did not evoke antibody production up to 3 weeks after immunization, it is suggested that this clearance was antibody-independent.
Fig. 4.
Aβ burden is decreased in hippocampus regions of APP/IFN-γ Tg mice immunized with Aβ10–24. (A) APP or APP/IFN-γ Tg mice were immunized with Aβ10–24 at 9 months of age. Six-micrometer brain sections were taken from the hippocampus and were immunolabeled for CD11b (green) and Aβ (red). TOTO-3 nuclei dye (blue) was used for counterstaining. Brain sections were analyzed by confocal microscopy as described in Materials and Methods. (Aa–Af) Two separate images and their merged image, showing the hippocampal–meningeal area of APP (Upper) and APP/IFN-γ (Lower) Tg mice taken at identical exposures. (B) Intensity representation of the Aβ and CD11b images shown in panels Aa and Ab and Ad and Ae was performed by using Zeiss lsm 510 software as described in Materials and Methods.
IFN-γ Enhances Microglia Motility and Uptake of Aβ, and Microglia-Induced T Cell Activation.
The meningoencephalitis we observed was followed by clearance of Aβ and was completely dependent on expression of IFN-γ in the CNS. We therefore established an in vitro system to examine the effect of IFN-γ on uptake of Aβ by microglia and early events associated with microglia-induced T cell activation. Aβ uptake (green in Fig. 8A, which is published as supporting information on the PNAS web site) was significantly enhanced in microglia pretreated with IFN-γ as compared with untreated cultures as indicated by the fluorescence images (Fig. 8A) and the related intensity analysis (Fig. 8B). Furthermore, filipodia formation as an indication of microglia motility was longer and occurred with higher frequency in the IFN-γ-treated cultures. (Fig. 8C).
We then cocultured IFN-γ-treated or -untreated microglia with resting Aβ-reactive T cells and examined T cell motility and immunological synapse formation in the presence of Aβ1–42 as an antigen. We demonstrate that IFN-γ not only increases uptake of Aβ by microglia, but also activates microglia to facilitate T cell motility and synapse formation (Movie 1 and Fig. 9, which are published as supporting information on the PNAS web site).
Discussion
The present study addresses the conditions under which Aβ vaccination elicits T cell responses directed at Aβ plaques in the CNS. We demonstrate that Aβ becomes an encephalitogenic antigen when three conditions are met: (i) when Aβ is present in the brain as occurs with aging; (ii) when the genetic background is conducive for T cell response to a high-affinity Aβ T cell epitope; and (iii) when there is a proinflammatory signal such as IFN-γ in the CNS. Of note, the immune infiltrates in the CNS we observed after Aβ vaccination were transient and were targeted primarily to sites of Aβ plaques.
In the present study, we demonstrate that Aβ is highly immunogenic in SJL and nonobese diabetic (NOD) mice and that the dominant T cell epitope resides between residues 10 and 24 of Aβ. In contrast, Aβ evokes a weak T cell response in C57BL/6 mice, as has been previously suggested (22) for which the T cell epitope is between residues 16 and 30. NOD congenic mice bearing the I-Abclass II allele also failed to mount strong T cell responses, suggesting that the low immunogenicity of Aβ16–30 in C57BL/6 is a result of a low-affinity epitope selected by the I-AbMHC class II and not because of T cell selection in the thymus. Overall, in this study and in our previous study (23), we found that the T cell epitopes in mice were localized between Aβ residues 7 and 30, and that no T cell responses were evoked by immunization with Aβ1–15 (23). An Aβ T cell epitope between residues 1 and 16 was detected in HLA-DR3/DQ8 double Tg but not in HLA-DR3 or HLA-DQ8 single Tg mice (22). In BALB/c, an Aβ T cell epitope was mapped between residues 6 and 20 (24). However, because T cell responses to Aβ10–24 were not measured, it is not clear whether Aβ6–20 is a new epitope or a partial epitope similar to Aβ7–21 detected in this study in SJL mice. In summary, MHC class II alleles are crucial in determining the strength and phenotype of the adaptive immune response evoked to Aβ after immunization.
Despite the high frequency of Aβ-reactive T cells induced after immunization, we did not observe encephalitis in APP-Tg mice (B6SJLF1 background), even though Aβ had accumulated in the brain and had induced microglial activation and expression of the CD86 costimulatory molecule at sites of Aβ plaques. A mild encephalomyelitis was reported in normal C57BL/6 mice after Aβ immunization using large amounts of PT; APP-Tg mice were not studied (25). In the current study, we found weak T cell reactivity to Aβ in the C57BL/6 strain as reported by others (13, 22) and did not observe encephalomyelitis. Nonetheless, meningoencephalitis in the hippocampus, cortex, and cerebellum was detected when we immunized APP/IFN-γ Tg mice. During the entire encephalitis course, T cell responses in the periphery were observed only to Aβ1–42, and only slight T cell proliferation was induced by the myelin antigens PLP139–151, MOG35–55, and whole MBP, suggesting that antigen spreading did not occur. Aβ-reactive T cells migrated primarily, but not only, to sites of Aβ accumulation and triggered transient inflammation. No inflammation was observed in the spinal cord as occurs in EAE after myelin immunization (26). We have observed no infiltrates in nonimmunized APP/IFN-γ Tg mice or after BSA or Aβ1–15 immunization, and PT was required to evoke the meningoencephalitis induced by Aβ10–24/CFA immunization. Because the blood–brain barrier was not disrupted to allow spontaneous or nonspecific T cell infiltrates, these data suggest that IFN-γ changed the milieu at sites of Aβ plaques to support migration of Aβ-specific T cells once they were activated in the periphery. It remains to be investigated whether parenchymal microglia, endothelial, and/or peripheral migrating dendritic cells (DCs) served as Aβ antigen-presenting cells (APCs) in the brain to support such migration and interaction with T cells (27), which seem to be dominantly suppressed in APP-Tg mice.
IFN-γ is a key cytokine that induces microglia differentiation to mature professional APCs (reviewed in ref. 28). A recent study has shown that IFN-γ promotes a genetic program in microglia cells toward their function as APCs (20). IFN-γ also affects immune cell trafficking into the CNS by means of regulation of chemokines in the CNS (29). As shown in the present study, IFN-γ indeed facilitates microglia motility and uptake of Aβ, as well as T cell motility and immunological synapse formation. These characteristics of IFN-γ promote the T cell responses in the brain observed in this study. Of note, it is unclear whether IFN-γ is expressed in the adult CNS (30); however, in aged mice, increased mRNA levels of IFN-γ, MHC class II, CD86, and CIITA, associated with decreased IL-10, were recently demonstrated, suggesting a shift toward a more proinflammatory environment in the brain (31). In the AD brain, there is primarily an innate immune response including activation of complement, secretion of the proinflammatory cytokines IL-1β, TNF-α, and IL-6, and the secretion of nitric oxide (NO) (32–37). However, microglia express IFN-γ receptors and, upon exposure to IFN-γ, as occurred in APP/IFN-γ Tg mice, they readily differentiate into professional APCs and thus promote a self-limited encephalitis after Aβ vaccination. Exposure of the brain to IFN-γ also may occur after viral or bacterial infection, predisposing to higher state of microglia activation in general and at sites of Aβ plaques in particular. It is worth notice that, at 9–10 months of age, expression of IFN-γ by itself in APP-Tg mice, although it changed the vulnerability of the brain to immune infiltrates, did not significantly affect the amounts of Aβ plaques.
The meningoencephalitis we observed in APP/IFN-γ Tg mice was similar to that which occurred in patients with AD vaccinated with Aβ (17, 19). The current study strongly suggests that the meningoencephalitis observed in AD patients after vaccination with Aβ1–42 was due to activation of Aβ-specific T cells. Using sensitive methods, we have found clearly increased Aβ-reactive T cells in elderly healthy individuals and patients with AD as compared with adult healthy individuals (38). The T cell reactivity in a subset of patients was particularly high and may explain why meningoencephalitis was induced only in 6% of the patients. We have further characterized these responses to be associated with certain HLA alleles and T cell epitopes (unpublished results). Immune infiltrates, however, were not observed in APP-Tg mice, and the question is whether a substantial difference exists between human and mice with regard to microglia activation and the dialogue with autoreactive Aβ T cells. In contrast to the case with APP-Tg mice, microglia in AD seem to be in a higher state of activation as they express significant amounts of MHC class II at sites of Aβ plaques (39, 40). This difference could be due to environmental factors and/or a genetic polymorphism that predisposes to a proinflammatory milieu in the human brain (37), possibly as part of immune surveillance of the CNS (41). It has not yet been reported whether AD patients with meningoencephalitis expressed IFN-γ in the affected regions. Furthermore, the number of T cells was increased in the brains of patients with AD compared with other neurodegenerative diseases and control individuals, analyzed postmortem (42). The phenotype of these T cells indicates that they were activated but not fully differentiated.
This finding raises the possibility that, in contrast to APP-Tg mice, the pathogenicity of Aβ in humans carrying certain HLA alleles triggers Aβ-specific T cell responses with yet unknown function. However, Aβ vaccination with the QS21 adjuvant could presumably induce a robust expansion of these cells in the periphery and consequently their accumulation in the CNS.
The meningoencephalitis observed in our study involved migration of T cells primarily to brain regions where Aβ is accumulated, similar to that observed in postmortem analysis of AD patients vaccinated with Aβ (19). This migration of T cells resulted in a substantial migration of macrophages, which further enhanced the proinflammatory response first in the meningeal tissue and subsequently in the parenchyma and facilitated the clearance of Aβ plaques. In contrast to the Aβ vaccination trial, Aβ antibodies were not induced in our study and thus clearance was B cell-independent although the role of macrophages and microglia remains to be elucidated. B cell-independent clearance of Aβ was also demonstrated recently by using nasal vaccination with a proteosome-based adjuvant and glatiramer acetate (43). Clearance was associated with microglia/macrophage activation induced directly by the adjuvant and T cells specific to glatiramer acetate. Taken together, although T cell responses in the brain can be detrimental (44, 45), in some instances, they are beneficial, as previously demonstrated in animal models of brain trauma (46, 47) or multiple sclerosis (48), and raise the possibility that some of the beneficial effects observed in the human trial were related to the induction of Aβ-reactive T cells, which can direct protective microglia/macrophage activation in the brain (49–51). Our study demonstrates that Aβ-reactive T cells can directly facilitate the clearance of Aβ and provides a model to investigate both their beneficial and detrimental outcomes after immunization.
Materials and Methods
Mice.
C57BL/6 and SJL mice were purchased from The Jackson Laboratory. NOD mice were purchased from Taconic Farms. APP-Tg J20 line in a C57BL/6 background expressing APP under the PDGF promoter were received from L. Mucke (6). Transgenic SJL mice expressing IFN-γ under the MBP promoter were received from T. Owens (21). Homozygous IFN-γ-Tg mice were bred with APP-Tg mice to generate double Tg B6SJLF1 mice. All APP/IFN-γ Tg mice and their control littermates were immunized at 9 months of age.
Antigens.
Aβ1–40 and Aβ1–42 peptides were synthesized in the Biopolymer Laboratory (Center for Neurologic Diseases, Brigham and Women's Hospital). FITC-labeled Aβ was purchased from BioSource International (Camarillo, CA). All other Aβ peptides, MOG35–55 peptide, and PLP139–151 peptide were synthesized by Quality Control Biochemicals (Hopkington, MA). For in vitro stimulation of lymphocytes, Aβ peptides were dissolved in DMSO (Sigma) at 2 mg/ml before final dilution in X-vivo media (BioWhittaker). MOG35–55, MBP, and PLP were dissolved in distilled water at 2 mg/ml. For immunization, Aβ peptides were dissolved in distilled water at 2 mg/ml.
Immunization and Measurement of Immune Responses.
Mice were immunized by footpad injection if killed on day 12; for longer periods, mice were injected in the flanks. At the indicated time points, popliteal draining lymph nodes (PLN) or spleens were excised and tested in vitro for antigen-induced proliferation and cytokine production. Antigen-induced cytokine production was measured by sandwich ELISA. Anti-Aβ antibodies in serum were measured by ELISA as described (23).
Immunohistochemistry and Confocal Imaging Analysis.
Sagital sections (6 μm) were taken to include full representation of the hippocampus and the dentate gyrus. Sections were examined under a Zeiss Laser Scanning Confocal Microscope. We assessed at least three sections per animal evenly spaced across a 2-mm-wide region of the hippocampus, which provides full representation of the dentate gyrus and CA1 regions in the brain. We have used two methods of Aβ quantification: (i) Intensity analysis of the entire hippocampus was performed by using 3D image analysis software (Zeiss). (ii) To obtain numerical values of fluorescence intensity, we have used the region of interest (ROI) feature of the advance imaging lsm software. We defined areas of 300 μm3 that comprised the entire neuropil or hippocampus. Twenty such areas were defined for three noncontiguous slides of each mouse and were analyzed at the same acquisition parameters. The pixel intensity of all areas in individual channels was obtained for statistical analysis by using one-way ANOVA with Bonferroni correction. These results were confirmed by measuring the covariance of both channels (CD11b and Aβ) in each area (see also supporting information).
Preparation of Cultures of Mouse Brain Microglia.
Glial cultures were prepared as described (52). On day 7, cultures were incubated with 100 pg/ml IFN-γ for 72 h, and, on day 10, the entire glial culture was trypsinized and microglia were labeled with phycoerythrin (PE)-conjugated anti-CD11b and sorted by using a FACS Vantage SE Cell Sorter.
Detection of Aβ by ELISA.
Brain tissues were homogenized in ice-cold 5 M guanidine thiocyanate/HCL, pH 8, and were then rotated at room temperature for 4 h. The homogenates were stored at −80°C. Brain homogenates were diluted and centrifuged, and Aβ levels were measured by ELISA according to the manufacturer's instructions (BioSource International).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health, the Alzheimer's Association, and the National Institute of Biotechnology Ben-Gurion University of the Negev.
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid β-peptide
- APP
amyloid precursor protein
- Tg
transgenic
- CFA
complete Freund's adjuvant
- MBP
myelin basic protein
- EAE
experimental autoimmune encephalomyelitis
- Th
T Helper
- PLP
proteolipid protein
- MOG
myelin oligodendrocyte glycoprotein
- NOD
nonobese diabetic
- APC
antigen-presenting cell
- PT
pertussis toxin.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Selkoe D. J. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z., Nadeau P., Song W., Donoviel D., Yuan M., Bernstein A., Yankner B. A. Nat. Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- 3.Price D. L., Sisodia S. S. Annu. Rev. Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D. J. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., Cooper N. R., Eikelenboom P., Emmerling M., Fiebich B. L., et al. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeifer M., Boncristiano S., Bondolfi L., Stalder A., Deller T., Staufenbiel M., Mathews P. M., Jucker M. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 8.Walsh D. M., Selkoe D. J. Protein Pept. Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- 9.Wu C. C., Chawla F., Games D., Rydel R. E., Freedman S., Schenk D., Young W. G., Morrison J. H., Bloom F. E. Proc. Natl. Acad. Sci. USA. 2004;101:7141–7146. doi: 10.1073/pnas.0402147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., et al. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 11.Morgan D., Diamond D. M., Gottschall P. E., Ugen K. E., Dickey C., Hardy J., Duff K., Jantzen P., DiCarlo G., Wilcock D., et al. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 12.Weiner H. L., Lemere C. A., Maron R., Spooner E. T., Grenfell T. J., Mori C., Issazadeh S., Hancock W. W., Selkoe D. J. Ann. Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 13.Spooner E. T., Desai R. V., Mori C., Leverone J. F., Lemere C. A. Vaccine. 2002;21:290–297. doi: 10.1016/s0264-410x(02)00464-4. [DOI] [PubMed] [Google Scholar]
- 14.Bard F., Cannon C., Barbour R., Burke R. L., Games D., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., et al. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 15.Dodart J. C., Bales K. R., Gannon K. S., Greene S. J., DeMattos R. B., Mathis C., DeLong C. A., Wu S., Wu X., Holtzman D. M., Paul S. M. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 16.McLaurin J., Cecal R., Kierstead M. E., Tian X., Phinney A. L., Manea M., French J. E., Lambermon M. H., Darabie A. A., Brown M. E., et al. Nat. Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 17.Orgogozo J. M., Gilman S., Dartigues J. F., Laurent B., Puel M., Kirby L. C., Jouanny P., Dubois B., Eisner L., Flitman S., et al. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 18.Hock C., Konietzko U., Streffer J. R., Tracy J., Signorell A., Muller-Tillmanns B., Lemke U., Henke K., Moritz E., Garcia E., et al. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 19.Nicoll J. A., Wilkinson D., Holmes C., Steart P., Markham H., Weller R. O. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 20.Moran L. B., Duke D. C., Turkheimer F. E., Banati R. B., Graeber M. B. Neurogenetics. 2004;5:95–108. doi: 10.1007/s10048-004-0172-5. [DOI] [PubMed] [Google Scholar]
- 21.Renno T., Taupin V., Bourbonniere L., Verge G., Tran E., De Simone R., Krakowski M., Rodriguez M., Peterson A., Owens T. Mol. Cell. Neurosci. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- 22.Das P., Chapoval S., Howard V., David C. S., Golde T. E. Neurobiol. Aging. 2003;24:969–976. doi: 10.1016/s0197-4580(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 23.Monsonego A., Maron R., Zota V., Selkoe D. J., Weiner H. L. Proc. Natl. Acad. Sci. USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cribbs D. H., Ghochikyan A., Vasilevko V., Tran M., Petrushina I., Sadzikava N., Babikyan D., Kesslak P., Kieber-Emmons T., Cotman C. W., Agadjanyan M. G. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furlan R., Brambilla E., Sanvito F., Roccatagliata L., Olivieri S., Bergami A., Pluchino S., Uccelli A., Comi G., Martino G. Brain. 2003;126:285–291. doi: 10.1093/brain/awg031. [DOI] [PubMed] [Google Scholar]
- 26.Waldner H., Whitters M. J., Sobel R. A., Collins M., Kuchroo V. K. Proc. Natl. Acad. Sci. USA. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greter M., Heppner F. L., Lemos M. P., Odermatt B. M., Goebels N., Laufer T., Noelle R. J., Becher B. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 28.Aloisi F. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 29.Tran E. H., Prince E. N., Owens T. J. Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 30.Jensen M. B., Hegelund I. V., Lomholt N. D., Finsen B., Owens T. J. Neurosci. 2000;20:3612–3621. doi: 10.1523/JNEUROSCI.20-10-03612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank M. G., Barrientos R. M., Biedenkapp J. C., Rudy J. W., Watkins L. R., Maier S. F. Neurobiol. Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.03.013. in press. [DOI] [PubMed] [Google Scholar]
- 32.Bradt B. M., Kolb W. P., Cooper N. R. J. Exp. Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Scarano F., Baltuch G. Annu. Rev. Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 34.El Khoury J., Hickman S. E., Thomas C. A., Cao L., Silverstein S. C., Loike J. D. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 35.Husemann J., Loike J. D., Kodama T., Silverstein S. C. J. Neuroimmunol. 2001;114:142–150. doi: 10.1016/s0165-5728(01)00239-9. [DOI] [PubMed] [Google Scholar]
- 36.Smits H. A., de Vos N. M., Wat J. W., van der Bruggen T., Verhoef J., Nottet H. S. J. Neuroimmunol. 2001;115:144–151. doi: 10.1016/s0165-5728(01)00254-5. [DOI] [PubMed] [Google Scholar]
- 37.Eikelenboom P., van Gool W. A. J. Neural Transm. 2004;111:281–294. doi: 10.1007/s00702-003-0055-1. [DOI] [PubMed] [Google Scholar]
- 38.Monsonego A., Zota V., Karni A., Krieger J. I., Bar-Or A., Bitan G., Budson A. E., Sperling R., Selkoe D. J., Weiner H. L. J. Clin. Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattiace L. A., Davies P., Dickson D. W. Am. J. Pathol. 1990;136:1101–1114. [PMC free article] [PubMed] [Google Scholar]
- 40.Perlmutter L. S., Scott S. A., Barron E., Chui H. C. J. Neurosci. Res. 1992;33:549–558. doi: 10.1002/jnr.490330407. [DOI] [PubMed] [Google Scholar]
- 41.Hickey W. F. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 42.Togo T., Akiyama H., Iseki E., Kondo H., Ikeda K., Kato M., Oda T., Tsuchiya K., Kosaka K. J. Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 43.Frenkel D., Maron R., Burt D. S., Weiner H. L. J. Clin. Invest. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitsch R., Pohl E. E., Smorodchenko A., Infante-Duarte C., Aktas O., Zipp F. J. Neurosci. 2004;24:2458–2464. doi: 10.1523/JNEUROSCI.4703-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones T. B., Ankeny D. P., Guan Z., McGaughy V., Fisher L. C., Basso D. M., Popovich P. G. J. Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moalem G., Leibowitz-Amit R., Yoles E., Mor F., Cohen I. R., Schwartz M. Nat. Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 47.Yoles E., Hauben E., Palgi O., Agranov E., Gothilf A., Cohen A., Kuchroo V., Cohen I. R., Weiner H., Schwartz M. J. Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerschensteiner M., Stadelmann C., Dechant G., Wekerle H., Hohlfeld R. Ann. Neurol. 2003;53:292–304. doi: 10.1002/ana.10446. [DOI] [PubMed] [Google Scholar]
- 49.Butovsky O., Talpalar A. E., Ben-Yaakov K., Schwartz M. Mol. Cell. Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A. E., Pluchino S., Martino G., Schwartz M. Mol. Cell. Neurosci. 2005;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Morgan D., Gordon M. N., Tan J., Wilcock D., Rojiani A. M. J. Neuropathol. Exp. Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- 52.Monsonego A., Imitola J., Zota V., Oida T., Weiner H. L. J. Immunol. 2003;171:2216–2224. doi: 10.4049/jimmunol.171.5.2216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.