Abstract
Type 1 diabetes is characterized by the infiltration of inflammatory cells into pancreatic islets of Langerhans, followed by the selective and progressive destruction of insulin-secreting beta cells. Islet-infiltrating leukocytes secrete cytokines such as IL-1β and IFN-γ, which contribute to beta cell death. In vitro evidence suggests that cytokine-induced activation of the transcription factor NF-κB is an important component of the signal triggering beta cell apoptosis. To study the in vivo role of NF-κB in beta cell death, we generated a transgenic mouse line expressing a degradation-resistant NF-κB protein inhibitor (ΔNIκBα), acting specifically in beta cells, in an inducible and reversible manner, by using the tet-on regulation system. In vitro, islets expressing the ΔNIκBα protein were resistant to the deleterious effects of IL-1β and IFN-γ, as assessed by reduced NO production and beta-cell apoptosis. This effect was even more striking in vivo, where nearly complete protection against multiple low-dose streptozocin-induced diabetes was observed, with reduced intraislet lymphocytic infiltration. Our results show in vivo that beta cell-specific activation of NF-κB is a key event in the progressive loss of beta cells in diabetes. Inhibition of this process could be a potential effective strategy for beta-cell protection.
Keywords: apoptosis, cytokine, diabetes, transgenic mice, insulin
Type 1 diabetes mellitus is an autoimmune disease characterized by an inflammatory response resulting in selective and progressive destruction of the insulin-secreting beta cells in the pancreas. The immune reaction against beta cells is a complex process involving both cellular and humoral elements of cytotoxicity (1–5). Proinflammatory cytokines, such as IL-1β and IFN-γ and the free radical nitric oxide (NO), play an important role in the initial destruction of beta cells, leading to the development of diabetes (2, 5–7). Beta cells are particularly sensitive to damage induced by the immune system, due at least in part to their low expression levels of cytoprotective enzymes (8–10). Although functional impairments of the beta cells are induced shortly after exposure to cytokines, apoptosis is detected only after several days of their coculture with IL-1β and INF-γ (6, 11–16). These findings indicate that an active process is taking place at the beta-cell level, a race between deleterious and protective mechanisms where the deleterious effects eventually prevail, leading to beta cell death and type 1 diabetes mellitus. Cumulative evidence suggests that transcription factor NF-κB is an important cellular signal in initiating the cascade of events culminating in beta-cell death (16, 17). NF-κB has been shown to regulate the expression of numerous genes that play important roles in cellular stress responses, cell growth, survival, and apoptosis (18, 19). As such, the specificity and temporal control of gene expression by NF-κB are of crucial physiological significance. Furthermore, realization of NF-κB's potential as a drug target for type 1 diabetes mellitus prevention depends on an understanding of the mechanism(s) governing NF-κB-controlled gene expression in beta cells.
Five related mammalian gene products participate in NF-κB functions: RelA/p65, cRel, RelB, p50 (a processing product of p105), and p52 (a processing product of p100). NF-κB/Rel proteins exist as homodimers or heterodimers, but the predominant species in most cell types is the p65:50 heterodimer. NF-κB/Rel proteins share a highly conserved 300-aa-long N-terminal Rel homology domain, which is responsible for DNA binding, dimerization, and association with the inhibitor of NF-κB (IκB) proteins (20). In resting cells, most of the NF-κB/Rel dimers are bound to three major IκB isoforms (IκBα, IκBβ, and IκBε) in the cytoplasm, preventing their nuclear translocation and DNA association. Signals from various stimuli are transduced to a specific IκB kinase complex (18, 21), which phosphorylates the N-terminal domain of the IκBs, tagging them for polyubiquitination. Upon ubiquitination, the IκB proteins are rapidly degraded by the proteosome, thereby freeing NF-κB, which then enters the nucleus, binds to DNA and activates transcription.
To study the role of NF-κB in mediating the deleterious effects of cytokines in beta cells, an “IκB superrepressor” (also known as a dominant negative IκB-DNIκBα) capable of inhibiting NF-κB translocation to the nucleus has been used. In rat beta cells, this blockade prevented the cytokine-induced expression of deleterious genes, protecting, to a large extent, beta cells from apoptosis (22). A similar approach was used to protect human and mouse islets from the detrimental effect of IL-1β (23). These results strongly suggest that NF-κB plays an important proapoptotic role in cytokine-induced beta cell destruction, which is at odds with studies in other cell types where NF-κB has mostly an antiapoptotic effect (19, 20). Of note, a transgenic mouse model expressing such a dominant negative IκB throughout pancreatic development under the control of the Pdx-1/Ipf1 promoter was recently reported (24). The adult mice were hyperglycemic and had altered glucose-stimulated insulin secretion, suggesting that the prolonged blockade of NF-κB, initiated during embryonic pancreas development, both reduced the expression of key genes in the insulin secretion pathway and reduced the total number of endocrine cells in the adult pancreas.
To clarify the role of NF-κB in beta cells, we developed an in vivo model system where NF-κB signaling is specifically inhibited in beta cells. To this end, we generated a transgenic mouse designated ToI-β (for Tet-On ΔIκB in beta cells), in which a dominant negative form of IκBα (ΔNIκBα) is expressed in beta cells under the control of the tetracycline (on/off) gene regulatory system. This dominant inhibitor was described as affecting the entire family of NF-κB/Rel transcription factors (25). This approach enabled us to control the timing and duration of the expressed inhibitor and investigate the role of NF-κB in beta cell death both in vitro and in vivo.
In this report, we show that inhibition of the NF-κB pathway protects pancreatic beta cells from cytokine-induced apoptosis in vitro and in vivo from multiple low-dose streptozocin (STZ)-induced diabetes. Our results highlight the key role played by NF-κB in beta-cell destruction and progression to diabetes and suggest a potential effective strategy for beta cell protection.
Results
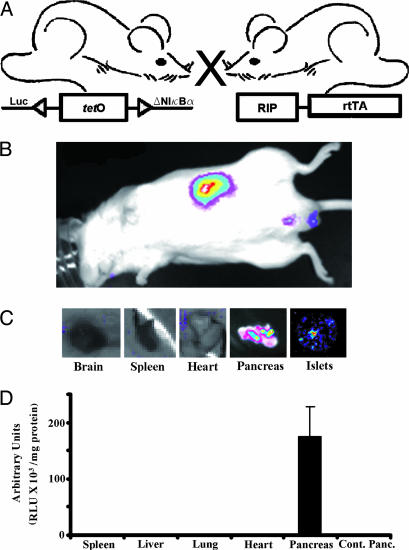
Selective beta cell inhibition of the NF-κB pathway is achieved by administration of the tetracycline analog doxycycline (Dox) to double transgenic mice (RIP7-rtTA/ΔNIκBα-Luc) carrying the inversely positioned ΔNIκBα (the N-terminal deleted IκBα) and luciferase genes (ΔNIκBα-Luc) and the reverse tetracycline transactivator (rtTA) under the control of the rat insulin promoter (Fig. 1A). These mice develop normally, and no differences were observed between them and the nontransgenic animals. When Dox is administered in the drinking water, rtTA is specifically expressed in beta cells, where it induces the expression of ΔNIκBα-Luc genes, resulting in both blockade of NF-κB nuclear translocation and expression of the luciferase gene. Bioluminescence is specifically detected in (i) the pancreas of anesthetized live animals (Fig. 1B), (ii) the excised pancreas and isolated islets (Fig. 1C) after luciferin injection and by using a light-detection cooled and charged-coupled device camera, and (iii) in vitro by luciferase assay (Fig. 1D). To determine the site and levels of ΔNIκBα expression in double-transgenic mice, Western blot analysis was performed by using cell lysates prepared from different organs, including pancreatic islets isolated from animals treated with Dox for 3 days. These lysates were compared with islet lysates from untreated mice from the same litter. Fig. 2 shows a strong band corresponding to the induced ΔNIκBα protein detected only in islets of Dox-treated animals (lane 2) and not in other tested organs (spleen, liver, and lung) (lanes 3–5) or in untreated islets (lane 1) where only expression of the wild-type IκBα was observed. To assess the conditional and reversible expression of ΔNIκBα protein, we followed its levels at different time points (1, 3, 7, 21, and 64 days) in islets Dox-treated mice (Fig. 8A, which is published as supporting information on the PNAS web site). Although higher expression was observed after 3 days of exposure to Dox as compared with 1 day, the levels were roughly similar for up to 64 days. On the other hand, when Dox administration was discontinued for 7 days after a 3-day period of treatment, the expression of the transgene was shut off and only the endogenous IκBα was detected, i.e., as in untreated animals.
Fig. 1.
Conditional expression of a mutant IkBα transgene in mouse beta cells. (A) The tet-on regulated system. Left, mouse with a transgene consisting of an inversely positioned ΔNIκBα and luciferase (Luc) genes, regulated by a bi-directional tetracycline operator-based promoter (tetO). Right, mouse with a transgene consisting of a reverse tetracycline-regulated transactivator (rtTA) under the control of the rat insulin II promoter (RIP). Cross-breeding of the two transgenic lines yielded a double-transgenic mouse (ToI-β mouse, for Tet-On ΔIκB in beta cells) capable of beta cell-specific expression of the ΔNIκBα-luciferase transgene. When rtTA is produced after administration of Dox, it binds specifically and with high affinity to tetO and activates transcription of both target genes (tet-on system). (B) Tissue-specific expression of the ΔNIκBα-luciferase transgene. Recorded image of the luciferase gene expression is shown, using a light detection cooled and charged coupled device camera. Double-transgenic mice treated with Dox for 3 days were injected with luciferin, anesthetized, and monitored for emitted photons and the bioluminescence signal in the mouse originates in the pancreas (n = 3). (C) Bioluminescence in the excised pancreas and isolated islets as compared with that in brain, spleen, and heart (n = 3). The intensity of the signal is represented by a range of “pseudocolors” (blue, least intense; red, most intense) corresponding to the luminescent organ at a gain point. (D) Tissue expression of the transgene measured by the luciferase assay (n = 5; RLU, relative light units/mg of protein).
Fig. 2.
Islet-specific expression of the ΔNIκBα gene in transgenic mice. Lysates from tissues (+) (lanes 3–5) or from isolated islets from double-transgenic mice treated with Dox for 3 days (+) (lane 2) or untreated (−) (lane 1) were tested for ΔNIκBα expression by Western blot analysis by using antibodies against the C-terminal domain of IkBα.
The impact of NF-κB blockade on the response of ToI-β mice to an acute glucose challenge showed that glucose tolerance (Fig. 9A, which is published as supporting information on the PNAS web site) and glucose-stimulated insulin secretion (Fig. 9B) were similar in the Dox-treated mice and untreated mice. Of note, glucose tolerance and blood glucose levels in fasting or fed mice did not differ statistically between the two groups of animals, even after 50 days of induction with Dox (data not shown). Finally, immunohistochemical analysis revealed a similar expression pattern of insulin, glucagon, and the transcription factors PDX-1, PAX6, and Nkx6.1 for both Dox-treated (+) and untreated (−) animals (Fig. 10, which is published as supporting information on the PNAS web site).
Inhibition of Cytokine-Induced NF-κB Nuclear Translocation in Dox-Treated Pancreatic Islets from ToI-β Mice.
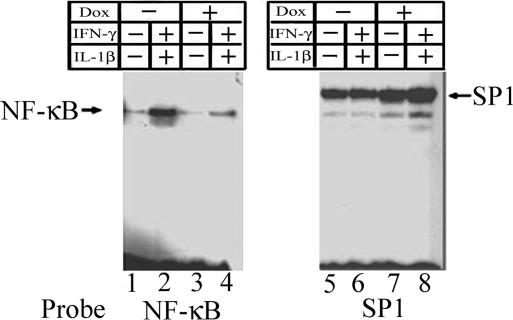
To examine the efficiency of ΔNIκBα in inhibiting cytokine-induced NF-κB translocation after exposure to cytokines, isolated islets from Dox-treated or untreated ToI-β mice were incubated in the presence or absence of IL-1β alone or in combination with IFN-γ. Nuclear extracts were prepared and tested for NF-κB-binding activity by EMSA by using the labeled NF-κB consensus sequence as a probe. Fig. 3 shows the presence of a faint band corresponding to the endogenous NF-κB complex in untreated islets (lane 1). Upon exposure to cytokines, strong NF-κB activation was observed in the islet cells nuclei (lane 2). This stimulation was significantly dampened in Dox-treated islets (lane 4), and a reduction in the endogenous NF-κB complex was also observed (lane 3). In parallel, we used as a control the labeled consensus binding sequence for the ubiquitous transcription factor SP1 (lanes 5–8). This inhibition of activation was completely reversed 7 days after Dox-withdrawal (data not shown). The major activated NF-κB-binding complex is composed mainly of p65:p50 heterodimers, because it is recognized by both anti-p65 and anti-p50 antibodies in a supershift assay (data not shown).
Fig. 3.
Effect of cytokines on nuclear translocation and DNA-binding activity of NF-κB analyzed by EMSA. Isolated islets from double transgenic (RIP7-rtTA+/ΔNIκBα) mice, untreated (lanes 1, 2, 5, and 6) or treated with Dox for 3 days (lanes 3, 4, 7, and 8) were exposed to the cytokines IL-1β and IFN-γ for 30 min. Nuclear proteins were incubated with labeled oligoprobes representing the NF-κB or the SP1 consensus sequences.
Inhibition of Cytokine-Induced NO Production in Dox-Treated Pancreatic Islets from ToI-β Mice.
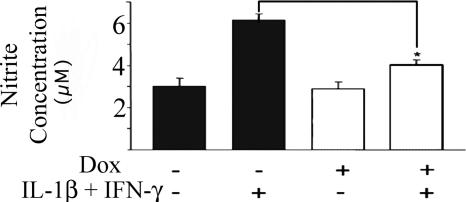
One of the mechanisms by which cytokine-induced NF-κB activation can impair beta-cell functions and, in part, mediate beta-cell death is via induction of the expression of the inducible form of nitric oxide synthase and subsequent NO production (12, 13, 26, 27). We measured NO formation as nitrite accumulation in the culture media of islets isolated from Dox-treated or untreated mice and incubated for 48 h in the presence or absence of IL-1β and INF-γ (with or without Dox). Fig. 4 shows that untreated islets exposed to cytokines released 6.1 ± 0.2 μM nitrite, whereas Dox-treated islets secreted only 4 ± 0.28 μM nitrite (n = 7–14; P < 0.05), indicating that NO formation was significantly inhibited as a result of NF-κB blockade.
Fig. 4.
Medium nitrite levels secreted from islets exposed in vitro to IL-1β (50 units/ml) and IFN-γ (1,000 units/ml) for 48 h in the presence (open bars) or absence (filled bars) of Dox. Data pooled from three separate experiments are presented as the mean ± SE (total number of untreated mice, n = 14; Dox-treated mice, n = 7; and Dox-treated mice exposed to the cytokines, n = 9. *, P < 0.05 vs. islets exposed to the cytokines only).
Reduction of Cytokine-Induced Apoptosis in Dox-Treated Pancreatic Islets from ToI-β Mice.
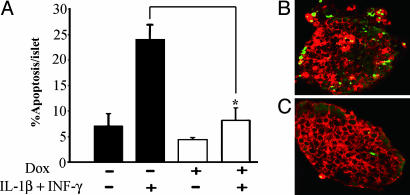
To ascertain whether the observed blockade of NF-κB activation in beta cells was accompanied by protection from cytokine-induced apoptosis, Dox-treated or -untreated isolated islets were exposed to the mentioned cytokines for 3 days, and DNA damage was assessed by using the TUNEL assay. Histological analysis and counts of apoptotic nuclei costained for insulin confirmed that Dox-treated beta cells were significantly more resistant to cytokine-induced apoptosis than the control (8.1% vs. 24% apoptotic beta cells per islet; P < 0.01, n = 4; Figs. 5 A–C).
Fig. 5.
Prevalence of apoptosis in islets exposed to cytokines. (A) Isolated islets were cultured in the presence or absence of IL-1β (50 units/ml) and IFN-γ (1,000 units/ml) for 72 h in medium with (open bars) or without (filled bars) Dox. Apoptosis was evaluated by using the TUNEL assay and is presented as the percentage of apoptotic beta cells per islet. The data are presented as the mean ± SE of four mice in each group, 35–45 islets per group and 2,800–3,000 beta cells counted per group. *, P < 0.01 vs. islets not exposed to Dox. Representative staining of TUNEL-positive nuclei (green) and insulin (red) from islets exposed to cytokines in the absence (B) or presence (C) of Dox.
Induced ToI-β Mice Are Resistant to Multiple Low Doses of STZ (MLDS).
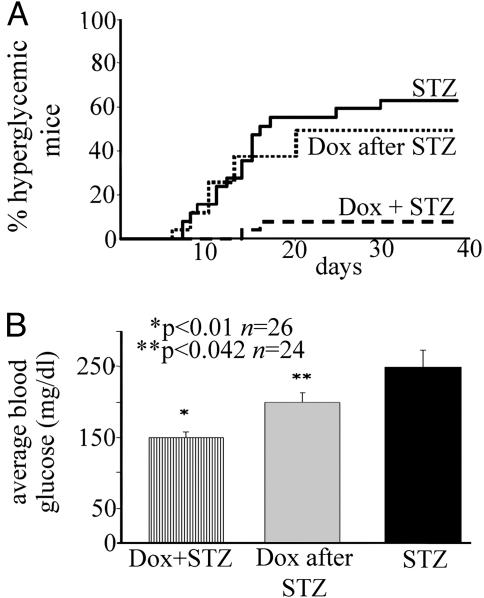
The importance of NF-κB activation in vivo was tested by injecting ToI-β mice with MLDS. MLDS is an immune-mediated murine diabetes model in which hyperglycemia and diabetes are achieved after five daily injections of subdiabetogenic doses of STZ (28, 29), leading to insulitis and selective beta-cell loss, most likely by apoptosis (28). We therefore monitored the appearance of diabetes as defined by blood glucose concentrations of >200 mg/dl on at least three occasions and insulitis in ToI-β mice. Our results show a striking resistance to the development of diabetes after MLDS in Dox-treated as compared with untreated animals. In fact, 16 of 25 mice from the untreated group gradually developed hyperglycemia 5–10 days after the last injection of STZ as opposed to only 2 of 26 Dox-induced mice (Fig. 6A; P < 0.05, as determined by Fisher's exact test comparing the percentage of diabetic mice in each group, or Fig. 6B; P < 0.01, using Student's t test comparing average blood glucose in untreated/STZ vs. Dox-treated/STZ mice). These results correlated with reduced staining for insulin and reduced pancreatic insulin content in the pancreata from the untreated vs. Dox-treated mice (data not shown).
Fig. 6.
The transgene ΔNIκBα protects mice from MLDS-induced diabetes. (A) Percentage of hyperglycemic mice in untreated (bold line, n = 25), Dox-treated (dashed line, n = 26) (P < 0.05 by using Fisher's exact test and comparing percent diabetic mice in each group) and animals where Dox was administered 1 day after the last injection of STZ (n = 24, dotted line). (B) MLDS-induced diabetes presented as average blood glucose (mg/dl) from day 10. Blood glucose levels were significantly lower in the STZ-injected mice treated with Dox simultaneously (Dox + STZ; *, P < 0.01) or 1 day after the last injection (Dox after STZ; **, P = 0.042) as compared with that in untreated mice (STZ), using Student's t test.
To assess the effect of NF-κB blockade after initiation of the damage induced by MLDS, we administered Dox 24 h after the last injection and measured glycemia during the following 10 weeks. Although there was only mild protection from diabetes (Fig. 6A, Dox after STZ), the levels of hyperglycemia were significantly lower (Fig. 6B; P < 0.05, as determined by Student's t test) as compared with that of the untreated group.
To exclude a protective effect against MLDS through a possible ΔNIkBα-mediated reduction in STZ entry into the beta cell, we administered a single high-dose injection of STZ to Dox-treated or -untreated mice. The result was a rapid and similar development of overt diabetes in both groups (n = 5–6). The preserved sensitivity to a single high-dose injection suggests that adequate cytoplasmic concentrations and toxic effects of STZ were achieved, making it unlikely that NF-κB blockade confers its protective effect through interference with STZ transport and toxicity.
Induced ToI-β Mice Have Reduced Intraislet Lymphocyte Infiltration in Response to MLDS.
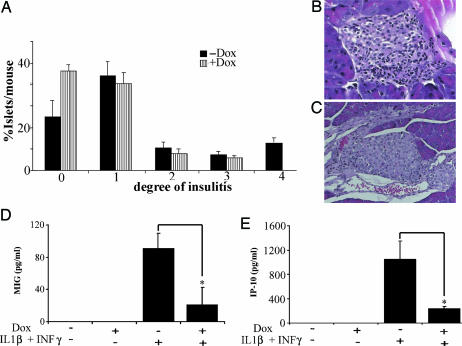
To determine whether the observed protective effect in mice expressing the dominant inhibitor ΔNIkBα was associated with a drop in insulitis, pancreata from MLDS-injected mice were removed at days 11–13 after the first injection of STZ and examined histologically for the degree of insulitis by using the following scale: 0, no infiltration; 1, minor infiltration; 2, minor periinsular lymphocytic infiltration; 3, clear periinsular lymphocytic infiltration; 4, clear intraislet lymphocytic infiltration (30). Interestingly, although the lower degrees of insulitis (grades 2 and 3) were similar in both groups, severe infiltration (grade 4) was observed only in uninduced mice (Fig. 7 A–C; n = 6, P < 0.01).
Fig. 7.
Reduced degree of intraislet infiltrate in MLDS model of diabetes and cytokine-induced chemokine secretion. (A) Degree of insulitis. Insulitis was calculated as percent of islets per mouse in each stage of insulitis (0–4 as described in Methods): P < 0.05 by Pearsons' χ2 test comparing the distribution of the two populations. (B and C) Hematoxylin/eosin staining of histologic sections of pancreata from MLDS-treated mice. (B) Staining of a representative islet from untreated mice, showing severe lymphocyte intraislet infiltration. (C) An islet from a Dox-treated mouse showing only mild periinsular lymphocyte infiltration. (D and E) Level of secreted chemokine MIG (D) or IP-10 (E) accumulated in the culture media of islets exposed to IL-1β (50 units/ml) and IFN-γ (1,000 units/ml) for 24 h in the presence or absence of Dox. Data pooled from three separate experiments are presented as the mean ± SE (total number of mice, n = 11; *, P < 0.04 vs. islets exposed to cytokines only).
Cytokines and chemokines are known mediators of immune responses, owing to their ability to recruit and activate leukocytes. Therefore, we determined the levels of two representative chemokines, namely the cytokine IFN-Inducible Protein-10 (IP-10) and the monokine induced by IFN-γ (MIG) secreted in the culture media of islets from Dox-treated or untreated mice in the presence or absence of cytokines. The results presented in Fig. 7 D and E show that cytokine-induced chemokine secretion is significantly reduced in islets from Dox-treated compared with that in untreated mice (Fig. 7D; n = 8, P = 0.032; and Fig. 7E; n = 8, P = 0.028).
Discussion
We present a transgenic mouse model, the ToI-β mouse, in which controlled inhibition of NF-κB activation can be obtained in beta cells, both in vivo and in vitro, in an inducible and reversible manner. This mouse line is a valuable means for studying the role of NF-κB activation in beta-cell apoptosis in vitro and its involvement in the in vivo process leading to diabetes (7, 16, 22, 23). Of note, a previous attempt to create a mouse with unconditional inhibition of NF-κB throughout pancreatic development (under the control of the Pdx-1/Ipf1 promoter) resulted in hyperglycemic mice with altered glucose-stimulated insulin secretion (24). In contrast, induced ToI-β mice are normoglycemic, even after prolonged exposure to Dox and display a normal response to an intraperitoneal glucose bolus. These findings indicate that blockade of NF-κB activation in mature mice does not immediately hinder beta-cell function, enabling further examination of the role of NF-κB in beta cells exposed to diabetogenic conditions. Another important benefit of this model is the ability to visualize the transcriptional activity of the tetO promoter in beta cells by measuring light emission through the abdomen of live anesthetized ToI-β mice. Although not fully exploited in this study, this method may prove to be a useful tool for future indirect assessment of beta-cell mass in live animals in response to exogenous stimuli or after islet transplantation.
As described above, immune-mediated beta-cell apoptosis is the hallmark of type 1 diabetes. Using the ToI-β mouse, we set out to clarify the distinct roles of NF-κB in this process. Several inflammatory cytokines, such as IL-1β and INF-γ, are implicated in promoting beta-cell death through activation of NF-κB and initiation of beta-cell apoptosis (11–15, 17). Therefore, we sought to confirm these in vitro results at three different levels of the cytokine signal cascade: NF-κB nuclear translocation, NO production, and beta-cell apoptosis. As described in Results, NF-κB nuclear translocation was induced by cytokines but inhibited by induction of ΔNIκBα in islets derived from ToI-β mice. Previous reports suggested that the main source of NO after islet stimulation by cytokines may be the nonendocrine cells present in and around the islet, such as macrophages (31), endothelial cells (32), and ductal cells (33). Here we show that cytokine-induced NF-κB-mediated NO production was significantly reduced in Dox-treated ToI-β islets, with a subsequent decrease in apoptosis. These results agree with previous data by using an exogenous super repressor IκB (22) and illustrate the central role played by NF-κB activation in the beta cells in mediating cytokine-derived signals leading to beta-cell death.
To further study the role of NFκB in vivo, we used the MLDS-injected mice as a murine model for immune-mediated diabetes. STZ enters the beta cells through the Glut2 transporter (34, 35) and causes diabetes in mice and rats (29) by inducing DNA alkylation (34, 36), and perhaps also by releasing NO (37, 38), resulting in beta-cell death (39). There are two main animal models of STZ-induced diabetes: administration of a single high dose, which directly destroys beta cells, or multiple low doses, which progressively damage the beta cells (28, 29), eventually leading to insulitis and selective beta-cell loss by apoptosis (40). It previously was described that systemic p50 or c-RelA null mice were resistant to MLDS (41, 42). These mice, however, had an impaired immune system, and no protective effect was observed in isolated islets exposed to cytokines, making it difficult to interpret the data (41, 42). In our experiments, when ToI-β mice were exposed to MLDS, a clear protective effect was observed, with significantly fewer diabetic mice among the induced ToI-β mice as compared with those in the uninduced controls. Moreover, protection was partly achieved even when NF-κB inhibition was induced 1 day after the last STZ injection, with a significant decrease in average blood glucose. Thus, progression of diabetes was inhibited by NF-κB blockade, even after the onset of a beta cell-directed inflammatory attack.
A similar effect was noted when the severity of insulitis after MLDS was assessed. Interestingly, islets from Dox-induced ToI-β mice had significantly lower levels of severe (grade 4) lymphocyte infiltration as compared with that in the uninduced controls. This observation may be the result of the lower expression of NF-κB-regulated chemokines, adhesion molecules, and cytokines by the beta cells (43), thus decreasing the recruitment and homing of lymphocytes to the islets. This hypothesis is, in part, supported by the present results from the in vitro experiments showing that islets expressing ΔNIκBα secreted significantly less IP-10 and MIG chemokines when exposed to cytokines than did islets from untreated animals. Prevention of severe insulitis was, however, only partial, indicating that the main effects of beta cell-specific NF-κB blockade are achieved via beta-cell protection from apoptosis induced by cytokines and/or other inflammatory agents.
In conclusion, we describe here a transgenic mouse model in which NF-κB activation is specifically modulated in beta cells for relatively short periods of time, providing a valuable tool for further examination of the complex pathways mediating beta-cell damage and death in diabetes. Our findings clearly show beta-cell protection from different diabetogenic agents, namely cytokines and MLDS and a remarkable agreement between the in vitro and in vivo observations. We provide direct evidence that NF-κB activation at the beta-cell level is a key event for beta-cell death during in vivo islet inflammation. Indeed, manipulation of NFκB activation in beta cells allowed these cells to withstand and survive a direct noxious immune reaction. These findings open avenues for future interventions to prevent beta-cell death in the early stages of type 1 diabetes mellitus or after islet transplantation.
Methods
Generation of Transgenic Mice.
The ToI-β mouse model was generated by cross-breeding two transgenic lines of mice: one carrying the inversely positioned ΔNIκBα (with the N-terminal deleted) and luciferase genes (ΔNIκBα-Luc), regulated by a bidirectional tetracycline-responsive element against a C57B/6 × BALB/c background (44) and the second expressing the reverse tetracycline transactivator (rtTA) under the control of the rat insulin II promoter, consisting of the 9.5-kb 5′ flanking region of the gene (RIP7-rtTA) against the C3H × BALB/c background (45). Transmission of both transgenes was monitored by PCR analysis from tail DNA by using the appropriate primers. The transgenes were activated in vivo by administrating 2 mg/ml of Dox in 2.5% sucrose in the drinking water (the control mice received only 2.5% sucrose) or in vitro by adding 5 μg/ml Dox to the culture media. All animals were maintained in a specific pathogen-free research animal facility, and the experiments were approved by the Hebrew University Institutional Animal Care and Use Committee and conducted in accordance with local ethical guidelines.
Isolation and Culture of Mouse Pancreatic Islets.
A solution of 1 mg/ml collagenase XI (Sigma) was first injected into the pancreas before its removal and subsequent digestion at 37°C for 35 min. Individual islets were hand-picked under a stereomicroscope and cultured in RPMI medium 1640 (46) at a concentration of 200 islets/ml in the presence or absence of human recombinant IL-1β (50 units/ml) and mouse recombinant INF-γ (1,000 units/ml) for the periods of time indicated in the legends.
Gel EMSA.
Islet nuclear protein extraction and DNA-binding assays were performed as described in ref. 47. The NF-κB binding mixture contained 30 mM Hepes, pH 7.9/100 mM NaCl/0.3 mM EDTA/2.5 mM DTT/1.5 mM MgCl2/2 μg of poly (dI-dC)/10% glycerol. The SP1 binding reaction was as described in ref. 47. Double-stranded oligonucleotides corresponding to the SP1-binding site (47) or to the NF-κB consensus binding sequence, 5′-GGCTTCAGAGGGGACTTTCCGAGACCG-3′ were end-labeled by a fill-in reaction by using the Klenow fragment of DNA polymerase I and used as probes. Nuclear extracts were incubated for 15 min with the labeled probe at 4°C. DNA–protein complexes were resolved in a 6% polyacrylamide gel as described in ref. 47.
Western Blot Analysis.
Tissues or islets were lysed in RIPA lysis buffer. A total of 100 μg of protein lysate were separated on 10% SDS/PAGE and transferred to a nitrocellulose membrane. After blocking in 5% nonfat dry milk, the membranes were incubated with the appropriate antibodies, namely anti-IκBα antibody (C-21; Santa Cruz Biotechnology) and anti-horseradish peroxidase-conjugated anti-rabbit IgG, followed by enhanced chemiluminescence detection (Biological Industries, Beit Haemek, Israel).
Medium Nitrite-Concentration Measurement.
Medium (100 μl) from an islet culture containing 200 islets/ml were added to an equal volume of Greiss reagent, as described in ref. 48.
Determination of Apoptosis.
Islets were incubated with cytokines for a period of 3 days and fixed overnight in a solution of 4% formaldehyde (Biological Industries). The islets were then centrifuged in 1% agarose solution and embedded in paraffin. Five-micrometer sections were prepared and apoptosis was determined by using the TUNEL assay (Roche, Mannheim, Germany). The sections were counterstained with anti-mouse insulin antibody (Zymed). Some 3,000 beta cells per treatment were counted by using a Zeiss fluorescent microscope. The frequency of apoptotic cells was calculated as the percentage of apoptotic beta cells per islet.
i.p. Glucose Tolerance Test.
Mice were injected with 2 g/kg d-glucose after an overnight fast. Tail blood samples were collected at 0, 15, 30, 60, and 120 min after injection and glucose levels were determined by using a glucometer (Accu-Check, Roche Diagnostics). In separate experiments, serum insulin levels were determined at 0, 2, 5, and 30 min after glucose injection by using the Rat insulin RIA kit (Linco Research, St. Charles, MI)
Immunohistochemistry.
Immunochemistry is described in Zahn et al. (49).
STZ-Induced Diabetes.
For the MLDS model, mice aged 8–10 weeks were injected i.p. for 5 consecutive days with either STZ (Sigma) dissolved in citrate buffer, pH 4.5, at a concentration of 50 mg/kg or citrate buffer alone. Day 0 was defined as the first day of injection of STZ. For the single high-dose model, STZ was injected i.p. at a concentration of 250 mg/kg (50). Blood glucose concentrations were measured after 4 days and up to 42 days by using a glucometer. Hyperglycemia was defined as a nonfasting blood glucose level >200 mg/dl in three sequential measurements.
Insulitis.
To determine the degree of insulitis, pancreata were removed 11–13 days after the last STZ injection. Specimens were fixed in 4% paraformaldehyde and embedded in paraffin. Twenty sections were prepared from three parts of the pancreas (head, body, and tail) and stained with hematoxylin/eosin for pathologic evaluation. Islet insulitis was determined as described in ref. 30. In brief, the degree of lymphocyte infiltration was set by using the following score: 0, no infiltration; 1, minor infiltration; 2, minor periinsulitis; 3, clear periinsulitis; 4, clear lymphocyte infiltration. Results are presented as the percentage of islets per mouse in each category.
Medium IP-10 and MIG Concentration Measurements.
One hundred microliters of medium from an islet culture containing 200 islets/ml was analyzed for MIG and IP-10 concentrations by using a specific ELISA (R & D Systems) according to the manufacturer's instructions.
Data Presentation and Statistical Analysis.
The data are presented as the means ± SE. Statistical analysis of apoptosis and i.p. glucose tolerance test was performed by using the paired Student t test, and statistical analysis of medium nitrite concentration was performed by using the Mann–Whitney test and the Kruskall Wallis test. Statistical analysis of MLDS was performed by using the paired Student's t test, based on a comparison of the average glucose measurements per mouse. This analysis was complemented by Fisher's exact test for the percent of diabetic mice in each group. Statistical analysis of the insulitis score performed by using Pearson's χ2 test. In all of the tests, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Shimon Efrat (Tel Aviv University, Tel Aviv) for helpful discussions and advice, Dr. Ben-Zion Tsuberi for mice embryo transfers and for his constant help, Evelyne Zeira for her expert assistance with the bioluminescence imaging, and Roy Abel for his excellent assistance. This work was supported by Juvenile Diabetes Research Foundation Grant 1-2003-772 and grants from the Diabetes Care in Israel and the Russell Berrie Foundation (to D.M.).
Abbreviations
- Dox
doxycycline
- IκB
inhibitor of NF-κB
- MLDS
multiple low-dose streptozocin
- rtTA
reverse tetracycline transactivator
- STZ
streptozocin
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.O'Brien B. A., Harmon B. V., Cameron D. P., Allan D. J. Diabetes. 1997;46:750–757. doi: 10.2337/diab.46.5.750. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D., Vence L., Benoist C. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 3.Pipeleers D., Ling Z. Diabetes Metab. Rev. 1992;8:209–227. doi: 10.1002/dmr.5610080303. [DOI] [PubMed] [Google Scholar]
- 4.Kay T. W., Thomas H. E., Harrison L. C., Allison J. Trends Endocrinol. Metab. 2000;11:11–15. doi: 10.1016/s1043-2760(99)00210-6. [DOI] [PubMed] [Google Scholar]
- 5.Kurrer M. O., Pakala S. V., Hanson H. L., Katz J. D. Proc. Natl. Acad. Sci. USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eizirik D. L., Mandrup-Poulsen T. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 7.Mauricio D., Mandrup-Poulsen T. Diabetes. 1998;47:1537–1543. doi: 10.2337/diabetes.47.10.1537. [DOI] [PubMed] [Google Scholar]
- 8.Grankvist K., Marklund S. L., Taljedal I. B. Biochem. J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenzen S., Drinkgern J., Tiedge M. Free Radical Biol. Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 10.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 11.Mandrup-Poulsen T., Bendtzen K., Nielsen J. H., Bendixen G., Nerup J. Allergy. 1985;40:424–429. doi: 10.1111/j.1398-9995.1985.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 12.Corbett J. A., Sweetland M. A., Wang J. L., Lancaster J. R., Jr., McDaniel M. L. Proc. Natl. Acad. Sci. USA. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoorens A., Stange G., Pavlovic D., Pipeleers D. Diabetes. 2001;50:551–557. doi: 10.2337/diabetes.50.3.551. [DOI] [PubMed] [Google Scholar]
- 14.Delaney C. A., Pavlovic D., Hoorens A., Pipeleers D. G., Eizirik D. L. Endocrinology. 1997;138:2610–2614. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovitch A. Diabetes Metab. Rev. 1998;14:129–151. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Cnop M., Welch N., Jonas J.-C., Jorns A., Lenzen S., Eizirik D. L. Diabetes. 2005;54(Suppl. 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 17.Baker M. S., Chen X., Cao X. C., Kaufman D. B. J. Surg. Res. 2001;97:117–122. doi: 10.1006/jsre.2001.6121. [DOI] [PubMed] [Google Scholar]
- 18.Karin M., Ben-Neriah Y. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 19.Karin M., Lin A. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S., May M. J., Kopp E. B. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 21.Karin M. J. Biol. Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 22.Heimberg H., Heremans Y., Jobin C., Leemans R., Cardozo A. K., Darville M., Eizirik D. L. Diabetes. 2001;50:2219–2224. doi: 10.2337/diabetes.50.10.2219. [DOI] [PubMed] [Google Scholar]
- 23.Giannoukakis N., Rudert W. A., Trucco M., Robbins P. D. J. Biol. Chem. 2000;275:36509–36513. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- 24.Norlin S., Ahlgren U., Edlund H. Diabetes. 2005;54:125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- 25.Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 26.Darville M. I., Eizirik D. L. Diabetologia. 1998;41:1101–1108. doi: 10.1007/s001250051036. [DOI] [PubMed] [Google Scholar]
- 27.Eizirik D. L., Flodstrom M., Karlsen A. E., Welsh N. Diabetologia. 1996;39:875–890. doi: 10.1007/BF00403906. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien B. A., Harmon B. V., Cameron D. P., Allan D. J. J. Pathol. 1996;178:176–181. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Like A. A., Rossini A. A. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 30.Flodstrom M., Tyrberg B., Eizirik D. L., Sandler S. Diabetes. 1999;48:706–713. doi: 10.2337/diabetes.48.4.706. [DOI] [PubMed] [Google Scholar]
- 31.Kroncke K. D., Kolb-Bachofen V., Berschick B., Burkart V., Kolb H. Biochem. Biophys. Res. Commun. 1991;175:752–758. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- 32.Steiner L., Kroncke K., Fehsel K., Kolb-Bachofen V. Diabetologia. 1997;40:150–155. doi: 10.1007/s001250050656. [DOI] [PubMed] [Google Scholar]
- 33.Pavlovic D., Chen M. C., Bouwens L., Eizirik D. L., Pipeleers D. Diabetes. 1999;48:29–33. doi: 10.2337/diabetes.48.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Elsner M., Guldbakke B., Tiedge M., Munday R., Lenzen S. Diabetologia. 2000;43:1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- 35.Schnedl W. J., Ferber S., Johnson J. H., Newgard C. B. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 36.Moreira J. E., Hand A. R., Hakan Borg L. A., Sandler S., Welsh M., Welsh N., Eizirik D. L. Virchows Arch. B Cell Pathol. 1991;60:337–344. doi: 10.1007/BF02899565. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji A., Sakurai H. Biochem. Biophys. Res. Commun. 1998;245:11–16. doi: 10.1006/bbrc.1998.8368. [DOI] [PubMed] [Google Scholar]
- 38.Turk J., Corbett J. A., Ramanadham S., Bohrer A., McDaniel M. L. Biochem. Biophys. Res. Commun. 1993;197:1458–1464. doi: 10.1006/bbrc.1993.2641. [DOI] [PubMed] [Google Scholar]
- 39.Szkudelski T. Physiol. Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 40.Like A. A., Appel M. C., Williams R. M., Rossini A. A. Lab. Invest. 1978;38:470–486. [PubMed] [Google Scholar]
- 41.Mabley J. G., Hasko G., Liaudet L., Soriano F., Southan G. J., Salzman A. L., Szabo C. J. Endocrinol. 2002;173:457–464. doi: 10.1677/joe.0.1730457. [DOI] [PubMed] [Google Scholar]
- 42.Lamhamedi-Cherradi S. E., Zheng S., Hilliard B. A., Xu L., Sun J., Alsheadat S., Liou H. C., Chen Y. H. J. Immunol. 2003;171:4886–4892. doi: 10.4049/jimmunol.171.9.4886. [DOI] [PubMed] [Google Scholar]
- 43.Cardozo A. K., Heimberg H., Heremans Y., Leeman R., Kutlu B., Kruhoffer M., Orntoft T., Eizirik D. L. J. Biol. Chem. 2001;276:48879–48886. doi: 10.1074/jbc.M108658200. [DOI] [PubMed] [Google Scholar]
- 44.Lavon I., Goldberg I., Amit S., Landsman L., Jung S., Tsuberi B. Z., Barshack I., Kopolovic J., Galun E., Bujard H., Ben-Neriah Y. Nat. Med. 2000;6:573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 45.Milo-Landesman D., Surana M., Berkovich I., Compagni A., Christofori G., Fleischer N., Efrat S. Cell Transplant. 2001;10:645–650. [PubMed] [Google Scholar]
- 46.Andersson A. Diabetologia. 1978;14:397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- 47.Ben-Shushan E., Marshak S., Shoshkes M., Cerasi E., Melloul D. J. Biol. Chem. 2001;276:17533–17540. doi: 10.1074/jbc.M009088200. [DOI] [PubMed] [Google Scholar]
- 48.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 49.Zahn S., Hecksher-Sorensen J., Pedersen I. L., Serup P., Madsen O. Hybrid Hybridomics. 2004;23:385–388. doi: 10.1089/hyb.2004.23.385. [DOI] [PubMed] [Google Scholar]
- 50.Soriano F. G., Pacher P., Mabley J., Liaudet L., Szabo C. Circ. Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.