Abstract
Injection of Caenorhabditis elegans polyA RNA into Xenopus laevis oocytes led to the expression of neurotransmitter receptors that generated some unique responses, including ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors as well as receptors that coupled to G proteins, such as those to octopamine, norepinephrine, and angiotensin, which activated the oocyte’s own phosphatidylinositol system and calcium-gated chloride channels. The oocytes also expressed chloride-conducting glutamate receptors, muscarinic acetylcholine receptors, and voltage-operated calcium channels. Unexpectedly, serotonin (5-hydroxytryptamine), dopamine, GABA, and kainate did not generate ionic currents, suggesting that the corresponding receptors were not expressed or were not functional in the oocytes. The use of X. laevis oocytes for expressing worm RNA demonstrates that there are many molecular components whose role remains to be clarified in the nematode. Among them are the nature of the endogenous agonists for the octopamine and angiotensin receptors and the subunits that compose the ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and the norepinephrine receptors that couple to the phosphoinositide cascade.
Keywords: nematode ion channels, norepinephrine, octopamine, voltage clamp
The nematode Caenorhabditis elegans has become a very useful model to study the development of the nervous system and the crucial process of interneuronal communication (1). The molecular bases underlying this process are highly conserved among metazoans, and neurotransmitters activate plasma membrane receptors that are phylogenetically related. Accordingly, much can be learned about the process of synaptic transmission everywhere by studying the receptors of C. elegans.
The genome of C. elegans was completely sequenced several years ago, and computer analyses of the DNA sequence revealed a number of genes coding for some of the classical vertebrate neurotransmitter receptors: acetylcholine, GABA, and glutamate (2). Acetylcholine is the main excitatory neurotransmitter controlling motor functions in C. elegans, and as many as 50 nicotinic and 3 muscarinic receptor genes have been identified (3, 4). Glutamate and GABA play both excitatory and inhibitory roles in the nematode, and several genes coding for many subunits have been already identified (2, 5)
In addition, several ion channels involved in the process of synaptic transmission, such as the potassium, calcium, and chloride channels, as well as a plethora of G protein-coupled receptor genes have been studied in C. elegans. Clearly, a complete analysis of the diverse products of these genes will require a functional approach using electrophysiological methods (1).
For years, many vertebrate ion channels and receptors have been expressed in frog oocytes, where their electrophysiological and pharmacological properties can be studied by using the two-microelectrode voltage-clamp technique (6, 7). However, relatively little has been done concerning the receptors of worms. Therefore, >5 years ago, we decided to determine which receptors and ion channels were expressed in oocytes after injecting them with C. elegans mRNA. Whereas some of the neurotransmitter receptors and ion channels that we expressed have now been cloned and expressed in heterologous systems (3, 4, 8), we found others, such as the angiotensin (AT) and norepinephrine receptors, whose genes are not known and which may play important roles in nematode physiology.
Results
A Glutamate-Gated Ion Channel.
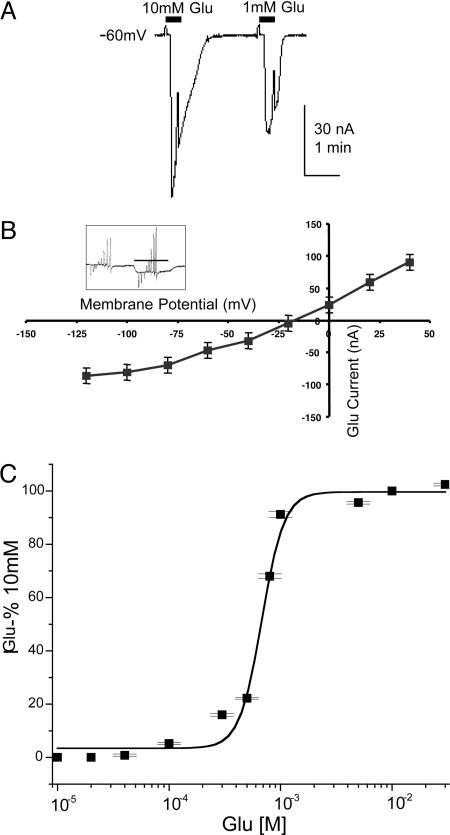
Like many other invertebrates, nematodes have glutamate receptors that conduct chloride ions (8), in contrast to the vertebrate ionotropic glutamate receptors, which have low chloride permeability. Fig. 1A illustrates a desensitizing glutamate-current response generated by an oocyte expressing C. elegans mRNA. To obtain some information about the ions that carry this current, we determined its reversal potential by changing the membrane potential from −120 to +40 in 20-mV steps while the receptor was activated by 1 mM l-glutamate (Fig. 1B Inset). The resulting I/V curve (Fig. 1B) shows slight rectification at potentials more negative than ≈−60 mV, and the average reversal potential was −20 ± 3 mV (n = 4), consistent with a calculated Nernst potential for chloride of −19 mV, assuming 50 mM internal chloride (9). Furthermore, this current was also generated in calcium-free Ringer’s medium (data not shown). The glutamate concentration/current response relation (Fig. 1C) gave an EC50 of 1.1 mM (SE ± 0.3, n = 6) and a Hill number of 2.
Fig. 1.
Expression of glutamate receptors. (A) Inward currents generated by 10 and 1 mM l-glutamate applied to an oocyte injected with C. elegans mRNA. In this and following figures, horizontal bars denote the beginning and end of exposures to the neurotransmitters. (B) I/V relation (n = 4, two frogs) for glutamate (1 mM) action, showing an equilibrium potential ≈−20 mV. (Inset) The currents generated by 20-mV incremental voltage steps before and in the presence of 1 mM glutamate. Holding potential is −60 mV. (C) Glutamate dose–response relation (n = 6, three frogs).
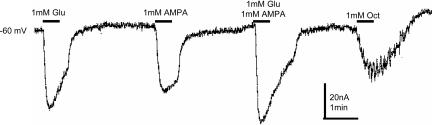
Several oocytes that responded to glutamate were exposed to 1 mM d-glutamate, kainate, l-aspartate, ivermectin, N-acetyl aspartate-glutamate, l-glutamate-diethyl ester, asparatate-aspartate or N-acetyl-acetate, and GABA, all of which did not generate obvious currents (n = 3–9, 2–3 frogs). In contrast, quisqualate (an agonist of metabotropic glutamate receptors), l-glutamate γ-methylester and l-α-aspartyl l-alanine generated small currents (n = 3–5, two frogs); in the range of 3–4% of that generated by 1 mM glutamate. On the other hand, 1 mM α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) activated receptors/channels that desensitized more slowly than those activated by glutamate alone (Fig. 2). Furthermore, as shown in Fig. 2, the mRNA-injected oocytes responded to octopamine by generating an oscillatory current. Therefore, we proceeded to explore the expression of this and other metabotropic G protein-coupled receptors (GPCRs).
Fig. 2.
Currents generated by glutamate, AMPA alone, or both applied simultaneously to an mRNA-injected oocyte. Octopamine (Oct) elicited an oscillatory current.
GPCRs.
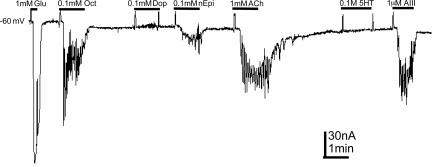
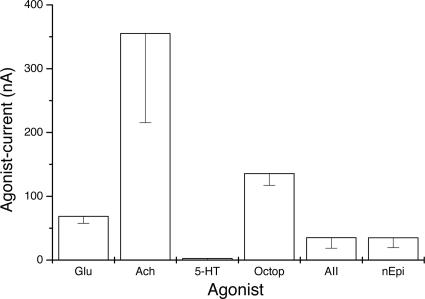
Activation of many GPCRs in oocytes triggers the diacylglycerol/inositol phosphate system and causes the release of calcium ions from intracellular stores, which then activate calcium-gated chloride channels (7, 10, 11). Fig. 3 shows current responses generated by an oocyte exposed consecutively to glutamate, octopamine, dopamine, norepinephrine, acetylcholine, 5-hydroxytryptamine (5-HT), and AT III (AIII). Except for dopamine, which failed to generated currents (n = 8, two frogs) and 5-HT, which elicited currents in only a few oocytes (n = 8, two frogs), the other neurotransmitters generated the characteristic metabotropic oscillatory currents, whereas glutamate gave the usual smooth ionotropic current. Fig. 4 summarizes the amplitudes of responses generated by oocytes (n = 4–9, one frog) that were exposed to various agonists. It is known that the worm has at least three genes that code for dopamine receptors (12). Therefore, the absence of responses to dopamine in oocytes injected with C. elegans mRNA could be due to an inability of those receptors to couple to the oocyte’s G proteins.
Fig. 3.
G protein-coupled receptors. Glutamate activated ionotropic receptors and elicited a smooth current that contrasts with the oscillatory currents generated by the metabotropic receptors: octopamine (Oct), dopamine (Dop), norepinephrine (nEpi), acetylcholine (ACh), and AIII. Note that dopamine and 5-HT did not elicit currents.
Fig. 4.
Average amplitudes of currents generated by several agonists in oocytes expressing C. elegans polyA RNA. These results were obtained from a single frog. Bars, SE; n = 4–9. octopamine (Octop), glutamate (Glu), acetylcholine (Ach), norepinephrine (nEpi).
Octopamine stimulates worm movements and inhibits egg laying, and, although an specific receptor has not been identified, the C. elegans tyramine receptors bind octopamine and increase the intracellular cAMP levels when expressed in eukaryotic cells in culture (13, 14). Several attempts to generate tyramine responses (n = 6, two donors) in the injected oocytes did not produce an evident current, suggesting that the receptor expressed after injecting the mRNA was assembled in such a way that octopamine activated G proteins with more efficiency than tyramine (n = 9, three frogs) or that there is a different, highly specific octopamine receptor that couples well to the oocyte G proteins.
Norepinephrine also activated the oscillatory chloride currents (n = 11, three frogs). The presence of norepinephrine has been established in other invertebrates, although thus far, we have not found any report of an invertebrate receptor activated by this neurotransmitter. Therefore, it will be important to determine the presence of norepinephrine or its analogues in the worm as well as the identity of the gene(s) coding for this receptor. Muscarinic acetylcholine receptor clones were identified in C. elegans and were functionally expressed in Xenopus oocytes, where they generated oscillatory chloride currents (3). We found that C. elegans mRNA induced the expression of these receptors (n = 19, four frogs) and that arecholine, a vermicide that is an agonist for both muscarinic and nicotinic receptors, activated with lower efficiency a GPCR (n = 4, 2 frogs).
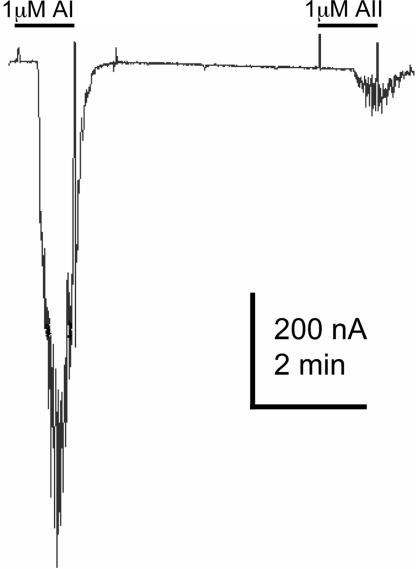
In this initial screen for neurotransmitter receptors, we also tested for responses to AT (AIII in Fig. 3). Several studies have already demonstrated that the mammalian AT receptors AT1 and -2) activate calcium-gated chloride channels when expressed in the oocyte and that, usually, the oocyte itself does not possess these kinds of receptors (15). We found that AI (n = 6, two frogs), AII (n = 16, four frogs), and AIII (n = 4, two frogs) activate receptors that trigger oscillatory chloride currents (Figs. 3 and 5). Searching in the genome data banks, we did not find DNA sequences with obvious homology between human or rat AT receptors and any GPCR of the worm.
Fig. 5.
The octapeptide AI and precursors ATII and ATIII induced oscillatory chloride currents. An AIII current is shown in Fig. 3.
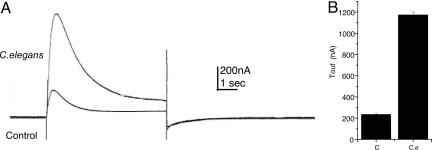
Finally, the expression of voltage-activated calcium channels was assessed by measuring the transient outward (Tout) current (10). Although the oocytes frequently have endogenous calcium channels that generate a Tout current, the expression of heterologous channels is evidenced by an increase in the amplitude of the Tout currents generated in the injected oocytes. Fig. 6A shows sample Tout currents generated by injected and noninjected oocytes. Averaged current amplitudes of 12 oocytes from four frogs are shown in Fig. 6B. The Tout was completely abolished by manganese, which blocks the influx of calcium ions through the channels, as well as by using calcium-free Ringer’s solution in the bath (data not shown).
Fig. 6.
Tout currents. (A) Tout currents in a control (noninjected) oocyte and in one injected with C. elegans mRNA. The records show the current that was blocked by manganese by using pulses from −80 to +20 mV. (B) Mean Tout currents of control (C) and injected (C.e) oocytes (n = 12).
Discussion
In this article, we describe membrane currents generated by several neurotransmitters applied to oocytes injected with C. elegans polyA RNA. Almost certainly, those currents arose from the activation of C. elegans neurotransmitter receptors expressed by the oocytes after injecting the mRNA, because control (noninjected) or water-injected oocytes from the same frogs did not elicit currents when treated similarly.
Because of the ion-selectivity and characteristics of the response to glutamate, the currents generated by the oocytes injected with C. elegans polyA RNA are probably due to activation of Glu–Clα2 receptors (16), alone or combined with other chloride-conducting glutamate-receptor subunits. However, in our experiments, ivermectin did not generate ionic currents in the injected oocytes, contrasting with the potent agonism that it exhibits on Glu–Clα2 receptors and with other reports where worm polyA RNA induced the expression of glutamate receptors in oocytes (17).
Receptor-subunit composition and subcellular localization play an important role in regulating synaptic strength. GLR-1 and GLR-2 are the C. elegans subunits most closely related to the mammalian AMPA-type receptors. These subunits are expressed in overlapping sets of interneurons and form heteromeric complexes that mediate synaptic transmission (18, 19). Although C. elegans GLR-1 and -2 do not express functional receptors when their clones are injected into oocytes (18), here, we provide evidence that AMPA receptors are actually produced after injecting raw polyA RNA. This finding suggests that other subunits, or accessory proteins, must be present to produce functional AMPA receptors. Such is the case with SOL-1, which is an accessory protein selectively required for glutamate/kainate-gated currents (20).
We found no evidence that dopamine gates ion channels in the injected oocytes. Although four putative dopamine receptors (DOP1–4) have been cloned from C. elegans, they have not been expressed in frog oocytes (12), whereas mammalian dopamine receptors couple to G proteins in this system (21). It has been shown that dopamine antagonizes serotonin action in worms via the 5-HT-gated ion channel MOD-1, suggesting that this channel activity couples both 5-HT and dopamine signaling (22). However, 5-HT did not consistently generate currents in the injected oocytes, even though its specific receptors have been known for years, and metabotropic serotonin receptors are potently expressed by many vertebrate brain mRNAs (7).
Recent evidence indicates that C. elegans has tyraminergic cells that are distinct from its octopaminergic cells (23). For years, tyramine was considered a biosynthetic precursor for octopamine, although the presence of receptors that respond to tyramine has led to the suggestion that it may, itself, act as a neurotransmitter. It is worth mentioning that it has not yet been demonstrated that tyramine is the endogenous ligand for these receptors. Two genes, SER-2 and TYRA-2, whose products respond to tyramine by mobilizing intracellular calcium, have been identified (14, 24), and cultured mammalian cells expressing these genes also bind octopamine. In contrast, our preparations of polyA RNA consistently induced the expression of an octopamine receptor that coupled to the phosphoinositide system, whereas the same oocytes did not respond to tyramine. An explanation for this finding still remains to be found. It may be that there is a highly specific octopamine receptor that couples efficiently to ion channels in frog oocytes and that is different from SER-2 and TYRA-2 receptors; or perhaps the products of these genes form heteromeric receptors with different properties.
Several components of the catecholaminergic system, including epinephrine and norepinephrine, have been found in some invertebrates, where they play diverse roles. For example, norepinephrine is required to reach the gastrulation stage in sea urchins (25) and for regulation of heart rate in the crustacean Triops longicaudatus (26). In C. elegans, the vesicular monoamine transporter is encoded by the gene cat-1, which has high affinity for norepinephrine, epinephrine, dopamine, and serotonin (27). However, a specific receptor for norepinephrine has not been reported, whereas we show here, that it would be possible to identify such a receptor by functional expression in oocytes.
The ATs I–III have been found in several animals, including invertebrates. Although the functional role of ATs in the latter organisms has not been well characterized, it is known that, in clams, ATII and ATIII regulate the water flow through the modulation of aquaporins (28). In the nematode, an enzyme with sequence and function related to the family of vertebrate AT-converting enzymes is necessary for molting and morphogenesis (29). We did not find in the literature evidence for the presence of AT peptides or the genes encoding them in C. elegans. It will be important, thus, to identify the molecular components and to define the role of an AT system in the nematode.
In conclusion, the X. laevis oocyte is well able to express C. elegans mRNA, revealing interesting new components of the worm’s synaptic transmission processes. Thus, the mRNA expressed AMPA and glutamate ionotropic receptors and octopamine, AT, acetylcholine, and norepinephrine metabotropic receptors as well as voltage-operated calcium channels. Clearly, more work is needed to determine in detail the molecular structure, function, and cellular localization of these receptors and channels. Interestingly, the oocytes injected with C. elegans mRNA did not respond to GABA, kainate, or serotonin, even though their corresponding receptors are among those more potently expressed by mammalian brain mRNA. The studies described in this article pave the way toward a better understanding of physiological and pharmacological features of C. elegans. Moreover, because these observations probably also apply to the nematode parasites of humans, plants, and farm animals, they may suggest nematode-specific genes as targets for new drugs, with C. elegans worms and Xenopus oocytes serving to evaluate candidate compounds.
Materials and Methods
Handling of Worms.
Worms were cultured following standard procedures (30). C. elegans strain N2 was obtained form the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis) and grown on nematode growth medium (NGM) agar plates seeded with Escherichia coli (OP50) at 25°C. Once the plates were saturated with worms (4–5 days), the worms were collected by washing the plate surface with NGM and centrifugation at 7,000 × g for 10 min. Pellets of worms were frozen in liquid nitrogen and whole-worm RNA was extracted by the phenol-guanidinium method (31). PolyA RNA was selected by affinity chromatography using an oligo(dT) cellulose column (Invitrogen).
Expression of mRNA in X. laevis Oocytes.
Female frogs [obtained from Nasco (Fort Atkinson, WI) or Xenopus I, Ann Arbor, MI)] were decapitated after anesthesia, and pieces of ovary were dissected. Full follicles were isolated manually, treated with collagenase type 1 (0.5 mg/ml) (Sigma-Aldrich) to remove the follicular cells, and maintained at 16°C in Barth′s solution supplemented with gentamycin (0.1 mg/ml) (Sigma-Aldrich). One day later, 50 nl of C. elegans polyA RNA (1 μg/μl) were injected into the oocytes and kept in Barth′s medium. One to 5 days later, the oocytes were transferred to a chamber continuously superfused with frog′s Ringer’s solution at ≈20°C. Neurotransmitters or drugs (glutamate, acetylcholine, octopamine, AT, norepinephrine, tyramine, and AMPA) were applied by bath perfusion, and the oocyte’s membrane current responses were recorded with the membrane held at −60 mV, unless otherwise indicated (10).
For voltage-activated calcium channels, we studied the Tout chloride current that is generated by depolarizing the oocytes from −100 to +20 mV. The consequent influx of calcium then causes the opening of calcium-gated chloride channels (10).
Acknowledgments
The Caenorhabditis Genetics Center supplied the worm N2 strain and E. coli OP50 strain. This work was supported by National Science Foundation (Neuronal and Glial Mechanisms) Grant 998285, Consejo Nacional de Ciencia y Tecnología Grant 41309Q, and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica Grant 212702.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AT
angiotensin
- GPCR
G protein-coupled receptor
- 5-HT
5-hydroxytryptamine
- Tout
transient outward
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Gally C., Bessereau J. L. Med. Sci. (Paris) 2003;19:725–734. doi: 10.1051/medsci/20031967725. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann C. I. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 3.Hwang J. M., Chang D. J., Kim U. S., Lee Y. S., Park Y. S., Kaang B. K., Cho N. J. Recept. Channels. 1999;6:415–424. [PubMed] [Google Scholar]
- 4.Park Y. S., Kim S., Shin Y., Choi B., Cho N. J. Biochem. Biophys. Res. 2003;308:961–965. doi: 10.1016/s0006-291x(03)01508-0. [DOI] [PubMed] [Google Scholar]
- 5.Schuske K., Beg A. A., Jorgensen E. M. Trends Neurosci. 2004;27:407–414. doi: 10.1016/j.tins.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Sumikawa K., Parker I., Miledi R. In: Membrane Control of Cellular Activity. Luttgau H., editor. Stuttgart: Fischer; 1986. pp. 127–139. [Google Scholar]
- 7.Miledi R., Parker I., Sumikawa K. In: Fidia Research Foundation Neuroscience Award Lectures Series. Smith J., editor. New York: Raven; 1989. pp. 57–90. [Google Scholar]
- 8.Brockie P. J., Maricq A. V. Neurosignals. 2003;12:108–125. doi: 10.1159/000072159. [DOI] [PubMed] [Google Scholar]
- 9.Kusano K., Miledi R., Stinnakre J. J. Physiol. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miledi R. Proc. R. Soc. London B; 1982. pp. 491–497. [DOI] [PubMed] [Google Scholar]
- 11.Parker I., Miledi R. Proc. R. Soc. London B; 1986. pp. 307–315. [DOI] [PubMed] [Google Scholar]
- 12.Suo S., Ishiura S., Van Tol H. H. Eur. J. Pharmacol. 2004;500:159–166. doi: 10.1016/j.ejphar.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Horvitz H. R., Chalfie M., Trent C., Sulston J. E., Evans P. D. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 14.Rex E., Komuniecki R. W. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- 15.Matute C., Pulakat L., Rio C., Valcarcel C., Miledi R. Proc. Natl. Acad. Sci. USA. 1994;91:3774–3778. doi: 10.1073/pnas.91.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pemberton D. J., Franks C. J., Walker R. J., Holden-Dye L. Mol. Pharmacol. 2001;59:1037–1043. doi: 10.1124/mol.59.5.1037. [DOI] [PubMed] [Google Scholar]
- 17.Arena J. P., Liu K. K., Paress P. S., Schaeffer J. M., Cully D. F. Brain Res. Mol. Brain Res. 1992;15:339–348. doi: 10.1016/0169-328x(92)90127-w. [DOI] [PubMed] [Google Scholar]
- 18.Chang H. C., Rongo C. J. Cell Sci. 2005;118:1945–1956. doi: 10.1242/jcs.02320. [DOI] [PubMed] [Google Scholar]
- 19.Glodowski D. R., Wright T., Martinowich K., Chang H. C., Beach D., Rongo C. Mol. Biol. Cell. 2005;16:1417–1426. doi: 10.1091/mbc.E04-10-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y., Mellem J. E., Brockie P. J., Madsen D. M., Maricq A V. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
- 21.Sumikawa K., Parker I., Miledi R. Proc. R. Soc. London B; 1984. pp. 255–260. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey C. M., Mackenzie S. M., Gargus A., Blanco G., Sze J. Y. Genetics. 2005;169:1425–1436. doi: 10.1534/genetics.104.032540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkema M. J., Hunter-Ensor M., Ringstad N., Horvitz H. R. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Rex E., Hapiak V., Hobson R., Smith K., Xiao H., Komuniecki R. J. Neurochem. 2005;94:181–191. doi: 10.1111/j.1471-4159.2005.03180.x. [DOI] [PubMed] [Google Scholar]
- 25.Anitole-Misleh K. G., Brown K. M. Comp. Biochem. Physiol. A. 2004;137:39–50. doi: 10.1016/j.cbpb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi H. Zoolog. Sci. 2003;20:841–846. doi: 10.2108/zsj.20.841. [DOI] [PubMed] [Google Scholar]
- 27.Duerr J. S., Frisby D. L., Gaskin J., Duke A., Asermely K., Huddleston D., Eiden L. E., Rand J. B. J. Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satou R., Nakagawa T., Ido H., Tomomatsu M., Suzuki F., Nakamura Y. Biosci. Biotechnol. Biochem. 2005;69:1221–1225. doi: 10.1271/bbb.69.1221. [DOI] [PubMed] [Google Scholar]
- 29.Brooks D. R., Appleford P. J., Murray L., Isaac R. E. J. Biol. Chem. 2003;278:52340–52346. doi: 10.1074/jbc.M308858200. [DOI] [PubMed] [Google Scholar]
- 30.Lewis J. A., Fleming J. T. Methods in Cell Biology. New York: Academic; 1995. pp. 4–29. [Google Scholar]
- 31.Chomczynski P., Sacchi N. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]