Abstract
The polypeptide snake toxin α-bungarotoxin (BTX) has been used in hundreds of studies on the structure, function, and development of the neuromuscular junction because it binds tightly and specifically to the nicotinic acetylcholine receptors (nAChRs) at this synapse. We show here that BTX also binds to and blocks a subset of GABAA receptors (GABAARs) that contain the GABAAR β3 subunit. These results introduce a previously unrecognized tool for analysis of GABAARs but may complicate interpretation of some studies on neuronal nAChRs.
Keywords: acetylcholine, neurotransmitter, synapse
Perhaps the best understood of all synapses is the vertebrate skeletal neuromuscular junction (1). Although the accessibility, simplicity, and large size of this synapse are often viewed as its principle advantages, another important factor has been the availability of a highly versatile ligand for the nicotinic acetylcholine receptors (nAChRs) that are the critical component of its postsynaptic membrane. This ligand, α-bungarotoxin (BTX), is a 74-aa polypeptide derived from the venom of the banded krait, Bungarus multicinctus. BTX binds quasi-irreversibly and highly specifically to the nAChRs at the neuromuscular junction and in electric organs of Torpedoes and rays (2, 3). An additional advantage is that it is readily derivatized without loss of activity; it has been conjugated to agarose for affinity-purification of AChRs (4), to 125I for quantification of AChRs (5), and to fluorophores for tracking of AChRs during synaptogenesis (ref. 6; see refs. 3 and 7 for reviews of early and recent work, respectively).
BTX binds not only to the α1 subunit contained in muscle nAChRs but also to a subset of neuronal nAChRs; it binds those containing the α7–α10 subunits but not those containing the α2–α6 subunits (8, 9). As at the neuromuscular junction, studies of the structure, function, and development of neuronal cholinergic synapses have benefited from BTX.
In studies on both muscle and neuronal cholinergic synapses, a guiding assumption has been that BTX binds only to nAChRs. During the course of studies to label neurotransmitter receptors that had been tagged with a BTX-binding motif (10), we obtained evidence that challenged this premise. We show here that BTX binds to a subset of GABAA receptors (GABAARs). In particular, BTX blocks GABAARs that contain interfaces between adjacent β3 subunits.
Results
BTX Binds to a GABAAR Subunit in Heterologous Cells.
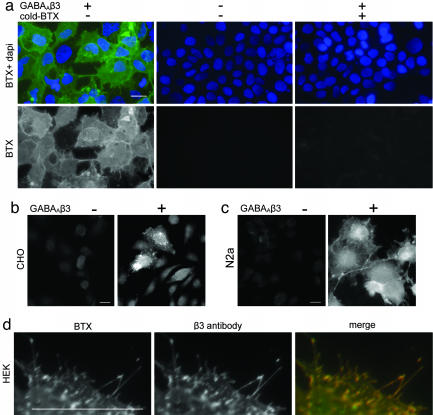
In initial studies, we found that fluorophore-conjugated BTX stained human embryonic kidney (HEK 293) cells that had been transfected with a cDNA encoding the rat GABAAR β3 subunit (Fig. 1a). This staining was not seen in untransfected cells (Fig. 1a). Staining by fluorophore-conjugated BTX was blocked by preincubation with unconjugated BTX, whereas staining was seen when cells were incubated successively with BTX, an affinity-purified antibody to BTX, and fluorophore-conjugated second antibody (Fig. 1a and data not shown). Similar results were obtained in several cell types, and specific staining was observed when cells were stained live or after fixation and permeabilization (Fig. 1 b and c). Staining by BTX and by an antibody to GABAAR β3 overlapped (Fig. 1d). These results show that BTX binds to GABAAR β3.
Fig. 1.
BTX binds to GABAAβ3. (a) Rhodamine-BTX labels HEK 293 cells transfected with a plasmid encoding GABAΑR β3. Labeling is blocked by preincubation with unconjugated BTX. Untransfected cells are not labeled. (b and c) BTX specifically labels GABAAR β3-transfected CHO and N2a cells. (d) Labeling of GABAΑR β3-transfected cells by BTX and anti-GABAAR β3 antibody is coincident. Cells in a and d were stained live and then fixed; cells in b and c were fixed and permeabilized before staining. (Scale bars, 5 μm.)
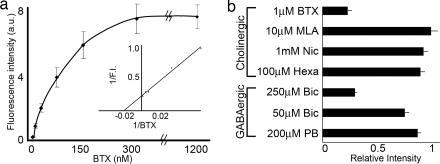
To assess the affinity of BTX for GABAAR β3, we used quantitative fluorescence microscopy, as described in ref. 10, to measure BTX binding to stably transfected HEK 293 cells. The apparent Kd determined in this way was ≈50 nM (Fig. 2a). We also used this assay to pharmacologically characterize BTX binding to GABAAR β3. Binding was affected little by ligands for nAChRs containing the α1 (hexamethonium or nicotine) or α7 (methyllycaconitine) subunits or by the GABAAR modulator pentobarbital (Fig. 2b). In contrast, binding of BTX to GABAAR β3-expressing cells was significantly reduced in the presence of the GABAAR antagonist bicuculline (Fig. 2b).
Fig. 2.
Quantitative assessment of BTX binding to GABAAR β3-expressing HEK 293 cells. (a) BTX binding to a stably transfected cell line was measured by quantitative fluorescence microscopy (10). (Inset) Double-reciprocal plot shows that Kd ≈ 50 nM. (b) BTX binding to this cell line was insensitive to preincubation with blockers of nAChRs containing α1 or α7 subunits [methyllycaconitine (MLA), nicotine (Nic), and hexamethonium (Hexa)] or the GABAΑR modulator pentobarbital (PB) but was blocked by the GABAΑR antagonist bicuculline (Bic).
BTX Binds Selectively to the GABAAR β3 Subunit.
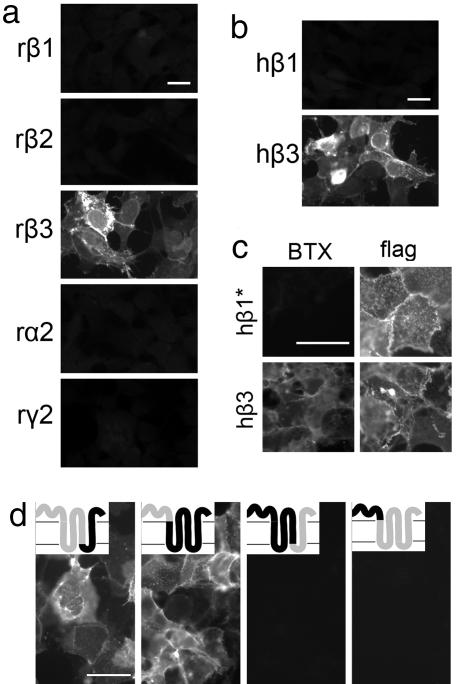
To determine whether other GABAAR subunits also bound BTX, we expressed rat GABAAR β1, β2, β3, α2, or γ2 subunits in HEK cells and then stained cells live or after fixation and permeabilization. Only cells that expressed GABAAR β3 detectably bound BTX (Fig. 3a). Because pharmacological and physiological differences between rodent and human GABAAR β-subunits have been reported (11–13), we also tested cells expressing human subunits. Human GABAAR β3 bound BTX, but human GABAAR β1 did not (Fig. 3b). Because some subunits ineffectively form channels when expressed on their own (13, 14), we also tested mutant GABAAR β1 subunits with an enhanced ability to form homooligomers and reach the cell surface (J.B. and J.H.S, unpublished data). These GABAAR β1 mutants reached the surface efficiently (as shown by staining for an extracellular epitope tag; see Fig. 3c Right) but failed to bind BTX (Fig. 3c Left), ruling out the possibility that differential binding of β1 and β3 subunits was secondary to an inability to multimerize.
Fig. 3.
Mapping of BTX-binding sites on GABAARs. HEK 293 cells transfected with cDNAs encoding indicated subunits were stained with BTX after (a, b, and d) or before (c) fixation and permeabilization. (a) Rat (r) GABAAR β3 binds BTX, but rat GABAAR β1, β2, α2, and γ2 do not. (b) Human (h) GABAAR β3 binds BTX, but human GABAAR β1 does not. (c) A GABAAR β1 subunit that had been mutated to enhance its ability to form pentamers (β1*) failed to bind BTX. The subunit bore an amino-terminal “flag” epitope tag; antibody to the tag stained live cells, confirming that the subunit reached the cell surface. A similarly tagged wild-type β3 subunit did bind BTX. (d) Chimeric receptors containing the amino-terminal extracellular domain of GABAAR β3 fused to the remainder of GABAAR β1 bind BTX, but chimeras containing the amino-terminal extracellular domain of GABAAR β1 fused to the remainder of GABAAR β3 do not. (Insets) Portions of receptors derived from β3 (gray) and β1 (black); fusions are at amino acid 314 in the first and third chimeras and at amino acid 213 in the second and fourth chimera (11). (Scale bars, 5 μm.)
BTX Binds to the Amino-Terminal Extracellular Domain of GABAAR β3.
We exploited the difference between GABAAR β1 and β3 subunits to localize the BTX-binding site. BTX bound to chimeric GABAAR subunits (11) in which the amino-terminal extracellular domain of GABAAR β1 was replaced by the corresponding domain of GABAAR β3, whereas no binding was detected to chimeras in which the amino-terminal extracellular domain of GABAAR β3 was replaced by the corresponding domain of GABAAR β1 (Fig. 3d). From these results, we conclude that BTX binds to the amino-terminal extracellular domain of GABAAR β3. Knowing that BTX also binds to an amino-terminal site in nAChR α1 and α7 subunits (15–17), which are distantly related to GABAARs, we compared nAChR and GABAAR sequences. Surprisingly, GABAAR β3 did not share residues critical for BTX binding to nAChRs, and no distinctive differences between GABAAR β1 and β3 sequences occurred in this domain (Fig. 8, which is published as supporting information on the PNAS web site). BTX may therefore bind to a previously uncharacterized motif on GABAAR β3. As discussed below, the binding site may involve an interface between adjacent β3 subunits.
BTX Blocks Chloride Conductance Through GABAAR β3 Receptors.
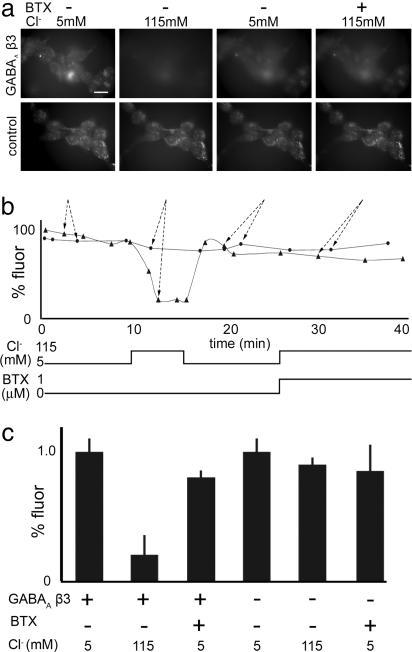
Knowing that BTX blocks activation of nAChRs, we asked whether BTX binding also affected the function of GABAARs. Because GABAARs are selectively permeable to chloride ions, we used the chloride indicator 6-methoxy-N-ethylquinolium iodide (MEQ), the fluorescence of which decreases as chloride concentration increases (18). When chloride levels in the medium bathing control HEK 293 cells were increased, a minimal change in MEQ fluorescence was observed (Fig. 4). In contrast, MEQ fluorescence decreased significantly when chloride was added to the medium bathing HEK 293 cells that had been stably transfected with GABAAR β3 (80 ± 10% decrease; Fig. 4). When chloride was added to the medium in the presence of BTX, MEQ fluorescence changed little (10 ± 5% decrease; Fig. 4). BTX had no effect on MEQ fluorescence in untransfected control cells. These results suggest that GABAAR β3 mediates a chloride conductance that can be blocked by BTX. The observation that homooligomeric β3-GABAARs are permeable to chloride in the absence of GABA is consistent with results of some (12) but not all (11) previous electrophysiological studies.
Fig. 4.
BTX blocks chloride influx in a GABAAR β3-expressing HEK 293 cell line. (a) Micrographs of cells incubated with the chloride sensor MEQ. (Upper) In cells stably transfected with GABAAR β3, MEQ fluorescence decreases when the extracellular chloride concentration is increased. The decrease is blocked by BTX. (Lower) Untransfected control HEK 293 cells show minimal response to changes in extracellular chloride concentration. (Scale bar, 5 μm.) (b) Quantitation of fluorescent signals from the experiment in a. •, control; ▴, GABAAR. Arrows show correspondence between micrographs in a and points in b. (c) Average MEQ fluorescence intensity in media containing 5 mM or 115 mM Cl− in the presence or absence of 1 μM BTX. Measurements in 115 mM Cl− were made 5 min after switching from 5 mM. Bars show mean ± SEM of measurements from 10–20 cells from five experiments.
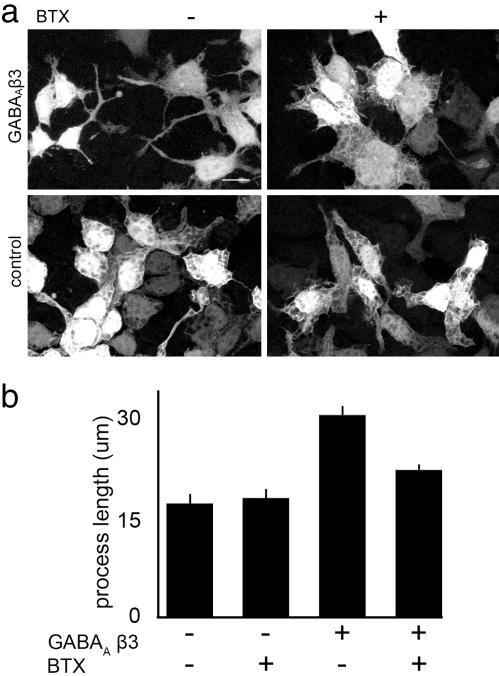
During the course of these experiments, we noted that cells expressing GABAAR β3 were more elongated than untransfected cells (Fig. 5a). We hypothesized that this difference resulted from the GABAAR-dependent chloride permeability documented above. To test this idea, we cultured cells in the presence or absence of BTX for 48 h and then measured cell shape. Chronic treatment with BTX decreased process length in GABAAR β3-expressing cells but had no effect on the shape of untransfected cells (Fig. 5).
Fig. 5.
BTX prevents the elongation of HEK 293 cells stably transfected with GABAAR β3. (a) Cellular processes in HEK 293 cells stably transfected with GABAAR β3 are longer than those in untransfected cells. Prolonged incubation with BTX shortens processes in cells stably transfected with GABAAR β3 but not in untransfected cells. All cells were transiently transfected with a plasmid encoding EGFP to label a subset of the cells in a field. (Scale bar, 5 μm.) (b) Quantification of process length. Measurements were made from the nucleus to the distalmost portion of a process. Bars show mean ± SEM of measurements from 150–200 cells from two experiments.
Analysis of Homooligomeric GABA β3 Receptors in Xenopus Oocytes.
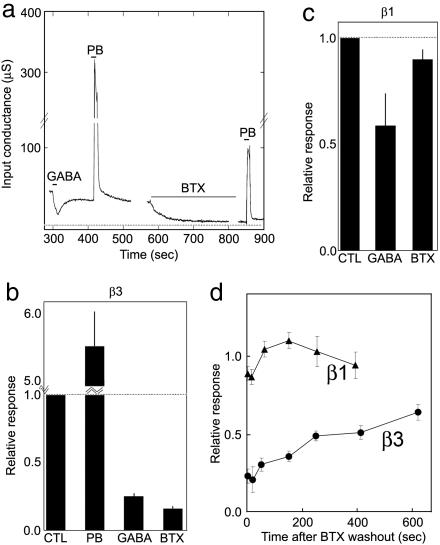
For a more detailed analysis of the effect of BTX on GABAAR function, we performed voltage clamp analysis of Xenopus oocytes that had been injected with GABAAR β3 mRNA. β3 subunits formed channels that were open in the absence of GABA: the conductance of β3-injected oocytes was ≈40-fold higher than that of controls (118 ± 68 μS, n = 16 vs. 3.0 ± 1.5 μS, n = 4; P < 0.00001 by t test). This conductance was blocked >90% by the GABAAR antagonist picrotoxin (reduced to 5.3 ± 3.2 μS, n = 16) and enhanced more than 5-fold by the GABAAR modulator pentobarbital (Fig. 6 a and b). Paradoxically, the picrotoxin-sensitive conductance was inhibited ≈70% by GABA; we cannot explain the discrepancy between this result and previous results (11–13), which showed GABAAR β3 homomeric channels to be either activated by or insensitive to GABA. Application of BTX had no detectable effect on the conductance of control eggs (P = 0.34) but decreased the picrotoxin-sensitive conductance of β3-injected eggs by >80% (Fig. 6 a and b). The effect appeared rapidly (forward rate constant = 23,000 M−1·s−1) and was slowly reversed after wash-off (t1/2 ≈ 10 min; Fig. 6d). The Kd calculated from these data, ≈100 nM, was similar to that determined directly (≈50 nM; Fig. 2a). BTX also decreased activation by pentobarbital (≈46% decrease, n = 10; Fig. 6a).
Fig. 6.
Effect of BTX on GABAAR currents recorded from Xenopus oocytes injected with mRNAs encoding GABAAR subunits. (a) Channels formed by GABAAR β3 are spontaneously open (12); conductance is blocked by 100 μM GABA or 1 μM BTX and potentiated by 100 μM pentobarbital (PB). Dotted line shows conductance after addition of 100 μM picrotoxin. (b) Quantification of effects of PB, GABA, and BTX on oocytes expressing GABAAR β3, obtained as in a; data are averaged from 13 oocytes. (c) Similar pharmacological analysis of channels formed by GABAAR β1 subunits; data are averaged from eight oocytes. (d) Recovery of picrotoxin-sensitive conductance in GABAAR β1- and β3-expressing oocytes after removal of BTX. Data are normalized to the preceding holding conductance.
We also tested the effect of BTX on GABAAR β1 homooligomers expressed in oocytes. β1 subunits formed channels that were blocked by picrotoxin (585 ± 470 μS vs. 30 ± 27 μS) and GABA (Fig. 6c). However, consistent with binding data reported above (Fig. 3), these channels were minimally sensitive to BTX (Fig. 6 c and d).
BTX Blocks GABAAR β3 Channels Containing a β3/β3 Interface.
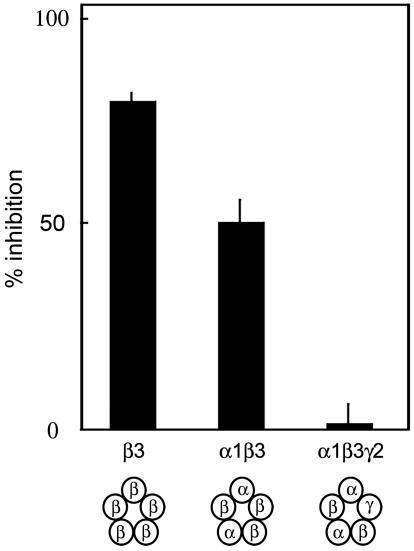
Although homooligomeric GABAARs are functional in nonneuronal cells, most GABAARs in neurons are composed of α-, β-, and γ-subunits (13). To ask whether BTX blocks heterooligomeric GABAARs, we analyzed oocytes that had been coinjected with GABAAR α1, β3, and γ2 RNAs. As expected (11, 13), GABA activated the resulting receptors, but BTX had no significant effect either on the ligand-independent conductance of the oocytes or on the response to GABA (Fig. 7).
Fig. 7.
BTX blocks conductance through GABAARs with a β3/β3 interface. RNAs encoding indicated combinations of GABAAR α1, β3, and γ2 subunits were injected into Xenopus oocytes, and current passage was measured before and after application of BTX. Schematics at bottom show predicted pentameric structures for each combination of GABAAR subunits. Note that a functional block was observed only for GABAARs predicted to contain an interface between two β3 subunits. Data are normalized to the response before BTX application. Bars show mean ± SEM from n = 4–13 oocytes.
Based on previous studies, the probable composition of these heterooligomeric receptors is [α1]2[β3]2[γ2]1 in the order α1-β3-γ2-α1-β3 (19–22). They are therefore unlikely to contain adjacent β3 subunits. The lack of effect of BTX on these receptors could indicate either that BTX is unable to antagonize the effects of GABA or that BTX binds to an interface between adjacent β3 subunits. To distinguish these possibilities, we analyzed oocytes injected with only GABAAR α1 and β3 RNAs. The oocytes exhibited GABA-activated currents, presumably mediated by GABAARs containing both α1 and β3 subunits. The composition of these receptors is likely to be [α1]2[β3]3 (19, 22), so they would be expected to bear a β3/β3 interface. BTX significantly blocked activation of these receptors by GABA (Fig. 7), supporting the idea that BTX binds to an interface between adjacent β3 subunits.
Discussion
We have shown that BTX binds not only to a subset of nAChRs but also to a subset of GABAARs, specifically, those containing an interface between two GABAAR β3 subunits. These results are significant in three respects.
First, although genetic and pharmacological studies clearly show that most BTX-binding sites in brain correspond to α7 nAChRs (23, 24), our results raise the possibility that some are GABAARs. This possibility is of particular interest, given the colocalization of nAChRs and GABAARs at a subset of GABAergic synapses (25, 26). Moreover, a brief report § demonstrates that embryonic muscle cells express GABAARs. Although the subunit composition of these receptors has not yet been reported, it may be necessary to consider whether some of the BTX binding to newly formed myotubes is to GABAARs.
Second, because the amino-terminal extracellular domain of GABAAR β3 does not contain significant homology to sequences on AChRs known to bind BTX (Fig. 8), GABAAR β3 may contain a previously uncharacterized BTX-binding sequence. This sequence could add to existing knowledge on the structures underlying BTX-binding peptides (27) and help to elucidate structural similarities between AChRs and GABAARs.
Third, BTX appears to bind to GABAARs with adjacent β3 subunits but not to heterooligomeric receptors composed of α-, β-, and γ-subunits. Most GABAARs reported in neurons to date are believed to include α-, β-, and γ- or α-, β-, and δ-subunits (13). However, we are aware of no studies that have directly tested the possibility that α/β-containing GABAARs or homomeric β-containing GABAARs are present in the brain (13). It is important to note that homomeric β3-GABAARs inactivated by GABA would almost certainly have escaped detection in cells that also bear heterooligomeric receptors, which are activated by GABA. BTX may be a useful reagent with which to seek such receptors in neurons.
Methods
Transfection and Staining of Cell Lines.
Eight-well chamber slides (Lab-Tek) were coated with rat-tail collagen in 30% ethanol. HEK 293, CHO, and N2a cells (American Type Culture Collection) were plated at 2 × 104 cells per well and cultured in DMEM containing 10% FCS. Glutamine (1 mM) was added to medium for N2a and CHO cells. After growth overnight, cells were transfected by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Plasmids encoding GABAAR subunits were obtained from the following sources: rat α1 and β2 were from A. Tobin (University of California, Los Angeles); human β1 and rat γ2L were from D. Weiss (University of Alabama at Birmingham); rat β3, α2, and γ2 were from P. Seeburg (Max Planck Institute for Medical Research, Heidelberg); human β3 was from G. White (Neurogen, Branford, CT); and rat β1 and β1/β3 chimeras were from L. Zhang (National Institutes of Health, Bethesda).
Cells were stained 36–48 h after transfection. Cells were stained in one of several ways: (i) For live staining, rhodamine-α-BTX (Molecular Probes) or anti-GABAAR β3 (MAB341, Chemicon) was added to media (2 μg/ml and 10 μg/ml final concentration, respectively) for 30 min at 37°C. Cells were then washed two times with media and fixed with 2% paraformaldehyde, rinsed with PBS containing a nuclear dye (DAPI), and mounted in 10% glycerol containing p-phenylenediamine. Where appropriate, a second antibody was added after fixation and before mounting. (ii) Alternatively, cells were fixed, washed, and incubated for 1 h in 0.1% Triton X-100/2% normal goat serum/2% BSA in PBS and then incubated in rhodamine-α-BTX (as above). (iii) In some cases, cells were incubated live with BTX and then fixed and incubated successively with rabbit antibodies to affinity-purified BTX (28) and Cy3-conjugated secondary antibody (Jackson ImmunoResearch). Images were taken on a Zeiss Axiovert epifluorescence microscope.
Quantitative Assessment of BTX Binding.
The apparent affinity of BTX for GABAARs was assessed as described in ref. 10. HEK cells were transfected with a GABAAR β3 plasmid that incorporated a neomycin resistance gene. Stably transfected cells were selected with G418, single cells were cloned, and clones expressing the construct in most or all cells were identified. Cells were plated in eight-well chamber slides, stained live at room temperature for 30 min with rhodamine-BTX, and then washed and fixed as above. Images were taken by using a ×10, 0.3 numerical aperture objective. Exposure times and illumination settings were selected to avoid saturated pixels in the wells stained with the highest concentration of probe. Using the same settings, multiple images were taken for each concentration of probe. Using metamorph software (Universal Imaging, Downingtown, PA), background values taken from images of identically stained untransfected cells were subtracted from images of transfected cells. Intensity histograms of the entire field were taken, and the most frequent pixel intensity of the histogram was recorded. Kd values were calculated from double reciprocal plots.
To assess pharmacological blockade of BTX binding, the GABAAR β3-expressing HEK 293 cell line was preincubated with blocker in DMEM for 20 min and then incubated in the same concentration of blocker along with 1 μg/ml rhodamine-BTX. Fluorescence intensity was then assessed as above.
Sequence Alignment.
Sequences of rat AChR α1, AChR α7, GABAAR β1, β2, and β3, and Torpedo AChR α1 were obtained from GenBank. Amino-terminal extracellular domains were identified by Swiss-Prot, and their sequences were aligned by using the AlignX algorithm of vectornti 9.0 (Invitrogen).
Imaging Chloride Concentrations in HEK Cells.
Stably transfected and control HEK 293 cells were plated on plastic tissue culture dishes (Nunc) coated with collagen, as described above. diH-MEQ (Molecular Probes) was prepared and applied to HEK 293 cells according to the manufacturer's protocol. Chloride concentration was manipulated by changing the extracellular solution with a syringe pump (Harvard Apparatus) and aspirator. The high- and low-chloride solution contained 110 mM NaCl or NaNO3, respectively, plus 5 mM KCl in 25 mM Hepes buffer (pH 7.2–7.4). Quantitative fluorescence was performed as above by using a water immersion objective (×40, 0.8 numerical aperture).
Morphological Change by BTX Incubation of Stably Transfected Cells.
HEK cells stably expressing GABAAR β3 were transiently transfected with a plasmid encoding EGFP (Clontech) to facilitate evaluation of cell shape. After transfection, cells were incubated in 4 μg/ml unlabeled BTX (Molecular Probes) for 48 h and then washed, fixed, and imaged. Process length of transfected cells was quantified by using Zeiss lsm 510 software.
Expression in Xenopus Oocytes and Physiology.
Receptors were expressed in Xenopus oocytes by injecting cRNA produced by using the mMessage mMachine T7 Ultra kit (Ambion, Austin, TX). Oocytes were injected with 19 nl of solution containing GABAAR β1 or GABAAR β3 cRNA at ≈1 μg/ml and incubated at 17°C in ND-96 solution. The GABAAR β3 subunit forms functional homomeric receptors with high efficiency. Therefore, when mixtures of subunits were injected, weight ratios of 2:1 and 2:1:4 were used for α1:β3 and α1:β3:γ2L, respectively, to enhance formation of heteromeric receptors.
Responses were recorded 1–3 days after injecting the oocytes by using a two-electrode oocyte clamp (Warner Instruments, Hamden, CT). Electrodes were filled with 3 M KCl and had resistances of 0.5–1 MΩ. The bath solution contained 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 10 mM Hepes; pH was adjusted to 7.5 with NaOH. The bath had a volume of ≈0.1 ml and was perfused with saline or drug solutions at ≈7 ml/min.
Responses and steady-state activity were measured by estimating the oocyte input conductance by using 400-ms, 10-mV hyperpolarizing pulses. Pulses were applied by using pclamp software (Axon Instruments, Union City, CA), and currents were filtered at 20 Hz and acquired by using a Digidata-1200B (Axon Instruments) at 20 samples per s. Subsequently, the current during the last 150 ms of each pulse was averaged, and then values were interpolated and subtracted to provide an estimate of input conductance by using pclamp and excel (Microsoft). Analysis was performed by using excel and sigmaplot (SSPS, Chicago).
Membrane conductance was measured continuously during the exposure to BTX. The effect of BTX was quantified by calculating the amount of picrotoxin-sensitive holding current present at the end of an ≈4-min application of 1 μM BTX relative to that immediately before the application (ratio). The apparent association rate was estimated from a semilogarithmic plot of the first 30 s of BTX exposure (β1: 0.002 ± 0.0004 s−1, n = 8; β3: 0.023 ± 0.0027 s−1, n = 13).
The recovery of baseline conductance after BTX treatment was relatively slow for β3 receptors. Because of the presence of a consistent diminution of both baseline conductance and pentobarbital responses (“rundown”) over time, recovery could not be quantified directly. However, the decline in picrotoxin-sensitive conductance could be described by a single exponential. Accordingly, the recovery was assessed by comparing the observed baseline current to the predicted current.
Oocytes expressing heteromeric receptors had significantly lower input conductance than homomeric β3 receptors (α1β3: 2.0 ± 0.2 μS, n = 7; αβ3γ2L: 15 ± 4 μS, n = 6; for both, P < 0.00002 for difference to β3 homomers) and a lower fraction of picrotoxin-sensitive conductance (28% and 57%, respectively). Responses were tested by using 10 μM GABA to avoid any contributions from possible homomeric β3 receptors.
Supplementary Material
Acknowledgments
We thank P. Seeburg, A. Tobin, D. Weiss, and G. White for providing GABAΑR cDNAs; L. Zhang for the GABAΑR β1/β3 chimeras; and J. Brubaker for preparation of diH-MEQ. This work was supported by grants from the National Institutes of Health (to J.R.S. and J.H.S.). J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology.
Abbreviations
- BTX
α-bungarotoxin
- GABAAR
GABAA receptor
- nAChR
nicotinic acetylcholine receptor
- MEQ
6-methoxy-N-ethylquinolium iodide
Footnotes
Conflict of interest statement: No conflicts declared.
Borodinsky, L. N. & Spitzer, N. C. (2005) 2005 Abstract Viewer and Itinerary Planner (Society for Neuroscience, Washington, DC), Program 27.1 (abstr.).
References
- 1.Sanes J. R., Lichtman J. W. Annu. Rev. Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Lee C. Y., Tseng L. F., Chin T. H. Nature. 1967;215:1177–1178. doi: 10.1038/2151177a0. [DOI] [PubMed] [Google Scholar]
- 3.Fambrough D. M. Physiol. Rev. 1979;59:165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Changeux J. P., Meunier J. C., Huchet M. Mol. Pharmacol. 1971;7:538–553. [PubMed] [Google Scholar]
- 5.Fertuck H. C., Salpeter M. M. J. Cell Biol. 1976;69:144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson M. J., Cohen M. W. J. Physiol. (London) 1977;268:757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kummer T., Misgeld T., Sanes J. R. Curr. Opin. Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Gotti C., Clementi F. Prog. Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Colquhoun L. M., Patrick J. W. Adv. Pharmacol. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- 10.McCann C. M., Bareyre F. M., Lichtman J. W., Sanes J. R. BioTechniques. 2005;38:945–952. doi: 10.2144/05386IT02. [DOI] [PubMed] [Google Scholar]
- 11.Miko A., Werby E., Sun H., Healey J., Zhang L. J. Biol. Chem. 2004;279:22833–22840. doi: 10.1074/jbc.M402577200. [DOI] [PubMed] [Google Scholar]
- 12.Wooltorton J. R., Moss S. J., Smart T. G. Eur. J. Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 13.Sieghart W., Sperk G. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 14.Connolly C. N., Wooltorton J. R., Smart T. G., Moss S. J. Proc. Natl. Acad. Sci. USA. 1996;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levandoski M. M., Lin Y., Moise L., McLaughlin J. T., Cooper E., Hawrot E. J. Biol. Chem. 1999;274:26113–26119. doi: 10.1074/jbc.274.37.26113. [DOI] [PubMed] [Google Scholar]
- 16.Balass M., Katchalski-Katzir E., Fuchs S. Proc. Natl. Acad. Sci. USA. 1997;94:6054–6058. doi: 10.1073/pnas.94.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinou M., Tzartos S. J. Biochem. J. 2003;372:543–554. doi: 10.1042/BJ20021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biwersi J., Verkman A. S. Biochemistry. 1991;30:7879–7883. doi: 10.1021/bi00246a001. [DOI] [PubMed] [Google Scholar]
- 19.Baumann S. W., Baur R., Sigel E. J. Biol. Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 20.Baumann S. W., Baur R., Sigel E. J. Biol. Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y., Wang R., Barot S., Weiss D. S. J. Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tretter V., Ehya N., Fuchs K., Sieghart W. J. Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr-Urtreger A., Goldner F. M., Saeki M., Lorenzo I., Goldberg L., De Biasi M., Dani J. A., Patrick J. W., Beaudet A. L. J. Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D., Patrick J. W. J. Biol. Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- 25.Kawai H., Zago W., Berg D. K. J. Neurosci. 2002;22:7903–7912. doi: 10.1523/JNEUROSCI.22-18-07903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabian-Fine R., Skehel P., Errington M. L., Davies H. A., Sher E., Stewart M. G., Fine A. J. Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs S., Kasher R., Balass M., Scherf T., Harel M., Fridkin M., Sussman J. L., Katchalski-Katzir E. Ann. N.Y. Acad. Sci. 2003;998:93–100. doi: 10.1196/annals.1254.011. [DOI] [PubMed] [Google Scholar]
- 28.Merlie J. P., Sebbanne R. J. Biol. Chem. 1981;256:3605–3608. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.