Abstract
Carbon monoxide (CO), an endogenous cytoprotective product of heme oxygenase type-1 regulates target thrombotic and inflammatory genes in ischemic stress. Regulation of the gene encoding early growth response 1 (Egr-1), a potent transcriptional activator of deleterious thrombotic and inflammatory cascades, may govern CO-mediated ischemic lung protection. The exact signaling mechanisms underlying CO-mediated cytoprotection are not well understood. In this study we tested the hypothesis that inhibition of mitogen-activated protein kinase-dependent Egr-1 expression may be pivotal in CO-mediated ischemic protection. In an in vivo isogeneic rat lung ischemic injury model, inhaled CO not only diminished fibrin accumulation and leukostasis and improved gas exchange and survival but also suppressed extracellular signal-regulated kinase (ERK) activation, Egr-1 expression, and Erg DNA-binding activity in lung tissue. Additionally, CO-mediated inhibition of Egr-1 reduced expression of target genes, such as tissue factor, serpine-1, interleukin-1, and TNF-α. However, CO failed to inhibit serpine-1 expression after unilateral lung ischemia in mice null for the Egr-1 gene. In RAW macrophages in vitro, hypoxia-induced Egr-1 mRNA expression was ERK-dependent, and CO-mediated suppression of ERK activation resulted in Egr-1 inhibition. Furthermore, CO suppression of ERK phosphorylation was reversed by the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one but was insensitive to cAMP-dependent protein kinase A inhibition with H89 and NO synthase inhibition with l-nitroarginine methyl ester. This finding indicates that CO suppresses ERK in a cGMP-dependent but cAMP/protein kinase A- and NO-independent manner. Together, these data identify a unifying molecular mechanism by which CO interrupts proinflammatory and prothrombotic mediators of ischemic injury.
Carbon monoxide (CO) is liberated endogenously by the inducible catabolic enzyme heme oxygenase type-1 (Hmox-1) in response to heat shock or oxidant stress. Although antiinflammatory effects of Hmox-1 (1, 2) were initially attributed to the antioxidants biliverdin and bilirubin (3), recent evidence suggests an important role for CO in vasodilation (4), suppressing cellular proliferation (4), and potentiating fibrinolysis (5). These effects may contribute to Hmox-1-mediated protection of the kidneys (6), liver (7), and lungs (5) against ischemia/reperfusion injury, contribute to long-term cardiac allograft acceptance (8), and promote reduction of hyperoxic lung injury (9).
CO is thought to elicit cellular protection during cellular stress by binding to the heme prosthetic group and activating soluble guanylate cyclase (sGC) (5). In addition, CO suppresses induction of plasminogen activator inhibitor-1 (PAI-1) (serpine-1) (5) after ischemia or lipopolysaccharide (LPS) challenge. Although sGC inhibition abrogates the effects of CO on PAI-1 expression, the signal transduction mechanism remains obscure. Furthermore, antiinflammatory (10), antithrombotic (5), and antiproliferative effects of CO (4) suggest regulation of a common signaling pathway mediating its protective effects.
To identify the mechanism underlying protective actions of CO, we evaluated the promoter region of serpine-1 gene based on published sequences in GenBank (accession no. M33961) and found two putative early growth response 1 (Egr-1) binding sites. Egr-1, a zinc finger transcription factor induced in ischemia, activates inflammatory and coagulant cascades. Stable transfection of Egr-1 gene into a subclone of fibrosarcoma cells induces serpine-1 secretion in vitro (11), and an absence of Egr-1 gene abrogates ischemic induction of serpine-1 in vivo (12). Furthermore, Egr-1 drives hypoxia-mediated induction of tissue factor (TF) (13), inflammatory cytokines, chemokines, and adhesion receptors (12) in ischemic injury.
Biological actions of CO in ischemia-associated thrombosis, fibrinolysis, and inflammation prompted us to ask whether mitogen-activated protein kinase (MAPK) and Egr-1 regulation is the principal intermediary link between tissue production of CO and ischemic protection. To test our hypothesis, we chose an established model of lung ischemia in which CO effects may be of greater importance, because protective levels of NO gas are virtually undetectable (14), and suppression of Egr-1 expression abrogates tissue injury (15). To further examine CO-mediated signaling mechanisms, we applied an in vitro cell culture model of RAW macrophages subjected to hypoxia. Our data demonstrate that CO-mediated inhibition of extracellular signal-regulated kinase 1 and 2 (ERK1/2)-driven Egr-1 expression and regulation of its downstream target genes is cGMP-dependent and central to CO-mediated protection against ischemia.
Results
CO Increases cGMP Levels in Transplanted Rat Lung.
CO activation of sGC produces cGMP within target cells. Under nonpreservation conditions (tissue samples obtained immediately upon lung harvest), inhaled CO elicited a dose-dependent increase in tissue cGMP levels; lungs excised from rats treated with 0.1% (1,000 ppm) CO for 16 h had a 70% increase in cGMP levels compared with naive (untreated) lungs (Fig. 1A). Tissue cGMP levels declined to very low levels during the 6-h hypothermic preservation in naive lungs. Inhalation of CO before lung harvest significantly increased cGMP levels, which remained elevated even at the end of preservation; cGMP levels were 0.8 pmol/mg of protein after 16 h of CO and 6 h of preservation, compared with lungs from naive rats 6 h after preservation (0.18 pmol/mg of protein). The guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (2 mg/kg), given to rat donors before lung harvest, significantly reduced cGMP levels after preservation despite concomitant CO (Fig. 1A).
Fig. 1.
CO increases cGMP and activates MAPK in transplanted lungs. (A) cGMP levels of lungs before [“Preservation (−)”] and after [“Preservation (+)”] 6-h hypothermic ischemic preservation. Lungs were excised from rats inhaling either RA (RA/normoxia), CO (for 16 h), or treated for 16 h with CO and then flushed/preserved with ODQ (2 mg/kg). n = 6 for each group. ∗, P < 0.05. (B) ERK1/2 phosphorylation by hypoxia and its suppression by CO were examined 45 min after lung transplantation. UnTx, nontransplanted lungs excised from naive rats breathing RA; RA, transplanted lungs from donor rats breathing RA; CO, transplanted lungs from rats given 0.1% CO 16 h before donor harvest; CO/ODQ, transplanted lungs from rats treated with 0.1% CO for 16 h and given ODQ (2 mg/kg) just before donor lung harvest. n = 4 for each group. ∗, P < 0.05, CO compared with RA/normoxia; ∗∗, P < 0.05, CO compared with CO/ODQ.
CO-Mediated Regulation of ERK in Transplanted Rat Lung and RAW Cells.
Because NO activates several MAPKs through a cGMP-dependent mechanism (16), we investigated whether CO could activate the three major mammalian MAPK families, namely, ERK1/2, c-Jun N-terminal kinase (JNK), and p38 MAPK. Four groups of rat lungs were studied: (i) untreated lungs rapidly excised from anesthetized/untreated rats, (ii) transplanted lungs from donor rats breathing room air (RA) (RA/normoxia), (iii) transplanted lungs from donors treated with CO (0.1%/6 h), or (iv) transplanted lungs from donors who inhaled CO but were injected with ODQ (2 mg/kg). Although total ERK1/2 protein was unchanged, phosphorylated ERK1/2 was remarkably increased after transplantation (≈11-fold for ERK1/2,). Inhaled CO significantly suppressed ERK1/2 phosphorylation and activation (Fig. 1B). ODQ completely reversed CO-mediated inhibition of ERK1/2 phosphorylation (Fig. 1B). JNK and p38 MAPK were also activated in transplanted lungs (≈4-fold increase of phospho-JNK and 6-fold increase of phospho-p38 MAPK) but were not significantly regulated by CO (Fig. 7, which is published as supporting information on the PNAS web site).
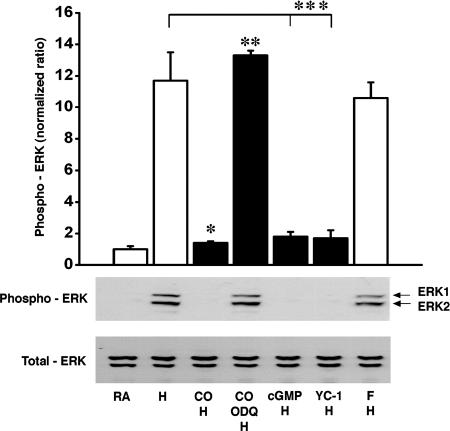
Macrophages are critical regulators of inflammatory and coagulant responses in ischemic (5, 12) and transplanted (15) lungs. Therefore, we examined ERK suppression by CO in a mononuclear phagocyte (RAW) cell culture model in which hypoxia serves as a paradigm for ischemic stress. Optimal temporal profiles to study CO effects on MAPK phosphorylation and activation in vitro were performed as described in Methods and based on experimental protocols described in ref. 16. CO significantly suppressed hypoxia-induced ERK1/2 phospho-activation (≈10 fold) in RAW cells (Fig. 2); suppression was abrogated by ODQ, suggesting cGMP dependence (Fig. 2), as noted in vivo. Furthermore, a cell-permeant analogue of cGMP, 8-bromo cGMP, suppressed ERK activation (Fig. 2). Likewise, the SGC activator YC-1 also significantly suppressed ERK phosphorylation (Fig. 2). YC-1-mediated ERK inhibition was reversed by ODQ but was insensitive to protein kinase A (PKA) inhibition with H89 (data not shown). Parallel to the in vivo results, JNK and p38 MAPK were activated in hypoxic RAW cells but not significantly regulated by CO (Fig. 8, which is published as supporting information on the PNAS web site). Together, these results indicate that ERK activation in hypoxia represents the dominant MAPK regulated by CO in a cGMP-dependent manner.
Fig. 2.
CO-mediated activation of MAPK in RAW cells is cGMP-dependent. Suppressive effects of CO on ERK1/2 phosphorylation induced by hypoxia in RAW cells is shown. Control group RA, RA/normoxic cells; H group, treated with hypoxia (H) for 1 h; CO group, treated with 550 ppm CO followed by hypoxia; CO/ODQ group, treated with 550 ppm CO and ODQ (10 μM/2 h) and then with hypoxia. n = 4 for each group. ∗, P < 0.05; ∗∗, 0.05; ∗∗∗, 0.05. Treatment of hypoxic RAW cells with the cGMP analogue (8-bromo-cGMP 0.1 mM/1 h) and the sGC activator YC-1 (30 μM/30 min) mimics the CO-mediated suppression of ERK1/2 phosphorylation. F group was treated with forskolin (10 μM/20 min) followed by hypoxia.
CO Regulates ERK Independent of cAMP/PKA and NO.
It is plausible that CO exposure could result in elevation of intracellular cAMP levels partly by reducing cAMP breakdown through inhibition of phosphodiesterase III (17). We therefore tested the effects of stimulating the cAMP–PKA pathway on ERK phosphorylation in RAW cells. Increasing intracellular cAMP by adenylate cyclase stimulation (by using forskolin) activated ERK under normoxic (Fig. 9A, which is published as supporting information on the PNAS web site) and hypoxic (Fig. 2) conditions. Hypoxia-induced ERK phosphorylation was significantly suppressed by CO, and the CO effect was reversed by ODQ. However, forskolin-induced ERK activation was unaffected by CO (Fig. 9A), and CO-mediated suppression of ERK phosphorylation was insensitive to PKA inhibition by H89 (Fig. 9A). Thus, inhibition of PKA did not affect CO-mediated regulation of ERK after hypoxia but effectively inhibited cAMP-mediated ERK activation.
Because CO has been demonstrated to increase NO under certain conditions (18), we tested whether a CO-mediated increase in cGMP is NO-dependent. CO regulation of ERK in hypoxic RAW cells was insensitive to pretreatment with the pan-NO synthase inhibitor l-nitroarginine methyl ester (L-NAME) (Fig. 9B). Furthermore, L-NAME treatment did not alter hypoxia-induced ERK activation and had no effect on ERK under normoxic conditions (Fig. 9B). Together, these data indicate that CO suppression of ERK activation is cAMP/PKA- and NO-independent.
CO Regulation of Egr-1 Expression in RAW Cells and Transplanted Rat Lung Is cGMP-Dependent.
To further delineate the signal transduction cascade linking CO inhalation to repression of thrombosis and inflammation, we examined the regulation of the transcription factor Egr-1 and its downstream target genes by CO. Egr-1 was a logical candidate because it is induced by phospho-ERK1/2 (19) and is a major cause of postischemic lung injury (12, 15). Hypoxic exposure caused a 7.6-fold increase of Egr-1 mRNA in RAW cells (Fig. 3A). CO treatment before hypoxia decreased Egr-1 expression by 62% (Fig. 3A). Only the specific ERK1/2 inhibitor PD98059 completely suppressed hypoxia-induced Egr-1 induction, whereas SB202190, a specific p38 MAPK inhibitor, had no effect (Fig. 3A). These results indicate that hypoxic induction of Egr-1 is primarily ERK1/2- (but not p38 MAPK-) dependent. These data strongly suggest that CO suppresses hypoxic induction of Egr-1 by interrupting ERK1/2 activation.
Fig. 3.
CO-mediated regulation of Egr-1 expression in RAW cells and transplanted lung tissue is ERK-dependent. (A) Effect of CO, PD98059 (ERK inhibitor), and SB202190 (p38 MAPK inhibitor) on Egr-1 mRNA induction by hypoxia in RAW cells. n = 4 for each group. ∗, P < 0.05. Mean values (±SEM) were calculated by densitometric scanning of blots, with the intensity of each Egr-1 band normalized to the corresponding β-actin band. (B) Effect of CO, ODQ (10 μM), hemin (50 μM), or ZnPP IX (10 μM) on Egr-1 mRNA induction by hypoxia in RAW cells. Hemin, ZnPP IX, or ODQ was added to culture media 2 h before onset of the hypoxic period. Five separate experiments were performed, and a representative blot is shown. ∗, P < 0.05. (C) Effect of inhaled CO in the presence or absence of ODQ on graft Egr-1 mRNA levels. Although a different cohort of animals was used, experimental conditions/groups were the same as described in the legend to Fig. 1B. n = 6 for each group. ∗, P < 0.05. (D) Effect of CO on Egr-1 protein expression in nuclei from lung grafts, analyzed by Western blotting and normalized to untreated samples. n = 6 for each group. ∗, P < 0.05. (E) Electrophoretic mobility gel shift assay performed on nuclear extracts with a 32P-labeled consensus probe for Egr-1. Lane 1 was loaded solely with buffer containing free 32P-labeled Egr-1 probe. Lane 2, nuclear extract from naive (untreated) lung; lane 3, nuclear extract from CO-treated/transplanted lung; lane 4, nuclear extract from normoxic/transplanted lung; lane 5, same sample as lane 4 in the presence of a 100-fold molar excess of unlabeled consensus Egr-1. The arrow indicates migration of the band corresponding to the Egr-1–DNA complex. Four experiments were performed with similar results, one of which is shown.
To ascertain the contribution of endogenous CO in Egr-1 suppression under hypoxic conditions, we examined Egr-1 expression in RAW cells. Egr-1 mRNA was strongly induced in hypoxic RAW cells, as reported in ref. 19 (Fig. 3B). Hemin (50 μM), a potent inducer of Hmox-1 (20) and a promoter of endogenous CO production, suppressed Egr-1 induction. In contrast, zinc protoporphyrin IX (ZnPP IX) (10 μM), a competitive inhibitor of Hmox-1 activity (5) and therefore an inhibitor of endogenous CO production, increased Egr-1 mRNA to a greater extent than hypoxia alone (Fig. 3B), indicating that endogenous CO modulates Egr-1 during hypoxia.
To test the hypothesis that cGMP is a pivotal intermediary in CO-mediated regulation of Egr-1, we next examined the effect of ODQ treatment and CO inhalation in transplanted rat lung tissue. Involvement of sGC in CO-mediated Egr-1 inhibition was exemplified by abrogation of CO-suppressive effects on Egr-1 mRNA in the presence of ODQ (Fig. 3C). Furthermore, CO decreased Egr-1 protein levels in lung grafts (Fig. 3D). To confirm that the decreased Egr-1 mRNA and protein in the CO group were associated with reduced Egr-1/DNA binding, we performed an electrophoretic mobility gel shift assay on nuclear extracts of lung graft from control and CO-treated rats. Whereas transplanted control lungs showed increased DNA binding (Fig. 3E, lane 4), transplanted lung tissue harvested from CO-treated animals demonstrated significant obliteration of the gel retardation band, corresponding with Egr-1/DNA binding (Fig. 3E, lane 3). Specificity of Egr-1–DNA interaction is evidenced by competition experiments in which a 100-fold molar excess of unlabeled Egr-1 probe added to the same sample as lane 4 abolished the appearance of the gel shift band. These data clearly demonstrate that CO suppresses Egr-1 induction during lung ischemia.
Functional Effects of CO on Coagulation, Fibrinolysis, and Inflammation in Lung Ischemia.
To determine whether Egr-1 induction underlies protective effects of CO, we examined expression of Egr-1 target genes (TF and serpine-1) after lung transplantation. Serpine-1 suppression by CO has been shown to be a key survival mechanism in pulmonary ischemia (5). CO suppressed serpine-1 mRNA (Fig. 4A) and TF mRNA (Fig. 4B) increased by orthotopic transplantation. As shown in ref. 5, ischemia/reperfusion strongly up-regulates serpine-1 in wild-type (Egr-1+/+) mice (Fig. 4C). Interestingly, mice null for the Egr-1 gene (Egr-1−/−) exhibited a modest induction of serpine-1 after ischemia/reperfusion, indicating that Egr-1 is an important regulator of ischemia-driven serpine-1 induction. In this identical model, CO has been shown to suppress serpine-1 induction by 60% (5). However, in Egr-1−/− mice, CO is unable to suppress serpine-1 induction (Fig. 4C). These data strongly demonstrate that Egr-1 plays a critical role in the biological effects of inhaled CO.
Fig. 4.
CO down-regulates mediators of graft thrombosis in transplanted lungs. (A) Effect of CO (0.1% for 24 h) on serpine-1 expression (mRNA) in transplanted lungs. Data shown are representative of at least three separate experiments. ∗, P < 0.05. (B) Effect of CO on graft TF mRNA levels. n = 6 for each group. ∗, P < 0.05. (C) Effect of CO (0.1% for 24 h) on serpine-1 expression in ischemic lungs of Egr-1+/+ and Egr-1−/− mice. Five separate experiments were performed, and a representative blot is shown. ∗, P < 0.05. (D) Effect of CO on graft fibrin accumulation, analyzed by Western blotting. Specimens were obtained from either untreated/nontransplanted (UnTx) lungs or transplanted lungs excised from rats treated with CO (0.1% for 16 h) or those breathing RA. ∗, P < 0.05.
We next measured accumulation of fibrin by immunoblotting lung extracts from rats heparinized immediately ante mortem. Transplantation caused a 5.1-fold increase in fibrin accumulation compared with untreated intact lungs (Fig. 4D). CO treatment of lung donors markedly decreased tissue fibrin accumulation, illustrating that inhaled CO promotes fibrin dissolution.
Because Egr-1 also regulates ischemic induction of inflammatory genes (12), we examined effects of CO on the expression of proinflammatory cytokines (IL-1β) and TNF-α. Both IL-β mRNA (Fig. 5A) and TNF-α protein (Fig. 5B) were up-regulated by transplantation, and their induction was suppressed by CO. CO significantly reduced leukocyte recruitment after transplantation as measured by graft myeloperoxidase activity (Fig. 5C). These data indicate that CO potently suppresses the induction of proinflammatory cytokines and graft leukostasis after lung transplantation.
Fig. 5.
CO attenuates inflammatory activation in lung grafts. Effect of CO on pulmonary graft IL-1β mRNA levels (A), TNF-α protein expression (B), and graft neutrophil sequestration (C), measured by myeloperoxidase activity assay, is shown. Experimental conditions are as described for Fig. 4. All values are normalized to those of the UnTx group.
Effect of CO on Lung Function and Pulmonary Graft Survival.
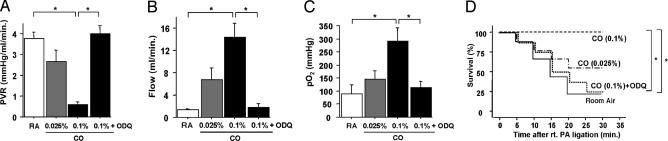
To further determine the physiological consequences of Egr-1 suppression by CO, we used a stringent model of lung ischemia (lung transplantation), because it enables instrumentation of animals for physiological measurements. Reduced graft microvascular thrombosis and leukostasis induced by CO results in distinct functional improvements after orthotopic lung transplantation. CO significantly reduced pulmonary vascular resistance of transplanted lungs and increased arterial blood flow (Fig. 6A and B) in a dose-dependent manner. In addition, inhaled CO improved arterial oxygenation and recipient survival (Fig. 6 C and D). ODQ treatment (2 mg/kg) abolished CO’s beneficial effects on both lung-graft function and recipient survival. These data emphasize that the beneficial effects of CO are likely mediated by activation of sGC.
Fig. 6.
CO improves lung function and graft survival. Effect of inhaled CO and guanylate cyclase inhibition with ODQ on transplanted lung function and survival are shown. ∗, P < 0.05. Effect of CO and ODQ on pulmonary vascular resistance (PVR) of transplanted lungs (A) and graft pulmonary arterial flow (B). n = 6 for each group. (C) Effects of CO and ODQ on pulmonary function measured as arterial oxygenation. n = 6 for each group. Recipients were ventilated with 100% O2 throughout the posttransplant period. (D) A separate cohort of animals was used for survival measurements. Effects of CO and ODQ on recipient survival are shown. Survival was recorded for 30 min after ligation of the right pulmonary artery. n = 9 for each group.
Discussion
The major finding of the current study is that CO inhibition of ERK-dependent Egr-1 expression interrupts proinflammatory and prothrombotic mediators in ischemic lung injury. In lung transplantation, levels of NO, the dominant beneficial gaseous mediator, fall precipitously because of quenching by superoxide. However, because CO is more stable and shares similar reactivity with heme prosthetic groups, some biological activities ascribed to NO during ischemia could be attributed to increased CO. Moreover, despite the primary recognition of CO as a lethal inhalant or respiratory asphyxiant, data from this study indicate that, at low doses, CO can be therapeutically useful in a clinically relevant model. Most notably, the antithrombotic and antiinflammatory activities of CO appear to confer functional benefit under ischemic stress.
We further demonstrate ERK1/2 phosphorylation as a proximal event after lung ischemia that leads to Egr-1 induction. The involvement of ERK1/2 in lung transplant injury is not surprising, given that, in addition to hypoxic/ischemic stress, MAPK pathways can be rapidly activated by other triggers of cellular stress, including heat shock, UV light, inflammatory cytokines, and endotoxin. Although three primary MAPKs have been reported in mammalian cells (ERK, JNK, and p38 MAPK), data shown here support a primary role for ERK1/2.
The signaling effects of CO through cGMP demonstrated here are similar to NO/Egr-1 signaling. NO down-regulates shear stress induction of Egr-1 by inhibition of ERK1/2 phosphorylation (21). NO donors not only suppress Egr-1 mRNA but also reduce Egr-1 protein expression and transcriptional activity (22). Moreover, NO also reduces Egr-1 mRNA induction in rat macrophages treated with LPS (23). cGMP-dependent CO suppression of ERK1/2 phosphorylation in RAW cells shown here is similar to the cGMP-dependent NO effects in human platelets and rat vascular smooth muscle cells (24). These findings are concordant with our data showing a cGMP-dependent CO suppression of ERK1/2. Regulation of ERK1/2 by CO results in Egr-1 attenuation and a consequential decrease in proinflammatory and prothrombotic gene targets of Egr-1 in ischemic tissue. Although CO increases NO under certain experimental conditions (18), here the pan-NO synthase blocker L-NAME failed to inhibit the suppressive effect of CO on ERK phosphorylation in hypoxic RAW cells. This finding indicates that cGMP-dependent CO effects on ERK activation are NO-independent, and generation of NO by CO does not appear functionally important in the models presented here.
Prolonged CO exposure may influence other signaling pathways, such as cAMP/PKA, through elevation of intracellular cAMP levels (17), resulting in secondary ERK regulation. Our data demonstrate that increasing cAMP-activated ERK in RAW cells and CO was unable to abrogate cAMP-mediated ERK activation. Furthermore, inhibition of PKA did not affect CO-mediated regulation of ERK phosphorylation after hypoxia while effectively inhibiting cAMP-mediated ERK activation. Together, these data support a cAMP/PKA-independent signaling by CO.
Opposing effects of hemin (inducer) and ZnPP IX (inhibitor) of Hmox-1 on endogenous CO production and regulation of Egr-1 demonstrated in this study illustrate how Hmox-1 may reduce postischemic inflammation. Our data are consonant with reports of potent antiinflammatory effects of Hmox-1 in other model systems. In vivo transfection of rat lungs increased Hmox-1 protein, attenuated neutrophil infiltration, and conferred cytoprotection in hypoxic injury (9). In a microvascular model of venular leukocyte adhesion, Hmox-1 induction reduced leukocyte adhesion, and blockade of its activity with ZnPP IX increased adhesion (1). Although this affect was ascribed to the bile pigment antioxidants generated by Hmox-1 activity, a role for CO was not excluded. The cytoprotective role of Hmox-1 was further substantiated in a rat model of endotoxemia, in which Hmox-1 induction before endotoxin challenge was beneficial, and exacerbation of outcome was observed after Hmox-1 inhibition (25).
Because beneficial effects of CO are cGMP-dependent, hypothetically, a brief exposure of CO should suffice in conferring protection. In this study, longer duration (up to 16 h) of CO stimulation was required to block downstream signaling through ERKs to suppress Egr-1. The exact reasons for this result are presently unclear. Arguably, effective attenuation of Egr-1 gene expression is likely dependent on sustained and sufficient levels of cGMP to adequately suppress ERK activation after hypoxia/ischemia. In contrast to known NO-mediated rapid but transient increases in cGMP, the effects of CO, which is more stable than NO, are likely temporally delayed but may remain sustained.
Our data indicate that cGMP levels rise substantially in lungs preserved with inhaled CO, in parallel with potent suppression of postischemic lung inflammation. Because functional benefits of CO were blocked by ODQ, cGMP formation likely represents an important intermediary step in the antiinflammatory and antithrombotic effects of CO in lung transplantation. These data are in contrast to in vitro evidence of cGMP-independent antiinflammatory effects exerted by CO in LPS-challenged macrophages (10). The reasons for the differences in our findings are not entirely clear. In a recent murine study, although both lung ischemia and LPS administration triggered induction of a similar profile of proinflammatory cytokines, adhesion receptors, and procoagulant mediators, only ischemia (not LPS) induced Egr-1 (12). Model-related differences may also underlie the divergent effects observed with CO administration and MAPK induction between the LPS-mediated effects versus ischemia. Although CO apparently did not affect ERK1/2 phosphorylation after LPS challenge in RAW cells (10), it potently did so in hypoxic RAW cells in our experiments. Our data are similar to effects reported in pulmonary artery smooth muscle cells, in which both NO donors and a cGMP mimetic suppressed phospho-activation of ERK, which was reversed by a cGMP/cGMP-dependent protein kinase inhibitor (16).
With regard to the physiological effects of endogenous and exogenous CO, the current experiments establish a paradigm to explain how CO suppresses thrombosis and inflammation. Here we demonstrate that CO suppressed ischemic induction of Egr-1 as well as Egr-1/DNA binding and abrogated expression of a prototypical Egr-1-responsive gene, serpine-1. In Egr-1 gene null mice, CO failed to suppress ischemia-induced serpine-1 gene expression. A cascade of events leading from Hmox-1 induction to CO generation, through an intermediary step of ERK phosphorylation, represses the Egr-1 “master switch” (12). Induction of Egr-1 by hypoxia (19) or ischemia (12) triggers procoagulant and inflammatory genes (12, 13, 19, 26) such as TF, serpine-1, and IL-1β. Modulation of these genes by CO (5, 10) may contribute to its clinically beneficial actions in lung transplantation (27). The present study underscores a cGMP-dependent mechanism by which CO prevents ischemic induction of downstream target genes through ERK/Egr-1 suppression, thereby reducing inflammatory and thrombotic mediators during ischemia.
Methods
Hypoxia and CO Exposure.
Mouse macrophage cells (RAW cells) were placed in a normobaric normoxic environment. See Supporting Text, which is published as supporting information on the PNAS web site, for more details. For the analysis of cell signaling the following were used: MAPK kinase/MEK (upstream of ERK) inhibitor PD98059 (10 μM/10 min; Calbiochem); p38 MAPK inhibitor, SB202190 (100 nM/10 min; Calbiochem); adenylate cyclase activator forskolin (10 μM/20 min; Sigma); PKA inhibitor H89 (20 μM/20 min; Sigma); NO synthase inhibitor L-NAME (1 mM/24 h; Sigma); guanylate cyclase activator (28) YC-1 [1-benzyl-3-(5′-hydroxymethyl-2′-furyl)indazole] (30 μM/30 min); 8-bromo-cGMP (0.1 mM/30 min to 2 h; Sigma); and guanylate cyclase inhibitor ODQ (24) (10 μM/2 h; Sigma) were added before hypoxia (1 h). Exposure to CO was accomplished by using a similar normobaric environment, except that the gas mixture was comprised of 550 ppm CO, 5% CO2, and the balance RA (5). For in vitro experiments, either media equilibrated with CO (550 ppm) for 16–24 h was added to RAW cells, or cells were incubated for 24 h in the CO chamber.
Rat Lung Transplant Experiments.
An orthotopic, isogeneic rat left lung transplant model was used, with donor preexposure to room air or CO (0.1% or 1,000 ppm for 16–24 h before surgery) as indicated. See Supporting Text for more details.
Murine Lung Ischemia Experiments.
The effect of inhaled CO on expression of serpine-1 was examined in 8- to 10-week-old male Egr-1−/− mice (12, 29) and compared with Egr-1+/+ littermate controls (genotype confirmed by Southern blotting as described in refs. 5, 30, and 31). CO exposure of mice was similar to rat experiments.
Electrophoretic Mobility Gel Shift Assay.
Gel shift assays were performed on nuclear extracts prepared immediately after lung harvest, as described in refs. 31 and 32.
Northern Blotting.
Twenty micrograms of total RNA from tissue or cell culture samples per lane was resolved on 0.8% agarose/formaldehyde gel, electrophoresed, and transferred to Duralon-UV membranes (Stratagene). Membranes hybridized with 32P-labeled cDNA probes for Egr-1 (33, 34), IL-1β (12), TF, or serpine-1. Human β-actin cDNA served as an RNA loading control, and quantification was performed with National Institutes of Health image analysis.
Western Blotting.
Immunoblotting was performed by using standard methods with primary antibodies; rabbit anti-phospho- or total p42/p44 MAPK IgG, rabbit anti-phospho- or total JNK IgG, rabbit anti-phospho- or total p38 MAPK IgG (Cell Signaling Technology, Beverly, MA), rabbit anti-Egr-1 IgG, goat anti-TNF-α (Santa Cruz Biotechnology), or mouse anti-fibrin IgG (Biodesign International, Saco, ME).
Myeloperoxidase and cGMP Assays.
Tissue myeloperoxidase activity was measured as an index of graft leukocyte accumulation as described in ref. 35.
Statistical Analysis.
The product limit (Kaplan–Meier) estimate of cumulative survival was assessed by the log-rank test; ∗, P < 0.05. Statistical significance was analyzed by ANOVA by using commercial software (statview; Cary, NC). For all experiments, data are shown as mean ± standard error of the mean; ∗, P < 0.05.
Supplementary Material
Acknowledgments
This study was supported in part by U.S. Public Health Service, National Institutes of Health Grants R01 HL55397, R01 HL69448, and R01 HL59488.
Abbreviations
- Egr-1
early growth response 1
- ERK
extracellular signal-related kinase
- ERK1/2
ERK 1 and 2
- Hmox-1
heme oxygenase type-1
- JNK
c-Jun N-terminal kinase
- L-NAME
l-nitroarginine methyl ester
- MAPK
mitogen-activated protein kinase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PKA
protein kinase A
- RA
room air
- sGC
soluble guanylate cyclase
- TF
tissue factor
- ZnPP IX
zinc protoporphyrin IX.
Footnotes
Conflict of interest statement: D.J.P. is an inventor on a CO-related patent application filed by Columbia University and may benefit according to Columbia University inventorship policies. D.J.P. also serves as a paid consultant to AGA Linde Healthcare.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hayashi S., Takamiya R., Yamaguchi T., Matsumoto K., Tojo S. J., Tamatani T., Kitajima M., Makino N., Ishimura Y., Suematsu M. Circ. Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 2.Bowry V. W., Mohr D., Cleary J., Stocker R. J. Biol. Chem. 1995;270:5756–5763. doi: 10.1074/jbc.270.11.5756. [DOI] [PubMed] [Google Scholar]
- 3.Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 4.Duckers H. J., Boehm M., True A. L., Yet S. F., San H., Park J. L., Clinton Webb R., Lee M. E., Nabel G. J., Nabel E. G. Nat. Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 5.Fujita T., Toda K., Karimova A., Yan S. F., Naka Y., Yet S. F., Pinsky D. J. Nat. Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 6.Maines M. D., Raju V. S., Panahian N. J. Pharmacol. Exp. Ther. 1999;291:911–919. [PubMed] [Google Scholar]
- 7.Amersi F., Buelow R., Kato H., Ke B., Coito A. J., Shen X. D., Zhao D., Zaky J., Melinek J., Lassman C. R., et al. J. Clin. Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock W. W., Buelow R., Sayegh M. H., Turka L. A. Nat. Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 9.Otterbein L. E., Kolls J. K., Mantell L. L., Cook J. L., Alam J., Choi A. M. J. Clin. Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otterbein L. E., Bach F. H., Alam J., Soares M., Tao Lu H., Wysk M., Davis R. J., Flavell R. A., Choi A. M. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 11.Liu C., Yao J., de Belle I., Huang R. P., Adamson E., Mercola D. J. Biol. Chem. 1999;274:4400–4411. doi: 10.1074/jbc.274.7.4400. [DOI] [PubMed] [Google Scholar]
- 12.Yan S. F., Fujita T., Lu J., Okada K., Shan Zou Y., Mackman N., Pinsky D. J., Stern D. M. Nat. Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 13.Yan S. F., Zou Y. S., Gao Y., Zhai C., Mackman N., Lee S. L., Milbrandt J., Pinsky D., Kisiel W., Stern D. Proc. Natl. Acad. Sci. USA. 1998;95:8298–8303. doi: 10.1073/pnas.95.14.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinsky D. J., Naka Y., Chowdhury N. C., Liao H., Oz M. C., Michler R. E., Kubaszewski E., Malinski T., Stern D. M. Proc. Natl. Acad. Sci. USA. 1994;91:12086–12090. doi: 10.1073/pnas.91.25.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada M., Fujita T., Sakaguchi T., Olson K. E., Collins T., Stern D. M., Yan S.F., Pinsky D. J. FASEB J. 2001;15:2757–2759. doi: 10.1096/fj.01-0490fje. [DOI] [PubMed] [Google Scholar]
- 16.Mitani Y., Zaidi S. H., Dufourcq P., Thompson K., Rabinovitch M. FASEB J. 2000;14:805–814. doi: 10.1096/fasebj.14.5.805. [DOI] [PubMed] [Google Scholar]
- 17.Lim I., Gibbons S. J., Lyford G. L., Miller S. M., Strege P. R., Sarr M. G., Chatterjee S., Szurszewski J. H., Shah V. H., Farrugia G. Am. J. Physiol. Gastrointest. 2005;288:G7–G14. doi: 10.1152/ajpgi.00205.2004. [DOI] [PubMed] [Google Scholar]
- 18.Thom S. R., Xu Y. A., Ischiropoulos H. Chem. Res. Toxicol. 1997;10:1023–1031. doi: 10.1021/tx970041h. [DOI] [PubMed] [Google Scholar]
- 19.Yan S. F., Lu J., Zou Y. S., Soh-Won J., Cohen D. M., Buttrick P. M., Cooper D. R., Steinberg S. F., Mackman N., Pinsky D. J., Stern D. M. J. Biol. Chem. 1999;274:15030–15040. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- 20.Marton L. S., Wang X., Kowalczuk A., Zhang Z. D., Windmeyer E., Macdonald R. L. Am. J. Physiol. 2000;279:H2405–H2413. doi: 10.1152/ajpheart.2000.279.5.H2405. [DOI] [PubMed] [Google Scholar]
- 21.Chiu J. J., Wung B. S., Hsieh H. J., Lo L. W., Wang D. L. Circ. Res. 1999;85:238–246. doi: 10.1161/01.res.85.3.238. [DOI] [PubMed] [Google Scholar]
- 22.Rupprecht H. D., Akagi Y., Keil A., Hofer G. Kidney Int. 2000;57:70–82. doi: 10.1046/j.1523-1755.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- 23.Guha M., O’Connell M. A., Pawlinski R., Hollis A., McGovern P., Yan S. F., Stern D., Mackman N. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- 24.Moro M. A., Russel R. J., Cellek S., Lizasoain I., Su Y., Darley-Usmar V. M., Radomski M. W., Moncada S. Proc. Natl. Acad. Sci. USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otterbein L., Sylvester S. L., Choi A. M. Am. J. Respir. Cell Mol. Biol. 1995;13:595–601. doi: 10.1165/ajrcmb.13.5.7576696. [DOI] [PubMed] [Google Scholar]
- 26.Yan S. F., Lu J., Zou Y. S., Kisiel W., Mackman N., Leitges M., Steinberg S., Pinsky D., Stern D. J. Biol. Chem. 2000;275:11921–11928. doi: 10.1074/jbc.275.16.11921. [DOI] [PubMed] [Google Scholar]
- 27.Song R., Kubo M., Morse D., Zhou Z., Zhang X., Dauber J. H., Fabisiak J., Alber S. M., Watkins S. C., Zuckerbraun B. S., et al. Am. J. Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamothe M., Chang F. J., Balashova N., Shirokov R., Beuve A. Biochemistry. 2004;43:3039–3048. doi: 10.1021/bi0360051. [DOI] [PubMed] [Google Scholar]
- 29.Lee S. L., Sadovsky Y., Swirnoff A. H., Polish J. A., Goda P., Gavrilina G., Milbrandt J. Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- 30.Morita T., Mitsialis S. A., Koike H., Liu Y., Kourembanas S. J. Biol. Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 31.Okada K., Fujita T., Minamoto K., Liao H., Naka Y., Pinsky D. J. J. Biol. Chem. 2000;275:21468–21476. doi: 10.1074/jbc.M002682200. [DOI] [PubMed] [Google Scholar]
- 32.Dignam J. D., Lebovitz R. M., Roeder R. G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan S. F., Tritto I., Pinsky D., Liao H., Huang J., Fuller G., Brett J., May L., Stern D. J. Biol. Chem. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- 34.Lemaire P., Vesque C., Schmitt J., Stunnenberg H., Frank R., Charnay P. Mol. Cell. Biol. 1990;10:3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinsky D. J., Oz M. C., Koga S., Taha Z., Broekman M. J., Marcus A. J., Liao H., Naka Y., Brett J., Cannon P. J., et al. J. Clin. Invest. 1994;93:2291–2297. doi: 10.1172/JCI117230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.