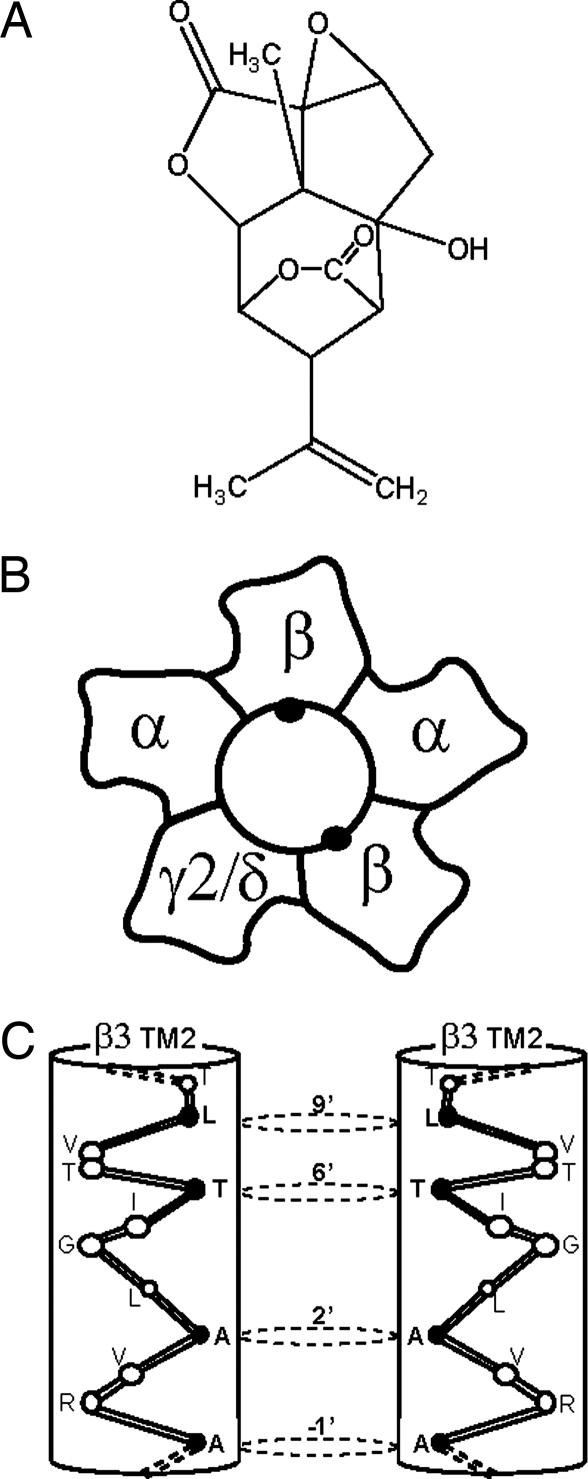
Picrotoxin (PTX) is the prototypic antagonist of GABAA receptors (GABARs), the primary mediators of inhibitory neurotransmission (rapid and tonic) in the nervous system. Picrotoxinin (Fig. 1A), the active ingredient in this plant convulsant, structurally does not resemble GABA, a simple, small amino acid, but it is a polycylic compound with no nitrogen atom. The compound somehow prevents ion flow through the chloride channel activated by GABA in the GABAR, a member of the cys-loop, ligand-gated ion channel superfamily. Unlike the competitive GABAR antagonist bicuculline, PTX is clearly a noncompetitive antagonist (NCA), acting not at the GABA recognition site but perhaps within the ion channel. Thus PTX appears to be an excellent example of allosteric modulation, which is extremely important in protein function in general and especially for GABAR (1). Recent advances in structural modeling of GABAR (Fig. 1 B and C) are consistent with action of PTX and analogous convulsants as NCAs. In a recent issue of PNAS, Chen et al. (2) describe how numerous drugs in this category, with a variety of pharmacological effects, can interact with the same domain of the GABAR protein within the ion channel.
Fig. 1.
Schematic view of the GABAA receptor–chloride channel and its blocker picrotoxinin. (A) Chemical structure of picrotoxinin. (B) Structure of a cys-loop ligand-gated ion channel receptor. Schematic view of a native GABAR heteropentamer, with the channel in the center, formed of two copies of an α subunit (α1–α6), two copies of a β subunit (β1, β2, or β3), and one additional type, like γ2 or δ. (C) Cytoplasmic end of the TM2/channel α-helix for two β3 subunits (black dots in B; the other three subunits are not shown) in GABAR, showing the PTX site residues near 2′–9′ (residues are numbered 1′–23′ from the N-terminal bottom of the helix to the C-terminal extracellular end of the helix).
PTX has an interesting history as a drug and research tool. Many naturally occurring and synthetic convulsive agents, also blockers of GABA-mediated inhibition, have some rather famous uses, such as stimulants, convulsants, chemical warfare agents, and insecticides, to name a few (3, 4). PTX is isolated from plants of the moonseed family, Menispermaceae, and its close relatives tutin and coriamyrtin, from the New Zealand tutu plant Coriaria arborea, known as a “loco weed” that caused occasional poisonings in cows and even in people. PTX was shown to be a stimulant and could cause tonic-clonic convulsions at fairly low doses, but was listed in the Merck Index as late as the 1970s as an “antidote for barbiturate overdose” (5). It was shown to antagonize inhibitory pathways in the nervous system activated by GABA, and GABA-enhancing drugs like barbiturates and benzodiazepines reverse its action. In vitro, PTX inhibited GABA-activated inhibitory currents in neurons and crayfish muscle (4, 6). Radioactive [3H]dihydropicrotoxinin bound to specific sites in crayfish muscle and mammalian brain that could be identified as GABARs (7).
Casida and Palmer (8) have synthesized and studied a series of synthetic potent neurotoxic chemicals, the “cage convulsants.” These compounds were developed as insecticides, aimed at the common target, acetylcholineresterase, like the well known malathion. These cage convulsants were not inhibitors of this enzyme, but they were equally toxic, like nerve gas, but were acting on another target in the nervous system. Instead of an acetylcholine (ACh) target, the cage convulsants are noncompetitive GABAR antagonists acting at the PTX site: they inhibit GABAR currents and synapses in mammalian neurons and inhibit [3H]dihydropicrotoxinin binding to GABAR sites in brain membranes (7, 9). A potent example, t-butyl bicyclophosphorothionate, is a major research tool used to assay GABARs by radio-ligand binding (10).
This drug target appears to be the site of action of the experimental convulsant pentylenetetrazol (1, 4) and numerous polychlorinated hydrocarbon insecticides, including dieldrin, lindane, and fipronil, compounds that have been applied in huge amounts to the environment with major agricultural economic impact (2). Some of the other potent toxicants/insecticides were also radiolabeled and used to characterize receptor action, allowing structure–function analysis of the chemical family on GABAR in both insects and mammals, and to define the receptor site (2, 4, 8). The point of ref. 2 is that this series of compounds has the same molecular target (“receptor”) despite considerable differences in chemical structure.
Thujone, a constituent of oil of wormwood, found in the beverage absinthe, was also shown by Casida and colleagues (11) to target the GABAR channels. Absinthe is the green-colored alcoholic beverage popular in fin de siecle Paris, now illegal because of its toxicity. Absinthe was not only the wildly popular favorite of Parisians (in addition to wine, of course, but there were more absinthe parlors than bread stores!), but considered then and now to impart a special creative stimulus to the cultural elite of the day. To paraphrase my commentary (12), why would a drug with toxic and convulsant actions possibly be considered pleasant or at least desirable? Thujone, like PTX, is excitatory on the brain (analeptic) [not a depressant, like marijuana]. Such an agent may produce mood elevation and antidepressant, anxiety-generating, and alerting effects, as opposed to the anxiolytic, sedative, and amnestic effects of GABA-enhancing drugs like benzodiazepines and ethanol (1). Do not forget, however, that in absinthe one is balancing the stimulatory effect of thujone with the intoxicating and depressant effects of ethanol.
Another important development in establishing the connection between PTX-like NCA and GABAR was the independent identification of the site of action of insecticides like dieldrin. In addition to the critical demonstration by Casida and Palmer (8) and others that many insecticides inhibited t-butylbicyclophosphoro[35S]thionate (TBPS) binding (and GABAR channels), a GABAR homolog gene was cloned from insects by using dieldrin resistance in houseflies as a screen. Comparing resistant and sensitive animals led to the identification of the Rdl gene in Drosophila, which turned out to be the insect orthologue of the mammalian GABAR β subunit (13). The mutation conferring resistance to dieldrin (which acts like PTX to noncompetitively antagonize GABAR) corresponds to A2′S, a residue in the TM2 domain/ion channel of the Rdl subunit of the GABAR protein, and is needed for [35S]TBPS binding (14–18). As described by Chen et al. (2), this residue and others lining the channel at the cytoplasmic (N-terminal) end (Fig. 1C) participate in the binding site for dieldrin, PTX, and the rest of the toxicants and insecticides in the NCA family. An important contribution showing that this domain (amino acids in the channel wall in TM2) actually provides a contact point for NCA ligands, rather than just conformational coupling, was the demonstration that an affinity-labeled sulfhydryl reagent based on the structure of fipronil bound covalently to the cysteine mutation at the 2′ residue (V257) in TM2 of the GABAR α1 subunit (17).
The type of structural modeling and analysis in Chen et al. (2) is especially valuable for the ligand-gated ion channel receptor superfamily, whose structures have not been solved by x-ray crystallography. Crystallography data on the snail ACh binding protein (19), which adapts a homopentameric structure that binds ACh, yielded a structure that is not only homologous to the nicotinic ACh receptor (nAChR), but verified the subunit arrangements and interfaces and agonist and modulator binding pocket placement: amino acids involved in agonist site binding have been identified in the extracellular domain at subunit interfaces by a combination of affinity labeling and mutagenesis (20–22). Homologous polypeptide loops are involved in GABAR ligand binding pockets. The GABARs have allosteric ligand binding pockets in the extracellular domain involving modified agonist sites at subunit interfaces for BZ ligands and possibly ethanol (1, 14, 15, 23).
The α-helical transmembrane domains of this receptor superfamily remain to be solved structurally at the atomic level, but computer-assisted analysis of cryoelectron microscopic images of Torpedo nAChR (24) have afforded a structure at 4-Å resolution. This approximation is consistent with biochemical evidence: first, noncompetitive channel blockers of nAChR were found to attach to residues in TM2 (20–22). Second, cysteine scanning accessibility mutagenesis showed that the residues proposed to line the channel on the lumen side of the TM2 α-helix were accessible to solvent, whereas those residues on the buried parts of the helix were not (21, 25). Channel blocking functional data were consistent with the pore being formed by the five TM2 domains of the heteropentameric protein. These residues (2′, 6′, 9′, etc.; Fig. 1C) include the pore size-determining residues, the impediment to ion flow in the middle of the pore (L9′), and site of NCA action, including binding, thus probably actual NCA binding sites (2, 17, 18, 23). Third, residues in TM2 are able to affect the agonist sensitivity and channel opening probability (14, 20–23), consistent with an allosteric coupling of this domain to the functional activation mechanism. All of the data on nAChR appear to be translatable to the other members of the receptor superfamily, including GABAR. Thus the TM2 channel domain is established for GABAR, and the action of PTX-like NCA is accepted by most workers as direct channel blocking. The residues involved in NCA action are all in TM2 and are consistent with the site of binding of these ligands.
The transmembrane domain of GABAR and related members of the receptor superfamily like the glycine receptor also have been shown to be sites of action of general anesthetics like the volatile agent isoflurane and high-dose (≥100 mM) ethanol (26) and i.v. anesthetics like etomidate and propofol (27). The anesthetics appear to bind in a water-filled pocket formed by the membrane-spanning helices, not within, but behind, the channel pore (26).
The model of Chen et al. (2) for the NCA binding site in the channel of GABAR is based on the β3 homopentameric GABAR, which has a high affinity for the NCAs, resembling the insect channel, and the symmetry of the protein facilitates modeling and ligand positioning. The model leads to the novel conclusion that several NCA molecules of diverse chemical structure all fit into the same ligand site in the 8.5-Å pore and block the channel, interacting with the same TM2 amino acid residues A2′, T6′, and L9′ (Fig. 1C), a valuable insight. Nevertheless, native mammalian GABARs are not homopentamers and differ from the β3 homomer in that the presence of additional subunits alters the structure–activity profile for NCAs, consistent with differences in heteromeric GABAR, and channel gating and allosteric interactions between sites for other modulators and NCAs. The PTX-type NCA drugs for GABAR remain interesting today.
Acknowledgments
I thank Jacob Hanchar for helpful discussions.
Conflict of interest statement: No conflicts declared.
See companion article on page 5185 in issue 13 of volume 103.
References
- 1.Martin D. L., Olsen R. W., editors. GABA in the Nervous System. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 2.Chen L., Durkin K. A., Casida J. E. Proc. Natl. Acad. Sci. USA. 2006;103:5185–5190. doi: 10.1073/pnas.0600370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter L. A. Chem. Rev. Washington, DC: 1967. pp. 441–464. [DOI] [PubMed] [Google Scholar]
- 4.Olsen R. W., Gordey M. In: Handbook of Experimental Pharmacology, Pharmacology of Ionic Channel Function: Activators and Inhibitors. Endo M., Kurachi Y., Mishina M., editors. Vol. 147. Heidelberg: Springer; 2000. pp. 497–515. [Google Scholar]
- 5.Windholz M., Budavari S., Stroumtsos L. Y., Fertig M. N., editors. The Merck Index. 9th Ed. Rahway, NJ: Merck; 1976. [Google Scholar]
- 6.Takeuchi A., Takeuchi N. J. Physiol. (London) 1969;205:377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ticku M. K., Olsen R. W. Neuropharmacology. 1979;18:315–318. doi: 10.1016/0028-3908(79)90132-1. [DOI] [PubMed] [Google Scholar]
- 8.Casida J. E., Palmer C. J. Adv. Biochem. Psychopharm. 1988;45:109–123. [PubMed] [Google Scholar]
- 9.Bowery N. G., Collins J. F., Hill R. G. Nature. 1976;261:601–603. doi: 10.1038/261601a0. [DOI] [PubMed] [Google Scholar]
- 10.Squires R. F., Casida J. E., Richardson M., Saederup E. Mol. Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- 11.Hold K. M., Sirisoma S. I., Ikeda T., Narahashi T., Casida J. E. Proc. Natl. Acad. Sci. USA. 2000;97:3286–3291. doi: 10.1073/pnas.070042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen R. W. Proc. Natl. Acad. Sci. USA. 2000;97:4417–4418. doi: 10.1073/pnas.97.9.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ffrench-Constant R. H., Steichen J. C., Rocheleau T. A., Aronstein K., Roush R. T. Proc. Natl. Acad. Sci. USA. 1993;90:1957–1961. doi: 10.1073/pnas.90.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen R. W., Chang C.-S. S., Li G., Hanchar H. J., Wallner M. Biochem. Pharmacol. 2004;68:1675–1684. doi: 10.1016/j.bcp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Sigel E. Curr. Top. Med. Chem. 2002;2:833–840. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- 16.Xu M., Covey D. F., Akabas M. H. Biophys. J. 1995;69:1858–1867. doi: 10.1016/S0006-3495(95)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perret P., Sarda X., Wolff M., Wu T. T., Bushey D., Goeldner M. J. Biol. Chem. 1999;274:25350–25354. doi: 10.1074/jbc.274.36.25350. [DOI] [PubMed] [Google Scholar]
- 18.Jursky F., Fuchs K., Buhr A., Tretter V., Sigel E., Sieghart W. J. Neurochem. 2000;74:1310–1316. doi: 10.1046/j.1471-4159.2000.741310.x. [DOI] [PubMed] [Google Scholar]
- 19.Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van der Oost J., Smit A. B., Sixma T. K. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 20.Corringer P.-J., LeNovere N., Changeux J.-P. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 21.Karlin A. Nat. Rev. Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 22.Chiara D. C., Trinidad J. C., Wang D., Ziebell M. R., Sullivan D., Cohen J. B. Biochemistry. 2003;42:271–283. doi: 10.1021/bi0269815. [DOI] [PubMed] [Google Scholar]
- 23.Chang Y., Weiss D. S. In: GABA in the Nervous System. Martin D. L., Olsen R. W., editors. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 127–139. [Google Scholar]
- 24.Miyazawa A., Fujiyoshi Y., Unwin N. Nature. 2003;423:949–958. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 25.Horenstein J., Wagner D. A., Czajkowski C., Akabas M. H. Nat. Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- 26.Yamakura T., Bertaccini E., Trudell J. R., Harris R. A. Annu. Rev. Pharmacol. Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph U., Mohler H. Annu. Rev. Pharmacol. Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]