Historically, ecology has focused on continuous distributions and smooth transitions. Only recently have discontinuities and thresholds become an explicit focus in some areas of ecology, especially in the realm of complex systems. The study of animal body mass distributions has been recognized for its potential to provide insight into the underlying processes shaping animal communities. Hutchinson (1) formalized the understanding of species niches and the potential for competition to shape body mass distributions. However, despite a long history of theoretical and empirical pursuit, the mechanisms driving patterns in body mass distributions remain poorly understood. The work of Scheffer and van Nes (2) in this issue of PNAS demonstrates that community interactions alone can create discontinuous, lumpy distributions of simulated species along a niche axis. Their contribution comes at a time of heightened interest in understanding the mechanisms that may lead to discontinuities in body mass or biomass distributions.
Much of the renewed interest in body mass distributions has followed the publication of a provocative ecological monograph that suggested animal body mass distributions are entrained by landscape structure (3). Holling’s paper (3) initially spawned skepticism that body mass distributions are characterized by what Holling termed “lumps” and “gaps.” Currently, many ecologists accept that body size distributions are discontinuous, but there remains disagreement regarding the mechanisms responsible. One mechanism proposed focuses on interactions among species living in the same habitat. The strongest and clearest species interaction, other than predation, is competition. However, facilitative interactions are also increasingly recognized for their potential to shape community structure. Scheffer and van Nes (2) demonstrate that species interactions may result in both repulsion and attraction along a niche axis. Attraction occurs when species are similar enough to avoid competition and results in aggregations (lumps) of species, and competition also repulses and disallows species of moderate similarity, resulting in species distributions that are both discontinuous and aggregated. Roughgarden (4) also recognized that species interactions have a strong effect on the distribution of species and that competitive interactions can lead to both aggregation and discontinuity along a niche axis.
Interestingly, similar patterns have been demonstrated for social–economic systems. Discontinuities have been found in international economic data (5), where the variable of interest was gross domestic product per capita. A discontinuous distribution was found to persist over time, and the overall structure seemed to bound the growth trajectories of individual countries. Explaining the mechanisms behind discontinuities in economic processes is difficult. Barro (6), for example, has hypothesized the existence of a limited number of “convergence clubs” in gross domestic product data, that is, aggregations of countries whose similar attributes “entrain” their economic performance, a finding that may have parallels in the results of Scheffer and van Nes (2). Further tests of the convergence and convergence club hypotheses have been performed using economic data from other scales, including states (7, 8) and counties in the U.S. Cross-country growth exhibits behavior that is best characterized by means of convergence clubs, in which the economy of the country is autocorrelated with other countries with similar growth, resulting in multiple steady states (9).
Species interactionsmay result in repulsionand attraction alonga niche axis.
City and firm size distributions are also discontinuous (10, 11), suggesting that discontinuities may be a general property of complex systems. Garmestani et al. (10) demonstrated that the hierarchical structure of urban systems is discontinuous despite variability in the growth dynamics of individual cities. Growth rates differ by city size (A. S. Garmestani, personal communication), and cities in the southeastern region of the U.S. cluster into size classes, in contrast to the expectation if Gibrat’s Law held for these data. Garmestani (personal communication) found that growth is correlated to size, with smaller cities exhibiting faster growth rates. It is possible that the interaction between endogenous comparative advantages and exogenous trade and transportation patterns triggers discontinuities in city growth rates, which manifest in cities clustering into distinct size classes. A similar mechanism may be responsible for the clustering of firms into size classes within industrial sectors (11). Growth within cities may be viewed as a competitive process leading to convergence and discontinuity, as demonstrated by Scheffer and van Nes (2).
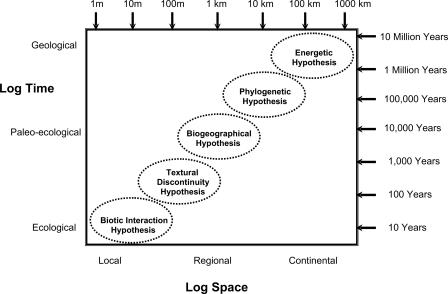
For animal communities, various hypotheses have been proposed to explain the patterns observed in body mass distributions. Energetic, phylogenetic, biogeographical, textural-discontinuity, and community-interaction hypotheses have been advanced to explain observed patterns (12). Energetic and textural-discontinuity hypotheses focus on the scaling of resource acquisition. Biogeographical and phylogenetic hypotheses focus on the role of either geographic or evolutionary constraints on the organization of communities. The community-interaction hypothesis focuses on biotic interactions within species communities, arguing that these interactions shape community structure. Much of the disagreement regarding the mechanisms responsible for discontinuities is due to the scale of the analyses and to the search for single simple, rather than complex interacting, sources of causation.
An explicit embrace of the problem and complexity imposed by scale is often absent from analyses seeking to understand community assembly and structure. The mechanistic hypotheses forwarded to explain discontinuities or other patterns in body mass distributions each applies over a limited domain of scale (Fig. 1; ref. 12), and each partly explains the observed patterns. Community-interaction hypotheses apply to spatially and temporally proximate interactions among species residing within local landscapes. Textural discontinuity and biogeographical hypotheses apply over regional spatial and paleoecological temporal scales, and phylogenetic and energetic hypotheses apply most appropriately over temporally slow and spatially broad domains. The scale of appropriate application, relevance, and interpretation varies among the hypotheses.
Fig. 1.
The scales at which the mechanistic hypotheses explaining discontinuity or multimodality in animal body mass patterns are likely to apply. No overlap among hypotheses is shown, but both the spatial and temporal dimensions of adjacent domains probably interact. Mechanisms acting at larger and slower scales provide nonrandom species pools on which faster and smaller mechanisms work. [Reprinted with permission from ref. 12 (Copyright 2006, Blackwell Publishing).]
There is a pressing need for an integration of theory relevant to discontinuities. Peterson et al. (13) provided a model that suggests scale has an important role in compartmentalizing species interactions, because species interacting with their environment at the same range of scale are most likely to compete. They suggested that this model would lead to a diversity of functions within a scale range and a redundancy of function across scales. That is to say, there would be aggregations of species along a size axis, and within body mass aggregations there would be a diversity of used niche space, whereas across aggregations (across scales) there would be apparent redundancy of used niche space. The model of Scheffer and van Nes (2) suggests a mechanism that could generate some of the patterns in the distribution of function within and across aggregations that have been theoretically proposed.
How might the model of Scheffer and van Nes (2) relate to the empirical results of Holling (3) and the numerous studies that suggest a relationship between landscape structure and body mass patterns (12)? Is it possible to reconcile results that suggest that structure in animal body mass distributions is imposed by the landscape with results that suggest structure emerges from interspecific interactions? Does the landscape provide a discontinuous distribution of structure that is the theater on which species interact? To consider species interactions without context can provide insight but will only partially mimic reality and may fail to capture the unexpected emergence of properties and structures that arise within complex systems such as ecosystems. Szabo and Meszena (14) provide some clues regarding the landscape template. They modeled species on a landscape characterized by resource distributions that vary with scale and discovered that more species were able to coexist when more scales of resource distribution were available and that successful species exploited their environment at scales matched with the distribution of resources.
Scheffer and van Nes (2) provide an elegant example of how species interactions can lead to discontinuous patterns of species distributions. Introducing the complexity of scale into niche interaction models and incorporating potentially self-organizing interactions between the environment and organisms within ranges of scale are the next critical steps toward understanding the structure and assembly of animal communities and the ecosystems on which they reside.
Acknowledgments
The Nebraska Cooperative Fish and Wildlife Research Unit is jointly supported by a cooperative agreement between the U.S. Geological Survey, the Nebraska Game and Parks Commission, the University of Nebraska-Lincoln, the U.S. Fish and Wildlife Service, and the Wildlife Management Institute.
Conflict of interest statement: No conflicts declared.
See companion article on page 6230.
References
- 1.Hutchinson G. E. Am. Nat. 1959;93:145–159. [Google Scholar]
- 2.Scheffer M., van Nes E. H. Proc. Natl. Acad. Sci. USA. 2006;103:6230–6235. doi: 10.1073/pnas.0508024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holling C. S. Ecol. Monogr. 1992;62:447–502. [Google Scholar]
- 4.Roughgarden J. Primer of Ecological Theory. Englewood Cliffs, NJ: Prentice–Hall; 1998. [Google Scholar]
- 5.Summers R., Heston A. Q. J. Econ. 1991;106:327–368. [Google Scholar]
- 6.Barro R. J. Determinants of Economic Growth: A Cross-Country Empirical Study. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- 7.Barro R. J., Salai-i-Martin X. J. Pol. Econ. 1992;100:223–251. [Google Scholar]
- 8.Kenworthy L. Soc. Sci. Q. 1999;80:858–869. [Google Scholar]
- 9.Durlauf S. N., Johnson P. A. J. Appl. Econ. 1995;10:365–384. [Google Scholar]
- 10.Garmestani A. S., Allen C. R., Bessey K. M. Urban Studies. 2005;42:1507–1515. [Google Scholar]
- 11.Garmestani A. S., Allen C. R., Mittelstaedt J. D., Stow C. A., Ward W. A. Environ. Dev. Econ. 2006;11 in press. [Google Scholar]
- 12.Allen C. R., Garmestani A. S., Havlicek T. D., Marquet P. A., Peterson G. D., Restrepo C, Stow C. A, Weeks B. E. Ecol. Lett. 2006;9:630–643. doi: 10.1111/j.1461-0248.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Peterson G. D., Allen C. R., Holling C. S. Ecosystems. 1998;1:6–18. [Google Scholar]
- 14.Szabo P., Meszena G. Ecosystems. 2006;9 in press. [Google Scholar]