Abstract
The first circadian-relevant kinase to be identified was DOUBLE-TIME (DBT) in Drosophila, a homolog of vertebrate CKIε, which regulates the progressive phosphorylation and stability of PERIOD (PER) proteins in animals. A negative feedback loop wherein PER directly inhibits the transcriptional activity of the CLOCK-CYCLE (CLK-CYC) heterodimer is central to the generation of molecular rhythms and normal progression of the clock in Drosophila. We show that DBT activity is required for the phase-specific hyperphosphorylation of CLK in vivo, an event that correlates with times of maximal repression in per RNA levels. The ability of DBT to hyperphosphorylate CLK, enhance its degradation, and evoke modest inhibition of CLK-dependent transactivation from circadian promoter elements was directly shown in cultured Drosophila cells. Intriguingly, DBT seems to function in close partnership with the PER-relevant protein phosphatase 2A, resulting in dynamic equilibrium between hypo- and hyperphosphorylated isoforms of CLK. This balancing mechanism might act to stabilize the limiting levels of CLK against stochastic fluctuations minimizing the propagation of “molecular noise” in the feedback circuitry. Also, the subcellular localization of CLK was altered from predominately nuclear to strong cytoplasmic staining in the presence of PER. These results suggest that, in contrast to mammalian clocks, circadian transcriptional inhibition in Drosophila involves displacement of the positive factors from chromatin. These results also demonstrate that DBT can target both negative and positive factors in circadian feedback loops and support a conserved role for dynamic regulation of reversible phosphorylation in directly modulating the activities of circadian transcription factors.
Keywords: circadian rhythms, protein phosphatase 2A, cycle, period
A highly conserved feature of circadian (≈24 h) clocks is that they are composed of cell-autonomous transcriptional/translational-based feedback loops that include both positive and negative elements, contributing to cyclical gene expression (reviewed in refs. 1 and 2). Normal progression of the clock is also highly dependent on integrating posttranslational regulatory pathways, most notably time-of-day-specific phosphorylation events that generate dynamic changes in clock protein stability and activity (reviewed in refs. 3–5).
In Drosophila melanogaster, a central circadian feedback loop is composed of the positively acting basic-helix–loop–helix (bHLH)/PER-ARNT-SIM (PAS) partners CLOCK (CLK) and CYCLE (CYC), which bind to E-box enhancer elements and stimulate transcription of period (per) and timeless (tim), among other core clock and downstream effector genes (recently reviewed in ref. 6). PER and TIM interact in the cytoplasm, and, after a time delay, they enter the nucleus where most likely PER acts as the key autoinhibitor by binding to CLK-CYC. The extent and timing of PER-mediated transcriptional inhibition is highly regulated by temporal changes in its phosphorylated state, which modulates PER's cytoplasmic accumulation rate, timing of nuclear entry, duration in the nucleus, and potency (7–12). A major kinase underlying these key aspects of PER's daily life cycle is DOUBLE-TIME (DBT), a homolog of vertebrate CKIε (8, 12). PER phosphorylation status is also regulated by protein phosphatase 2A (PP2A), presumably involving circadian changes in the levels of its regulatory subunits, twins (tws) and widerborst (wdb) (13). This tight regulation ensures that the negative regulation of CLK-CYC by interactions with PER is limited to a specific phase of the day (early-night to early-day), contributing to rhythmic expression of CLK-CYC-driven expression. Indeed, it is thought that the transactivation potential of CLK-CYC is mainly regulated by phase-specific interactions with PER. A similar circuit whereby (i) a heterodimer composed of CLOCK and BMAL1 (vertebrate homology of CYC) stimulates expression of autoinhibitors (Per1-3 and Cry1,2) and (ii) CKIε/δ regulates the phosphorylation/abundance of Per proteins is conserved in mammals (14).
Edery and colleagues (15–17) previously showed that there are differentially phosphorylated CLK proteins in Drosophila; however, it was not clear which kinase(s) phosphorylates CLK or what were the consequences of this posttranslational modification. Here, we show a role for DBT in regulating the phosphorylation and stability of CLK. Moreover, DBT-mediated hyperphosphorylation of CLK is intricately balanced by protein phosphatase activity, resulting in a dynamic equilibrium between hypo- to hyperphosphorylated CLK variants. This balance between phosphorylation and dephosphorylation presumably stabilizes total CLK levels, which is thought to be the limiting component in the transcriptional feedback circuitry (15).
Results and Discussion
A Phase-Specific Hyperphosphorylated Isoform(s) of CLK Is Highly Dependent on DBT Activity.
We previously showed that there are multiple electrophoretic mobility isoforms of CLK arising from differential phosphorylation, with the most hyperphosphorylated (slowest migrating) variants preferentially occurring during the late-night/early-day (15, 17). In addition, we also reported that the total levels of CLK cycle, with trough amounts observed during the mid- to late-day and peak amounts in the late-night/early-day (17), in sync with cycles in Clk RNA levels (18). During the course of our current studies, we noticed that the overall intensity of CLK staining as evaluated by immunoblotting was increased ≈2- to 3-fold when head extracts were prepared using more stringent extraction conditions (data not shown), consistent with results recently obtained by others (44). Under these conditions, although the relative abundance of highly phosphorylated CLK is still greater during the late-night/early-day, total CLK levels in WT head extracts are relatively constant (Fig. 1A and ref. 44). Presumably, insufficient extraction of CLK using our earlier, milder conditions likely contributed to the biochemical rhythm in staining intensity (44). However, a limitation of using WT flies to study posttranslational aspects of CLK metabolism is that its levels are very low (15), rendering it difficult to reproducibly distinguish electrophoretic mobility variants resulting from differential phosphorylation of CLK.
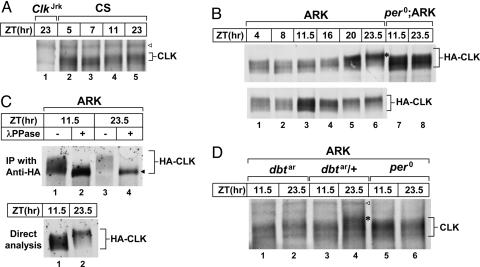
Fig. 1.
A phase-specific hyperphosphorylated isoform(s) of CLK is absent in dbt-deficient flies. Adult flies (genotypes indicated above each set of blots) were entrained to light/dark (LD) cycles and collected at the indicated zeitgeber times (ZT). Head extracts were prepared and either directly analyzed by immunoblotting (A, B, C Lower, and D) or first subjected to immunoprecipitation by using anti-HA antibodies (C Upper). (B) Results from two independent experiments are shown (Upper and Lower, lanes 1–6). (C) Immune complexes were incubated in the absence (−) or presence (+) of λ phosphatase (λPPase), followed by immunoblotting. Blots were incubated with antibodies against either HA (B and C) or CLK (A and D). ◁, nonspecific band; ◀, nonphosphorylated CLK; ∗, hyperphosphorylated CLK.
To circumvent some of these limitations, we used ARK transgenic flies, previously described by Kim et al. (16), whereby the Clk ORF is fused in frame with the hemagglutinin (HA) epitope tag and expression driven by per regulatory elements (16). The overall levels of HA-CLK are ≈3- to 5-fold greater compared with endogenous CLK, and by various biochemical and behavioral criteria the hybrid protein has a similar if not identical mode-of-action in the clock mechanism as WT CLK (16).
ARK flies were entrained under standard conditions of 12 h light/dark (LD) cycles [in which zeitgeber time (ZT) 0 is lights-on and ZT12 is lights-off] at 25°C, head extracts were prepared by using more stringent conditions, and HA-CLK were visualized by immunoblotting in the presence of anti-HA antibodies. By optimizing procedures (see Materials and Methods), we observed similar but higher resolution rhythms in the phosphorylated state of HA-CLK compared with what we previously described (Fig. 1B). Consistent with earlier work (16, 17), phosphatase treatment revealed that most, if not all, of the electrophoretic mobility differences result from differential phosphorylation, and that the majority of HA-CLK is at least somewhat phosphorylated in vivo (Fig. 1C). Although multiple phosphorylated isoforms of HA-CLK are detected throughout a daily cycle, the late-night is characterized by the specific appearance of a highly phosphorylated isoform(s) (Fig. 1B, lane 6; indicated by ∗) with concomitant disappearance of faster migrating species. We observed a low-amplitude fluctuation in the total levels of HA-CLK with late nighttime levels lower (Fig. 1C, compare lanes 2 and 4), possibly driven by the anti-phase cycling of per-HA-Clk RNA compared with endogenous Clk transcripts, as reported (16). In the arrhythmic per01 mutant (19) genetic background (per01;ARK), differential phosphorylation of CLK still occurs, but the pattern is constant throughout a daily cycle and, most notably, the hyperphosphorylated variant(s) normally observed in rhythmic flies is missing (Fig. 1B Upper, compare lanes 7 and 8 with 6). Similar results were obtained with anti-CLK antibodies (see below). per-HA-Clk hybrid RNA is ≈2-fold higher in per01;ARK flies compared with peak values for ARK flies (data not shown), likely contributing to the elevated levels of HA-CLK protein in the mutant background.
To evaluate the effects of DBT on CLK phosphorylation we used the dbtar mutant, a hypomorphic allele of this vital gene that has circadian effects but still provides sufficient activity to produce viable adults (20). To this end, we crossed flies to place the per-HA-Clk transgene in the dbtar genetic background (ARK;dbtar) and compared results with their heterozygote siblings (ARK;dbtar/+). Intriguingly, although the residual kinase activity of DBTAR (20) might affect CLK phosphorylation, it is clear that, in the homozygote mutant background, the phase-specific hyperphosphorylated variant(s) of CLK is either absent or present at very low levels (Fig. 1D, compare lane 4 with 1 and 2; similar results were obtained in three independent experiments by using two different anti-CLK antibodies; data not shown). Thus, we can distinguish at least two groups of CLK phospho-variants with regard to DBT activity: (i) those ranging from hypophosphorylated to medium phosphorylated that are independent or less dependent on DBT activity, and (ii) a phase-specific hyperphosphorylated isoform(s) that is highly dependent on DBT activity. It is reasonable to suggest that phosphorylated CLK is the physiological substrate for hyperphosphorylation by DBT (see below), because priming is typical for this kinase (21).
Hyperphosphorylation of CLK by DBT in a Drosophila Cell Culture Model System.
To better determine biochemical aspects of DBT on regulation of CLK metabolism and activity, we used cultured Drosophila Schneider (S2) cells, a simplified noncycling experimental system that alleviates potential complications from transcriptional feedback regulation. We previously showed that, under our experimental conditions, exogenous expression of DBT is required to generate progressive phosphorylation of PER and its rapid degradation by the 26S proteasome (22).
In this study, exogenous expression was driven from either the copper-inducible metallothionein promoter (pMT) or a constitutive promoter (pAct), and recombinant proteins were modified with the V5 or other epitope tags for enhanced protein surveillance. Similar to results obtained in vivo, the appearance of a hyperphosphorylated isoform(s) is detected with coexpression of DBT but not DBTAR (Fig. 2A). Furthermore, a large portion of CLK-V5 is phosphorylated to some degree in our cell culture system even in the absence of exogenous DBT (herein termed “medium” phosphorylation; Fig. 2D; also see Fig. 3C, compare lanes 1 and 2). We did not observe changes in medium phosphorylated CLK by reducing endogenous DBT levels using RNA interference (RNAi)-mediated strategies (data not shown), suggesting that other kinases are involved in generating these phospho-variants of CLK. This contention is further supported by treating cells with RNAi against dbt under conditions where DBT-mediated hyperphosphorylation of CLK is observed (Fig. 2B). Although we cannot rule out the possibility that even small amounts of DBT are sufficient for medium phosphorylation of CLK, the results clearly show that hyperphosphorylation of CLK is highly dependent on DBT levels/activity whereas medium phosphorylated CLK is not, similar to findings obtained with dbtar flies (Fig. 1D). Although naive S2 cells have residual DBT activity (e.g., see ref. 10), DBT's presumed stoichiometric mode-of-action (23) and the presence of counteracting protein phosphatases (see below, Fig. 3) might render the levels of endogenous DBT activity below the threshold required to attain detectable steady-state accumulation of hyperphosphorylated CLK. Likewise, a similar requirement for exogenous expression of DBT was also observed for the progressive phosphorylation of PER in S2 cells (22).
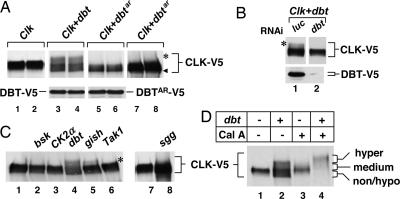
Fig. 2.
Hyperphosphorylation of CLK by DBT in S2 cells. S2 cells were transfected either singly with 50 ng of pMT-Clk-V5 (Clk) or in combination with 100 ng of pMT-based plasmids containing the indicated ORFs (top of each set of blots). Cells were incubated in the presence of Cu2+ to induce ectopic expression of recombinant proteins and collected after 26 h, and extracts were analyzed by immunoblotting in the presence of anti-V5 antibodies to visualize CLK-V5 (A Upper, C, and D), DBT-V5 (A Lower, lanes 3 and 4), or DBTAR-V5 (A Lower, lanes 5 and 6). (A) Samples from two independent transfections were analyzed side-by-side. No hyperphosphorylated CLK-V5 was detected in the presence of DBTAR (lanes 5 and 6) even after overexposure of the staining (lanes 7 and 8). (B) Cells were cotransfected with pAct-Clk-V5 and pAct-dbt-V5 in addition to dsRNA against either luc or dbt. CLK-V5 (Upper) and DBT-V5 (Lower) were visualized by immunoblotting in the presence of anti-V5 antibody. (D) Cells were incubated with 30 nM Cal A (lanes 3 and 4) or with identical volume of vehicle alone (DMSO; lanes 1 and 2) for 2 h before collecting. ◀, nonphosphorylated CLK; ∗, hyperphosphorylated CLK.
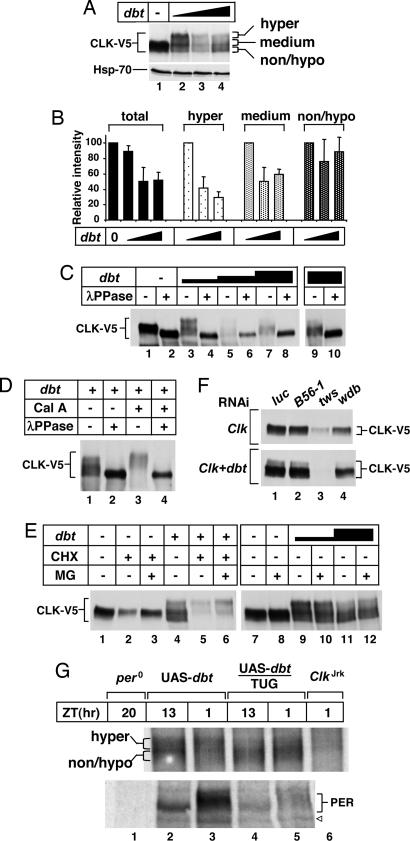
Fig. 3.
Effects of DBT and protein phosphatase activity on CLK phosphorylation and stability. (A–E) S2 cells were transfected either singly with 50 ng of pMT-Clk-V5 (Clk) or in combination with different amounts of pMT-dbt-V5 (dbt; indicated above each set of blots), incubated in the presence of Cu2+, and collected after 26 h. The different amounts of pMT-dbt-V5 used were as follows: 100 ng (A, lane 2; C, lanes 3 and 4; D, lanes 1–4; E, lanes 4–6 and 9 and 10), 300 ng (A, lane 3; C, lanes 5 and 6), and 600 ng (A, lane 4; C, lanes 7–10; E, lanes 11 and 12). Where indicated, Cal A (30 nM for 2 h), cycloheximide (CHX; 10 μg/ml for 4 h), or MG132 (MG; 50 μM for 4 h) were added to the media; in all cases, control cells were similarly incubated with an equal volume of DMSO. Extracts were prepared and either directly analyzed by immunoblotting (A, E, and F) or first subjected to immunoprecipitation (C and D) in the presence (+) or absence (−) of λ phosphatase. (A) Unaltered levels of the control protein Hsp-70 in the presence of increasing dbt. (B) The relative staining intensities for total CLK and the different phospho-variants obtained from averaging the results shown in A and two other independent experiments. For total CLK staining (Left), the intensity in the absence of dbt was set to 100, and all other values were normalized. For the different phospho-variants (identified in A Right), the intensity at 100 ng of dbt was set to 100, and all other values were normalized. (F) Cells were cotransfected with 50 ng of pAct-Clk-V5 and 100 ng of pAct-dbt-V5 in addition to the indicated dsRNAs (listed at the top). (G) Head extracts were prepared from adult flies (genotypes indicated at the top) collected at the indicated zeitgeber times (ZT) and immunoblotted in the presence of antibodies against CLK (Upper) or PER (Lower). ◁, nonspecific band.
Specificity for DBT-mediated hyperphosphorylation of CLK was further shown by the inability of several different kinases to noticeably alter the phosphorylation pattern of CLK-V5, including the clock-relevant kinases CK2 and GSK3β/SGG that target PER and TIM, respectively (24–26) [Fig. 2C; expression levels of the different kinases were comparable with that of DBT (Fig. 5, which is published as supporting information on the PNAS web site)]. Consistent with the high selectivity, a small fraction of hyperphosphorylated CLK-V5 was observed with the kinase GILGAMESH (GISH), a closely related CKI variant (γ isoform) (Fig. 2C, lane 5).
Even though we do not have definitive evidence that the DBT-dependent hyperphosphorylated isoform(s) of CLK [herein referred to as CLK(P*)] observed in flies and S2 cells is identical, the shared high dependency on DBT activity in both systems strongly suggests that our cell-culture based approach faithfully recapitulates physiologically relevant aspects of the effects of DBT on CLK.
DBT and Protein Phosphatase Activity Modulate CLK Stability.
Although hyperphosphorylation of CLK is clearly dependent on DBT activity, we did note a reproducible effect of expressing DBTAR that was also observed with WT DBT: namely, increases in nonphosphorylated or very hypophosphorylated variants of CLK-V5 having faster electrophoretic mobility compared with CLK-V5 expressed in the absence of ectopic DBT (e.g., Fig. 2D, compare lanes 1 and 2). Intriguingly, the appearance of this non-/hypophosphorylated CLK-V5 band was greatly diminished or eliminated by inhibition of protein phosphatase activity by using the cell-permeable calyculin A (Cal A), which also stimulated hyperphosphorylation (Fig. 2D, compare lanes 2 and 4). Presently, it is not clear how DBT, apparently in a manner that does not have a high requirement for its kinase activity, as it occurs with DBTAR, stimulates the appearance of non-/hypophosphorylated isoforms of CLK (Fig. 2A, lanes 3–6). Nonetheless, a series of experiments described below indicate that the phosphorylated state and stability of CLK are modulated by a tight balance between DBT and protein phosphatases.
To better evaluate whether DBT affects CLK stability, we varied dbt-V5 concentration and immunoprecipitated CLK-V5, followed by treatment of the pellet with λ phosphatase (λPPase) to more easily compare total staining intensity (Fig. 3 A–E). The results demonstrate that, although the total abundance of CLK-V5 is moderately reduced by increasing DBT levels, it plateaus at ≈50% reduction (quantitation of results shown in Fig. 3B). However, there is preferential disappearance of hyperphosphorylated variants, followed by medium phosphorylated CLK and little effect on the levels of non- or hypophosphorylated species (Fig. 3B). Although hyperphosphorylated isoforms of CLK were preferentially reduced in intensity with higher DBT levels, a variety of hyper- to non-/hypophosphorylated CLK could be observed over a range of exogenously expressed DBT levels (e.g., Fig. 3 A, lanes 2–4, and E, lanes 9–12), suggesting a close functional balance between DBT-mediated phosphorylation and dephosphorylation by means of endogenous protein phosphatases. Moreover, at very high concentrations of dbt, we noted a reproducible enhancement in the relative amount of hypo- to hyperphosphorylated CLK isoforms, which also correlated with higher total CLK protein abundance (Fig. 3 A–C; e.g., Fig. 3 A, compare lanes 3 and 4; C, compare lanes 6 and 8). These results suggest that, whereas very low concentrations of DBT are not sufficient to promote hyperphosphorylation of CLK (Fig. 2), at higher DBT concentrations, the net balance tilts toward dephosphorylation and stabilization of CLK. Indeed, addition of Cal A enhances DBT-mediated hyperphosphorylation and degradation of CLK (Fig. 3D, compare lanes 2 and 4). More rapid degradation of CLK in the presence of exogenous DBT was also revealed by adding cycloheximide to block de novo protein synthesis (Fig. 3E). Under the experimental conditions used, total CLK levels were reduced ≈50% more when coexpressed with DBT. Degradation of CLK in the presence or absence of DBT was partially blocked by the proteasome inhibitor MG132, with preferential stabilization of CLK(P*) (Fig. 3E, compare lanes 5 and 6).
Although not the focus of this study, prominent roles for protein phosphatase activity in regulating CLK levels were also obtained by using RNAi gene silencing to lower the levels of TWS and WDB, two regulatory circadian-relevant subunits of PP2A recently shown to target PER (see introduction) (13). Prior work showed that reductions in TWS and WDB destabilize PER in S2 cells (13). Likewise, we observed remarkably similar specificity whereby dsRNA against tws and wdb but not B56, another regulatory subunit of PP2A, yielded significant decreases in CLK levels (Fig. 3F). In our experimental system, it was difficult to evaluate a role for the catalytic subunit of PP2A [mutagenic star (mts)] by using RNAi-based strategies because cell viability was compromised (data not shown). Consistent with results using Cal A, larger magnitude reductions in the levels of CLK occurred in tws and wdb RNAi-treated cells coexpressing DBT (Fig. 3F, compare Upper and Lower). Although future studies will be required to determine the physiological significance of these and other protein phosphatase components in regulating CLK metabolism, it is clear that, similar to PER, the phosphorylated state and overall levels of CLK can be subject to dual regulation by counterbalancing kinases and phosphatase activities. The one notable difference is that DBT-mediated progressive phosphorylation of PER seems more unidirectional because there does not seem to be a noticeable balance with less phosphorylated isoforms in flies or S2 cells (22, 27). This presumptive mechanistic difference might underlie the robust daily changes in the levels of PER compared with the more static total abundance of CLK (Fig. 1A).
To further evaluate the effects of DBT on CLK protein in flies, we used the UAS/GAL4 binary system to drive overexpression of DBT in tim-expressing pacemaker cells using previously described tim-UAS-gal4 and UAS-dbt transgenic flies (28). The resultant progeny from the cross was compared with the parental strains (Fig. 3G). tim-UAS-gal4/UAS-dbt flies manifest robust circadian rhythms with near normal periods (ref. 28 and data not shown). We did not detect gross changes in the levels of CLK, in contrast to PER, where the levels were strongly reduced (Fig. 3G Lower). However, the phosphorylated profile of CLK was clearly altered with specific decreases in hyperphosphorylated variants and relative increases in hypophosphorylated CLK. These findings are remarkably consistent with our observations in S2 cells whereby augmenting DBT levels leads to the preferential disappearance of hyperphosphorylated CLK and fewer effects on non-/hypophosphorylated to medium phosphorylated variants (Fig. 3 B and C, compare lanes 3 and 9, and E, compare lanes 9 and 11). Additionally, in flies overexpressing DBT, there might also be contributions to the CLK phosphorylation pattern by means of the ability of DBT to somehow stimulate the steady-state accumulation of non-/hypophosphorylated CLK species (e.g., Fig. 2).
Thus, both in flies and in a simplified experimental system, we observed (i) a strong requirement for DBT in the production of a hyperphosphorylated isoform(s) but not medium phosphorylated CLK, and (ii) the ability of DBT to alter the dynamic equilibrium between hypo- and hyperphosphorylated variants of CLK. Our results in S2 cells also raise the interesting possibility that the effects of DBT on CLK levels are attenuated because its kinase activity is functionally counterbalanced by one or more highly interconnected protein phosphatases, resulting in a steady-state equilibrium of hypo- to hyperphosphorylated CLK isoforms. Presumably, this mechanism could stabilize total CLK levels throughout a daily cycle but still maintain the ability for dynamic regulation. In this regard, it is interesting to note that, during the night when CLK and PER are hyperphosphorylated and less stable, Clk RNA levels increase whereas those of per decrease (e.g., see ref. 18). This difference in expression profiles might also contribute to the observed smaller effect of DBT overexpression on the abundance of CLK compared with PER (Fig. 3G). Indeed, increases in Clk RNA during the night might function to resupply total CLK abundance during times when it is less stable, thus stabilizing the overall levels of CLK. Because CLK is a limiting component in the clock mechanism (15), stabilizing its abundance against stochastic fluctuations could minimize the propagation of molecular noise in the feedback circuitry (e.g., see ref. 29).
DBT-Mediated Hyperphosphorylation of CLK Does Not Require CYC or the DNA Binding Domain of CLK.
To determine whether DBT-mediated hyperphosphorylation of CLK depends on its heterodimeric partner CYC, cells were incubated with dsRNA targeting two different regions of cyc or a non-relevant dsRNA control. Addition of dsRNA against cyc did not produce noticeable effects on the DBT phosphorylation of CLK (Fig. 6A, which is published as supporting information on the PNAS web site; see lanes 4–6). Moreover, an inactive form of CLK missing the basic domain required for DNA binding (ΔDBD) was also phosphorylated by DBT to the same extent as its WT counterpart (Fig. 6C). These findings are in apparent contrast to the mammalian system where phosphorylation of CLOCK depends on its binding partner BMAL1 and presumably productive transactivation complex formation (30).
DBT Evokes Modest Reductions in CLK-Dependent Transactivation.
To test whether DBT modulates the transcriptional activity of CLK, we used the well established CLK-dependent transactivation assay developed in S2 cells, based on measuring luciferase (luc) activity from E-box containing per or tim promoter-luc reporters (31) (Fig. 7, which is published as supporting information on the PNAS web site). Ectopic expression of DBT but not several other non-relevant kinases resulted in a somewhat dose-sensitive repression of CLK-dependent transcriptional stimulation that flattened with a maximal repression of ≈60% (Fig. 7B and data not shown). Saturation of the inhibitory effects is strikingly similar to the response in total protein levels as a function of dbt dosage wherein the abundance of CLK was reduced by ≈50% (Fig. 3B). DBT had a slight stimulatory effect on basal non-CLK-driven expression in our system (Fig. 7A), supporting specific inhibition of CLK transactivation. In addition to reductions in CLK levels, DBT might have more direct effects on CLK activity, consistent with the observation that hyperphosphorylated CLK is present during times of maximal repression in per/tim expression. However, it is unlikely to involve DBT-induced changes in subcellular localization (see below, Fig. 4) or interactions with CYC because CLK is stably bound to CYC throughout a daily cycle (15). Phosphorylation could inhibit DNA-binding activity as recently shown for the WHITE-COLLAR complex (WCC) in the Neurospora clock (32, 33), although, in mammals, both phosphorylated and nonphosphorylated forms of CLOCK are specifically bound to chromatin (34). Further studies will be required to determine whether the transactivation potential of CLK is altered by phosphorylation.
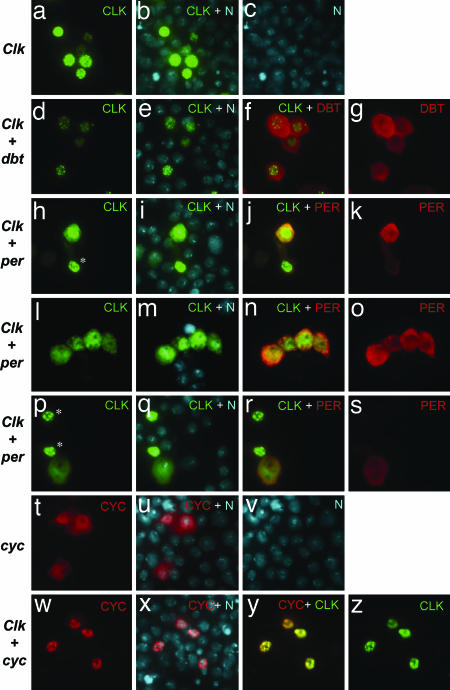
Fig. 4.
CLK cytoplasmic staining is increased in the presence of PER. (a–z) Cells were either individually transfected or cotransfected with the indicated plasmids (listed at left) and processed for in situ detection. Recombinant proteins were visualized as follows: CLK-V5 (FITC, green fluorescence), DBT-3xFLAG (Cy3, red fluorescence), PER and CYC [tetramethylrhodamine B isothiocyanate (TRITC), red fluorescence]. Nuclei were visualized by using Hoechst (blue fluorescence, which was converted to cyan). All images were captured under identical exposure conditions. In the overlay of images visualized with FITC (CLK-V5) and TRITC (PER or CYC), overlap results in a range from orange to yellow. (h–s) Representative examples are shown of the changes in CLK-V5 subcellular distribution by coexpressing PER, whereby strong PER staining is accompanied by significant cytoplasmic staining, a situation not observed in cells expressing low amounts of PER (∗, cells with little or no PER staining).
PER Elicits Substantial Changes in the Subcellular Localization of CLK.
Immunohistochemical analysis of CLK-V5 in S2 cells revealed immunoreactive staining that is predominately localized to the nucleus (Fig. 4 a–c). A virtually identical distribution, including the punctate nuclear staining, was observed with a DNA-binding defective variant of CLK that was missing the basic DNA binding domain (ΔDBD; Fig. 6 and data not shown), strongly suggesting that nuclear localization is not coupled to the formation of transcriptionally competent complexes. DBT expression was clearly accompanied by decreases in the intensity of CLK staining, without evident increases in cytoplasmic presence (compare Fig. 4 a and d), consistent with our biochemical studies showing enhanced degradation of total CLK (Fig. 3B). A diffuse staining pattern was observed for DBT (Fig. 4g), mainly localized in the cytoplasm with some nuclear staining, a distribution that could limit its effective concentration in targeting the predominately nuclear CLK.
A significant alteration in CLK subcellular localization was observed in the presence of PER, leading to very diffuse CLK distribution in the nucleus and strong cytoplasmic staining (Fig. 4 h–s; and Fig. 8 A and B, which is published as supporting information on the PNAS web site). Although the physiological significance of these results is not clear, these results are in agreement with our earlier in vitro studies showing that PER alone (i.e., in the absence of TIM) can block the ability of the CLK-CYC heterodimer to bind relevant DNA regulatory elements (35). Similar results were also recently obtained by others, whereby they showed that, during times in a day when CLK-CYC activity is repressed, the heterodimer is not stably bound to chromatin (44). In sharp contrast, the CLOCK-BMAL1 complex is stably bound to chromatin even during times of transcriptional repression (34, 36, 37), strongly suggesting a prominent difference between the fly and vertebrate clocks. Under similar experimental conditions, coexpression of TIM did not lead to cytoplasmic localization of CLK (Fig. 8C). Although we cannot rule out the possibility that under different conditions TIM can alter the subcellular localization of CLK, the results demonstrate high specificity for PER.
We were intrigued by the observation that CLK is predominately localized to the nucleus. Prior work in mammalian systems revealed that CLOCK is mainly cytoplasmic and requires physical interaction with its partner BMAL1 to enter the nucleus (30). We observed a role reversal whereby CYC is mainly located in the cytoplasm and requires CLK to accumulate in the nucleus (compare Fig. 4 t with w and y). Although the physiological significance of this observation is not clear, it further supports the contention that, whereas there is commonality in the design principles underlying circadian clocks, there is variation in how individual components operate within the circuitry.
Conclusions
Based on numerous lines of evidence accumulated over the past several years, it was generally thought that the positive factors (e.g., CLOCK and BMAL1/CYC) operating within eukaryotic circadian feedback loops were mainly, if not exclusively, regulated by stimulating the production of one or more negatively acting factors [e.g., PERs and mammalian cryptochromes (CRYs)] that would physically interact with the positive factors and somehow block or diminish transactivation. Later work suggested that the daily timing of this autoinhibition is largely based on posttranslational mechanisms that regulate when the negative factors enter the nucleus, how long they reside there, and their potency. In this model, the positive factors were generally treated as static players. Recently, this view has rapidly changed to one that involves more dynamic behavior of positive factors (reviewed in ref. 5). A most dramatic departure from the “old” model is based on recent findings in Neurospora indicating that the main autoinhibitor previously identified in this system, FREQUENCY (FRQ), does not block the positively acting WC1/2 complex (WCC) by physically interacting with it but in a more indirect manner by rhythmic phosphorylation-dependent inactivation of WCC (33).
Our findings support this growing viewpoint and demonstrate that DBT has multiple functions in Drosophila clocks, targeting both negative and positive factors in circadian feedback loops, a position it might also occupy in vertebrate clocks (23). Notwithstanding recent findings in Neurospora (33), why such a preoccupation with regulating CLK activity by phase-specific reversible phosphorylation and perhaps other modifications when PER is a respectable inhibitor and the timing of its inhibitory activity can be temporally restricted? Although the answer to this question is not clear, there is growing evidence that multisite phosphorylation can act to fine-tune the intensity of transcription factor activity to attain a calibrated response, rather than acting as a simple on/off switch (38). It is also possible that hyperphosphorylation of CLK is a mechanism that tags it for rapid promoter clearance, as recently suggested for the mammalian clock system (30). Finally, our findings suggest a close functional interaction between DBT and protein phosphatases (presumably PP2A) in maintaining a dynamic equilibrium between hypo- and hyperphosphorylated variants of CLK. This balance might serve as a noise-resistance mechanism, especially because CLK levels seem to be limiting (15) but still maintain a responsive system that likely modulates the oscillatory potential of the interconnected feedback circuits (e.g., see refs. 16 and 39).
Materials and Methods
Flies.
ARK, CS, Clkjrk, per01, dbtar, and tim-(UAS)-gal4 have been described (16, 20, 22, 40, 41). The UAS-dbt flies were kindly provided by Ying-Hui Fu (University of California, San Francisco) and have been described in ref. 28.
Constructs and Transfection.
Constructs were generated as described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site, and transfections were performed by using Cellfectin (GIBCO/BRL) as described (22). Induction of genes under the control of the pMT promoter was initiated by adding 500 μM CuSO4 to the media beginning 36 h after transfection.
Western Blot Analysis.
Immunoblotting was performed essentially as described (22), with modifications (see Supporting Materials and Methods).
Immunoprecipitation (IP) and Phosphatase Treatment.
IPs and phosphatase treatment of immune complexes were performed as described (17), with modifications (see Supporting Materials and Methods).
Immunocytochemistry.
In situ detection was performed as described (42) and is further detailed in Supporting Materials and Methods.
RNAi.
RNAi was applied to S2 cells as described (43). Briefly, 1 μg of dsRNA was cotransfected into S2 cells along with the indicated expression constructs by using Cellfectin as described. Cells were harvested 48 h after transfection and processed for Western blot analysis. The dsRNAs used were as follows (numbering of nucleotide sequence begins with start ATG): dbt, 503-1136; B56-1, 795-1482; twins, 1227–1998; widerborst, 399-1145, luciferase; 171–864 (13).
Note Added in Proof.
While this manuscript was being prepared for publication, similar results showing a role for DBT in phosphorylating CLK were published by Yu et al. (44).
Supplementary Material
Acknowledgments
We thank Paul Hardin for communicating unpublished results and Eunsung Junn for help in the immunocytochemical experiments. This work was supported by National Institutes of Health Grant NS34958 (to I.E.).
Abbreviations
- PP2A
protein phosphatase 2A
- HA
hemagglutinin
- S2
Drosophila Schneider
- pMT
metallothionein promoter
- RNAi
RNA interference
- Cal A
calyculin A.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Dunlap J. C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Young M. W., Kay S. A. Nat. Rev. Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 3.Edery I. Chronobiol. Int. 1999;16:377–414. doi: 10.3109/07420529908998716. [DOI] [PubMed] [Google Scholar]
- 4.Harms E., Kivimae S., Young M. W., Saez L. J. Biol. Rhythms. 2004;19:361–373. doi: 10.1177/0748730404268111. [DOI] [PubMed] [Google Scholar]
- 5.Hirayama J., Sassone-Corsi P. Curr. Opin. Genet. Dev. 2005;15:548–556. doi: 10.1016/j.gde.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Hardin P. E. Curr. Biol. 2005;15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Cyran S. A., Yiannoulos G., Buchsbaum A. M., Saez L., Young M. W., Blau J. J. Neurosci. 2005;25:5430–5437. doi: 10.1523/JNEUROSCI.0263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloss B., Price J. L., Saez L., Blau J., Rothenfluh A., Wesley C. S., Young M. W. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 9.Kloss B., Rothenfluh A., Young M. W., Saez L. Neuron. 2001;30:699–706. doi: 10.1016/s0896-6273(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 10.Nawathean P., Rosbash M. Mol. Cell. 2004;13:213–223. doi: 10.1016/s1097-2765(03)00503-3. [DOI] [PubMed] [Google Scholar]
- 11.Preuss F., Fan J. Y., Kalive M., Bao S., Schuenemann E., Bjes E. S., Price J. L. Mol. Cell. Biol. 2004;24:886–898. doi: 10.1128/MCB.24.2.886-898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price J. L., Blau J., Rothenfluh A., Abodeely M., Kloss B., Young M. W. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 13.Sathyanarayanan S., Zheng X., Xiao R., Sehgal A. Cell. 2004;116:603–615. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- 14.Hardin P. E. J. Biol. Rhythms. 2004;19:348–360. doi: 10.1177/0748730404268052. [DOI] [PubMed] [Google Scholar]
- 15.Bae K., Lee C., Hardin P. E., Edery I. J. Neurosci. 2000;20:1746–1753. doi: 10.1523/JNEUROSCI.20-05-01746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim E. Y., Bae K., Ng F. S., Glossop N. R., Hardin P. E., Edery I. Neuron. 2002;34:69–81. doi: 10.1016/s0896-6273(02)00639-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee C., Bae K., Edery I. Neuron. 1998;21:857–867. doi: 10.1016/s0896-6273(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 18.Bae K., Lee C., Sidote D., Chuang K. Y., Edery I. Mol. Cell. Biol. 1998;18:6142–6151. doi: 10.1128/mcb.18.10.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konopka R. J., Benzer S. Proc. Natl. Acad. Sci. USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothenfluh A., Abodeely M., Young M. W. Curr. Biol. 2000;10:1399–1402. doi: 10.1016/s0960-9822(00)00786-7. [DOI] [PubMed] [Google Scholar]
- 21.Eide E. J., Virshup D. M. Chronobiol. Int. 2001;18:389–398. doi: 10.1081/cbi-100103963. [DOI] [PubMed] [Google Scholar]
- 22.Ko H. W., Jiang J., Edery I. Nature. 2002;420:673–678. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- 23.Eide E. J., Vielhaber E. L., Hinz W. A., Virshup D. M. J. Biol. Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akten B., Jauch E., Genova G. K., Kim E. Y., Edery I., Raabe T., Jackson F. R. Nat. Neurosci. 2003;6:251–257. doi: 10.1038/nn1007. [DOI] [PubMed] [Google Scholar]
- 25.Lin J. M., Kilman V. L., Keegan K., Paddock B., Emery-Le M., Rosbash M., Allada R. Nature. 2002;420:816–820. doi: 10.1038/nature01235. [DOI] [PubMed] [Google Scholar]
- 26.Martinek S., Inonog S., Manoukian A. S., Young M. W. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- 27.Edery I., Zwiebel L. J., Dembinska M. E., Rosbash M. Proc. Natl. Acad. Sci. USA. 1994;91:2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Padiath Q. S., Shapiro R. E., Jones C. R., Wu S. C., Saigoh N., Saigoh K., Ptacek L. J., Fu Y. H. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 29.Forger D. B., Peskin C. S. Proc. Natl. Acad. Sci. USA. 2005;102:321–324. doi: 10.1073/pnas.0408465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondratov R. V., Chernov M. V., Kondratova A. A., Gorbacheva V. Y., Gudkov A. V., Antoch M. P. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darlington T. K., Wager-Smith K., Ceriani M. F., Staknis D., Gekakis N., Steeves T. D., Weitz C. J., Takahashi J. S., Kay S. A. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 32.He Q., Liu Y. Genes Dev. 2005;19:2888–2899. doi: 10.1101/gad.1369605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafmeier T., Haase A., Kaldi K., Scholz J., Fuchs M., Brunner M. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Lee C., Etchegaray J. P., Cagampang F. R., Loudon A. S., Reppert S. M. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee C., Bae K., Edery I. Mol. Cell. Biol. 1999;19:5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etchegaray J. P., Lee C., Wade P. A., Reppert S. M. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa T., Hirayama J., Kobayashi Y., Todo T. Genes Cells. 2002;7:1073–1086. doi: 10.1046/j.1365-2443.2002.00579.x. [DOI] [PubMed] [Google Scholar]
- 38.Holmberg C. I., Tran S. E., Eriksson J. E., Sistonen L. Trends Biochem. Sci. 2002;27:619–627. doi: 10.1016/s0968-0004(02)02207-7. [DOI] [PubMed] [Google Scholar]
- 39.Allada R., Kadener S., Nandakumar N., Rosbash M. EMBO J. 2003;22:3367–3375. doi: 10.1093/emboj/cdg318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allada R., White N. E., So W. V., Hall J. C., Rosbash M. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 41.Blau J., Young M. W. Cell. 1999;99:661–671. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- 42.Chang D. C., Reppert S. M. Curr. Biol. 2003;13:758–762. doi: 10.1016/s0960-9822(03)00286-0. [DOI] [PubMed] [Google Scholar]
- 43.Hammond S. M., Bernstein E., Beach D., Hannon G. J. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 44.Yu W., Zheng H., Houl J. H., Dauwalder B., Hardin P. E. Genes Dev. 2006;20:723–733. doi: 10.1101/gad.1404406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.