Abstract
Non-small cell lung cancer (NSCLC) demonstrates a strong etiologic association with smoking. Although nicotine is not carcinogenic, it can induce cell proliferation and angiogenesis and suppress apoptosis induced by certain agents. Here we show that nicotine inhibits apoptosis induced by the drugs gemcitabine, cisplatin, and taxol, which are used to treat NSCLCs. This protection correlated with the induction of XIAP and survivin by nicotine in a panel of human NSCLC cell lines, and depletion of XIAP and survivin ablated the protective effects of nicotine. The antiapoptotic effects of nicotine were mediated by dihydro β-erythroidine-sensitive α3-containing nicotinic acetylcholine receptors and required the Akt pathway. Chromatin immunoprecipitation assays demonstrated that nicotine stimulation caused an increased recruitment of E2F1 and concomitant dissociation of retinoblastoma tumor suppressor protein (Rb) from survivin promoter in A549 cells. Moreover, ablation of E2F1 levels caused abrogation of the protective effects of nicotine against cisplatin-induced apoptosis in A549 cells whereas ablation of signal transducer and activator of transcription 3 levels had no effect. These studies suggest that exposure to nicotine might negatively impact the apoptotic potential of chemotherapeutic drugs and that survivin and XIAP play a key role in the antiapoptotic activity of nicotine.
Keywords: E2F, retinoblastoma, Stat 3, IAP, lung cancer
Cigarette smoking is a major risk factor in the development of non-small cell lung cancer (NSCLC), which accounts for 80% of all lung cancers (1–3). Previous studies have implied that nicotine may be genotoxic, forming adducts with DNA, histone H1/H3, or H1b, thereby causing mutations in vital genes leading to neoplastic transformation (4, 5). However, recent evidence has shown that nicotine can also lead to sustained activation of mitogenic pathways, promote angiogenesis, and accelerate tumor growth and atherosclerosis (6–13). Nicotine and its related carcinogens, like 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, have been found to activate Raf-1-, EGFR-, c-Src-, Akt-, and 5-lipooxygenase-mediated growth stimulatory pathways (14–19). In addition, nicotine has been also found to inhibit apoptosis induced by opioids, etoposide, cisplatin, and UV irradiation in lung cancer cells (7, 10, 14). The inhibitory effect of nicotine on apoptosis has been attributed to its ability to activate and phosphorylate antiapoptotic proteins like Bcl-2, induction of NF-κB complexes, activation of the Akt pathway, as well as inactivation of proapoptotic proteins like Bax and Bad through phosphorylation in lung cancer cells (7, 20–24).
NSCLC is characterized by its poor prognosis and resistance to the apoptotic activity of antineoplastic drugs both in vivo and in vitro (25, 26). Although many signaling cascades have been implicated in the acquisition of chemoresistance in NSCLC (3, 26, 27), we conjectured that it is probable that the antiapoptotic effects of nicotine contribute to the process. Here we demonstrate that nicotine abrogates the apoptotic activity of gemcitabine, cisplatin, and taxol, which are standard therapy for NSCLC, in a variety of human NSCLC cell lines. The protective effects of nicotine correlated with the induction of IAP proteins XIAP and survivin in lung cancer cells; furthermore, depletion of XIAP and survivin by small interfering RNAs (siRNAs) abrogated the protective effects of nicotine on drug-induced apoptosis. The antiapoptotic effects of nicotine were mediated by activation of Akt via dihydro β-erythroidine (DhβE)-sensitive nicotinic acetylcholine receptors (nAChRs), which facilitated the stabilization of XIAP proteins and transcriptional induction of survivin. The treatment of A549 NSCLC cells with nicotine caused an increased binding of E2F1 to the survivin promoter, and transfection of E2F1–siRNA abrogated the protective effects of nicotine. In conclusion, the antiapoptotic effects of nicotine, mediated by XIAP and survivin, may negatively impact the chemosensitivity of NSCLC cells against antineoplastic drugs.
Results and Discussion
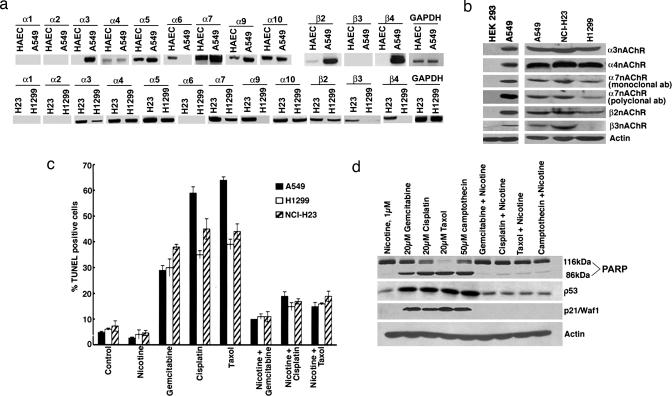
The biological effects of nicotine are mediated by nAChRs, which are widely expressed in neurons and at neuromuscular junctions (28); they are present on certain nonneuronal cells as well (29, 30). Attempts were made to identify the nAChR subunits present on three human NSCLC cells (A549, H23, and H1299) by using RT-PCR (14, 23). Human aortic endothelial cells were used as a positive control because the pattern of nAChRs of these cells is known (6). As shown in Fig. 1a, α1- and α2-subunits were absent in all of the three NSCLC cell lines whereas α3-, α4-, α5-, α7-, and α10-subunits were present in all. Similar results were obtained in two additional NSCLC lines, H226 and H441, as well as primary human microvascular endothelial cells from lung (HMEC-Ls) (Fig. 4, which is published as supporting information on the PNAS web site). The status of α8-subunit in nonneuronal cells is not known. Regarding β-subunits, β2 was expressed in A549, NCI-H23, and H1299 cells; β3 was present only in NCI-H23, and β4 was found on A549 and NCI-H23 cells. The expression of the receptors in A549, NCI-H23, and H1299 cells was confirmed by Western blotting using HEK293 cells as the negative control (Fig. 1b); the results from the RT-PCR and immunoblotting experiments are summarized in Table 1, which is published as supporting information on the PNAS web site.
Fig. 1.
Nicotine inhibits drug-induced apoptosis in lung cancer cells. (a) RT-PCR showing the expression of nAChR subunits in A549, NCI-H23, and H1299 NSCLC cells. cDNA from human aortic endothelial cells was the positive control; PCR for GAPDH was used as the loading control. (b) nAChR protein expression in A549, NCI-H23, and H1299 cells as seen by Western blotting. Lysates from HEK293 cells were used as the negative control. (c) Nicotine inhibits apoptosis induced by chemotherapeutic drugs. Quiescent A549, NCI-H23, and H1299 cells were treated with 20 μM gemcitabine, cisplatin, or taxol; the presence of 1 μM nicotine inhibited apoptosis, as seen in a TUNEL assay. (d) A similar experiment as in c where apoptosis in A549 cells was assessed by PARP cleavage; although there was a significant amount of PARP cleavage in cells treated with the drugs, it was greatly reduced when nicotine was present. Induction of p53 and p21/Waf1/CIP1 by the drugs was also inhibited by nicotine.
It was next examined whether nicotine could confer protection against apoptosis induced by gemcitabine, cisplatin, and taxol, which are widely used to treat NSCLC (1). As shown in Fig. 1c, 1 μM nicotine could suppress apoptosis induced by 20 μM gemcitabine, cisplatin, and taxol in quiescent A549, NCI-H23, and H1299 cells, as measured by TUNEL assay. Nicotine could also protect asynchronous A549 cells against apoptosis induced by these drugs (Fig. 5, which is published as supporting information on the PNAS web site). Apoptosis was further verified by poly(ADP-ribose)polymerase (PARP) cleavage. Treatment of A549 cells with 20 μM gemcitabine, cisplatin, or taxol or with 50 μM camptothecin caused robust PARP cleavage, which was inhibited by 1 μM nicotine (Fig. 1d). Drug treatment enhanced the levels of p53 and its transcriptional target, p21, as seen by Western blots; interestingly, this induction was also inhibited by nicotine.
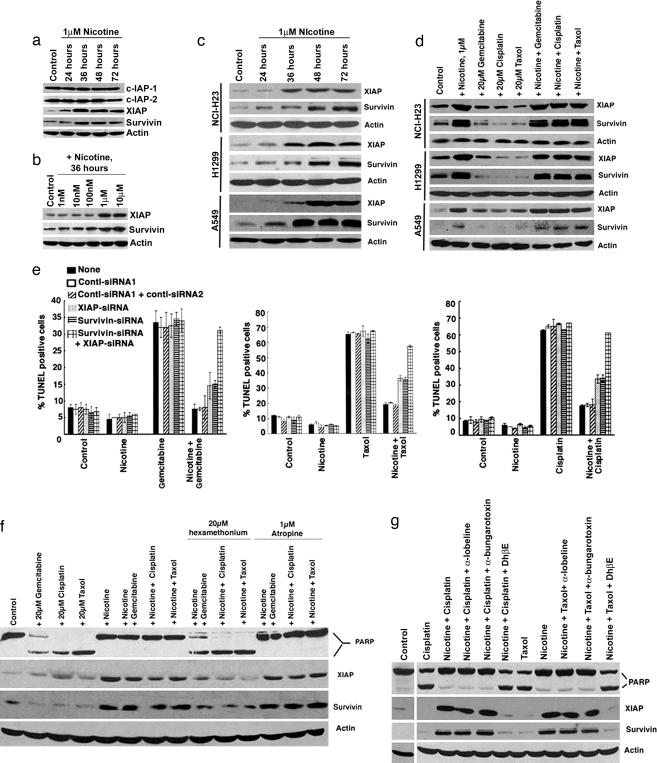
The effect of nicotine on the levels of the IAP family of proteins (31) was examined by Western blotting. The treatment of A549 cells with 1 μM nicotine did not affect the levels of c-IAP-1 and c-IAP-2 (Fig. 2a). However, XIAP and survivin were up-regulated by nicotine treatment of quiescent A549 cells in a time- and dose-dependent manner (Fig. 2 a and b), suggesting that nicotine might be inhibiting apoptosis by using these proteins. A similar up-regulation of XIAP and survivin was also observed upon treatment of quiescent NCI-H23 and H1299 cells with 1 μM nicotine (Fig. 2c). We next examined whether these molecules are altered upon drug treatment of A549, H23, and H1299 cells. As shown in Fig. 2d, XIAP and survivin levels were induced by nicotine; treatment of cells with the indicated drugs reduced the levels of these two proteins compared with untreated cells. However, the levels of XIAP and survivin were elevated when the drug treatment was done in the presence of 1 μM nicotine. The contribution of XIAP and survivin in nicotine-mediated protection from apoptosis was examined by using siRNAs. A549 cells were transfected with siRNAs to XIAP, survivin, or a combination of both; siRNAs ablated their protein levels selectively (Fig. 6, which is published as supporting information on the PNAS web site). The cells were rendered quiescent 18 h after transfection and treated with the above drugs in the presence or absence of nicotine, and apoptosis was measured by TUNEL assay. Transfection of siRNA for XIAP or survivin individually resulted in a partial abrogation of the antiapoptotic effects of nicotine, whereas transfection of both the siRNAs reversed the protection and restored the sensitivity to the anti-cancer drugs (Fig. 2e). Apoptosis was not affected by cotransfection of one or a combination of two nontargeting control siRNAs. Similar results were obtained by using a second set of siRNAs for XIAP and survivin (Fig. 7 a and b, which is published as supporting information on the PNAS web site). These experiments suggest that the up-regulation of XIAP and survivin by nicotine contributes to its antiapoptotic properties.
Fig. 2.
Nicotine induces XIAP and survivin. (a) Western blot analysis showing that nicotine induces XIAP and survivin levels in A549 cells but not the levels of c-IAP-1 and c-IAP-2. (b) Nicotine up-regulates the levels of XIAP and survivin in a dose-dependent manner in A549 cells. (c) A Western blot showing the induction of XIAP and survivin by nicotine in A549, NCI-H23, and H1299 cells. (d) Nicotine induces XIAP and survivin in cells treated with chemotherapeutic drugs. Quiescent A549, NCI-H23, and H1299 cells were treated with 20 μM gemcitabine, cisplatin, or taxol in the presence or absence of 1 μM nicotine. Nicotine enhanced the levels of XIAP and survivin irrespective of whether drugs are present. (e) The antiapoptotic effect of nicotine could be abrogated by siRNAs to XIAP and survivin but not by nontargeting control siRNAs. The siRNAs were transfected individually or in combination; at 18 h after transfection, the cells were rendered quiescent and treated with gemcitabine, cisplatin, or taxol in the presence or absence of 1 μM nicotine for 36 h. Apoptosis was measured by using a TUNEL assay. (f) Nicotine-mediated inhibition of apoptosis is mediated by nAChRs on A549 cells. Apoptosis induced by gemcitabine, cisplatin, and taxol was inhibited by 1 μM nicotine; this inhibition could be prevented by hexamethonium bromide but not by atropine, as measured by PARP cleavage. Induction of XIAP and survivin by nicotine was also prevented by hexamethonium bromide. (g) DhβE, an antagonist of α3/β2- and α4/β2-subunits, prevented nicotine-mediated inhibition of apoptosis as well as induction of XIAP and survivin in A549 cells. α-Lobeline, an antagonist of α4/β2, had no effect, suggesting that α3/β2-subunits play the predominant role.
It was examined whether the antiapoptotic effects of nicotine required nAChR function. Nicotine at 1 μM inhibited apoptosis induced by gemcitabine, cisplatin, and taxol in A549 cells, as seen by PARP cleavage (Fig. 2f Upper). Hexamethonium bromide, a general antagonist of nAChRs, at 20 μM prevented nicotine-mediated inhibition of apoptosis; at the same time, 1 μM atropine, an antagonist of muscarinic acetylcholine receptors, did not (Fig. 2f). In addition, nicotine-mediated induction of XIAP and survivin was inhibited by hexamethonium bromide but not by atropine. These results suggest that the inhibition of apoptosis by nicotine is mediated through nAChRs on lung cancer cells.
Attempts were made to identify the specific nAChR subunits mediating inhibition of apoptosis by using specific receptor subunit antagonists. A549 cells were treated with 20 μM cisplatin or taxol to induce apoptosis; 1 μM nicotine protected the cells. Specific antagonists of α7-subunits (α-bungarotoxin) or α4/β2-subunits (α-lobeline) (12, 14) had no effect on the inhibition; in contrast, an inhibitor of α3/β2- and α4/β2-subunits (DhβE) could prevent nicotine-mediated inhibition of apoptosis, suggesting that these subunits are involved in the inhibition (Fig. 2g). West et al. (14) showed that nicotine protects bronchial epithelial cells against apoptosis by etoposide through DhβE-sensitive α3/β2-subunits; our results show that the same subunits are involved in the inhibition of apoptosis in NSCLC cells as well (14). Furthermore, the induction of XIAP and survivin by nicotine (alone or in the presence of the anti-cancer drugs) was ablated by DhβE (Fig. 2g, middle two panels). This finding suggests that DhβE-sensitive-α3/β2- and α4/β2-subunits play a vital role in mediating nicotine-induced up-regulation of XIAP and survivin as well. Indeed, depletion of α3–nAChR or β2–nAChR by siRNA partially ablated nicotine-mediated protection from apoptosis (Fig. 8 a and b, which is published as supporting information on the PNAS web site), which was accompanied by concomitant down-regulation of levels of XIAP and survivin in A549 cells. However, a combination of α3–nAChR and β2–nAChR siRNAs totally abrogated the antiapoptotic effect of nicotine on A549 NSCLC cells. In addition, the transfection of both α3–nAChR and β2–nAChR siRNAs led to an increased suppression of XIAP and survivin levels compared with the individual siRNAs.
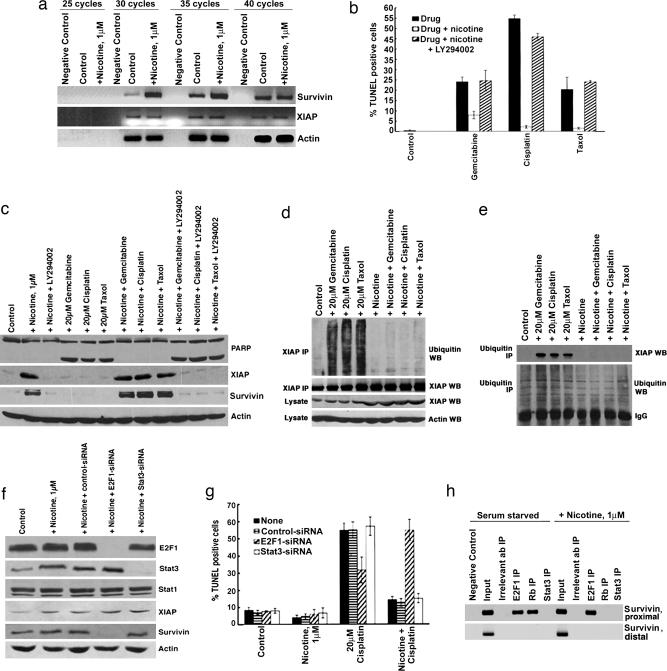
Attempts were made to understand the molecular mechanisms underlying the induction of XIAP and survivin by nicotine. It was first assessed whether the induction of XIAP and survivin occurs at the transcriptional level by semiquantitative RT-PCR by using RNA extracted from quiescent as well as nicotine-stimulated cells (32, 33). As shown in Fig. 3a, expression of XIAP or β-actin did not change upon nicotine stimulation whereas survivin levels were significantly up-regulated. These findings were verified by Northern blot analysis (Fig. 9, which is published as supporting information on the PNAS web site); the levels of survivin message were elevated, but there was no change in the levels of XIAP, suggesting that survivin is transcriptionally regulated whereas XIAP is regulated by posttranscriptional mechanisms.
Fig. 3.
Mechanism of nicotine-mediated induction of XIAP and survivin. (a) Semiquantitative RT-PCR showing the induction of survivin, but not XIAP mRNA, by 1 μM nicotine. cDNA prepared from starved and nicotine-stimulated A549 cells was examined by RT-PCR for XIAP and survivin; PCR for actin was done as a loading control. (b) Akt pathway is involved in nicotine-mediated inhibition of apoptosis. A 10 μM concentration of LY290042 ablated nicotine-induced inhibition of apoptosis in A549 cells as seen in a TUNEL assay; induction of XIAP and survivin was inhibited as well (c). (d) Ubiquitination of XIAP is induced by gemcitabine, cisplatin, and taxol but inhibited by nicotine. Quiescent A549 cells were treated with 20 μM gemcitabine, 20 μM cisplatin, or 20 μM taxol in the presence or absence of 1 μM nicotine for 36 h. Cell lysates were immunoprecipitated with a polyclonal anti-XIAP antibody, and the immunoprecipitates were probed by using an anti-ubiquitin monoclonal antibody by Western blot analysis. (e) The IP/Western blot experiment was performed in the reverse fashion by immunoprecipitating the indicated lysates by a ubiquitin monoclonal antibody and examining the presence of XIAP in the immunoprecipitates by immunoblotting. (f) Western blots demonstrating the selective ablation of E2F1 and Stat3 levels in A549 cells by specific siRNAs. A nontargeting siRNA served as control for all experiments. Eighteen hours after transfection, the cells were rendered quiescent and treated with 1 μM nicotine for 36 h. E2F1–siRNA abrogated nicotine-mediated induction of survivin, whereas levels of XIAP and Stat1 remained unchanged. In contrast, Stat3-siRNA did not affect the expression of either survivin or XIAP. (g) The protective effect of nicotine on cisplatin-induced apoptosis could be abrogated by siRNA to E2F1, but not by Stat3-siRNA or a nontargeting control-siRNA. A549 cells were transfected with the indicated siRNAs as described in the text. Eighteen hours after transfection, the cells were rendered quiescent and subsequently treated with 20 μM cisplatin for 36 h, in the presence or absence of 1 μM nicotine, and apoptosis was measured by TUNEL assay. (h) Chromatin IP assays showing the differential occupancy of E2F1, Rb and Stat3 on the survivin promoter. The stimulation of A549 cells with nicotine leads to increased E2F1 binding and concomitant dissociation of Rb from the proximal site (region +38 to +43) of the survivin promoter. In contrast, Stat3 was not found to be associated with either proximal (+38 to +43) or distal (−806 to −800) sites of the survivin promoter in either quiescent or nicotine-stimulated A549 cells.
The Akt pathway has been implicated in nicotine function, and its role in mediating its antiapoptotic effects was tested (14–16). As shown in Fig. 3 b and c Top, treatment with 10 μM LY290042, a phosphatidylinositol 3-kinase/Akt pathway inhibitor, ablated the antiapoptotic effects of nicotine in A549 cells. A role for the Akt pathway in protecting against cisplatin-induced apoptosis in an XIAP-dependent manner has been proposed in other systems (34); hence, we examined whether nicotine regulated the levels of XIAP in an Akt-dependent manner. LY290042 could abrogate induction of XIAP by nicotine (Fig. 3c); furthermore, the up-regulation of XIAP and survivin levels in A549 cells treated with nicotine and cisplatin (as well as taxol or gemcitabine) was ablated by LY290042. This finding suggests that an Akt-dependent increase in the level of antiapoptotic proteins contributes to nicotine-mediated inhibition of apoptosis. Because Akt could down-regulate apoptosis by blocking ubiquitination of XIAP, we examined whether nicotine affects ubiquitination of XIAP. An immunoprecipitation (IP)/Western blot experiment showed that the XIAP is ubiquitinated in the drug-treated A549 cells; ubiquitination was significantly reduced when nicotine was present during drug treatment (Fig. 3d). This decrease in ubiquitination correlated with increased levels of XIAP (Fig. 3d). These results were further confirmed by performing the IP/Western blots in the reverse fashion. The above A549 lysates were immunoprecipitated with an anti-ubiquitin antibody, and the presence of XIAP in the immunoprecipitates was assessed by immunoblotting (Fig. 3e). The presence of 1 μM nicotine decreased the levels of XIAP ubiquitination in drug-treated A549 cells, thereby preventing XIAP degradation and cell death. Nicotine did not affect the ubiquitination pattern of survivin (data not shown).
Recent studies have shown that putative E2F1 and signal transducer and activator of transcription (Stat)3 binding sites are present on the survivin promoter (35, 36). The contribution of these proteins in mediating the antiapoptotic effects of nicotine was ascertained by siRNA methodology. Western blotting showed that E2F1–siRNA or Stat3–siRNA selectively suppressed the levels of these proteins in A549 cells (Fig. 3f). Fig. 3g shows that the ablation of E2F1 levels effectively reversed the protective effects of nicotine in cisplatin-induced apoptosis in A549 cells, whereas Stat3–siRNA did not have any effect. This finding correlates with the reduction of survivin expression by E2F1–siRNA whereas XIAP levels remain unaffected. Although nicotine induced Stat3 levels, Stat3–siRNA does not affect the expression of either survivin or XIAP in A549 cells (Fig. 3f). Next, the differential recruitment of E2F1 and Stat3 on survivin promoter was examined by chromatin IP assays. As shown in Fig. 3h, both E2F1 and retinoblastoma tumor suppressor protein (Rb) were bound to the proximal site (−63 to −51) of the survivin promoter in quiescent A549 cells. However, nicotine stimulation caused the increased recruitment of E2F1 to proximal site, whereas Rb was dissociated. At the same time, Stat3 was not bound to proximal site (+38 to +43) or the distal site (−806 to −800) of the survivin promoter in quiescent or nicotine-stimulated A549 cells. Taken together, our data suggest that E2F1-mediated regulation of the survivin promoter contributes to the antiapoptotic activity of nicotine.
In summary, the data presented here show that the up-regulation of XIAP and survivin in NSCLC cells contributes to the protective effects of nicotine against anti-cancer agents. Although the antiapoptotic activity of nicotine is regulated by multiple signaling proteins like Bcl-2, NF-κB, Bax, and Bad (7, 20, 23, 24), our results for the first time, to our knowledge, reveal a role for the IAP proteins XIAP and survivin as significant players in this process. Our findings are in agreement with clinical studies showing that patients who continue to smoke have worse survival profiles than those who quit before treatment (26); they also raise the possibility that nicotine supplementation for smoking cessation might reduce the response to chemotherapeutic agents. Further elucidation of the signaling pathways underlying the antiapoptotic effects of nicotine may facilitate the design of improved therapeutic strategies to combat NSCLC in patients who are exposed to nicotine through cigarette smoking or nicotine supplements.
Materials and Methods
Cell Culture.
A549 cells and HEK293 cells were cultured in DMEM (Mediatech Cellgro) containing 10% FBS. NCI-H441 and NCI-H226 were cultured in RPMI medium 1640 containing 10% FBS. HMEC-Ls were obtained from Clonetics and cultured in endothelial growth medium containing 5% FBS. The studies using nicotine, anti-cancer drugs, or signal transduction inhibitors were done on cells that were rendered quiescent by serum starvation for 24 h. Thereafter, cells were stimulated with 1 μM nicotine (Sigma) in the presence or absence of the indicated drugs and/or inhibitors for 36 h.
RT-PCR, Semiquantitative RT-PCR Analysis, and Northern Blotting.
Reverse transcriptase coupled PCR was done to map the subtypes of nAChRs on A549 cells. Total RNA was isolated from asynchronous A549, NCI-H23, H1299, NCI-H441, NCI-H226, and HMEC-L cells (RNEasy Kit; Qiagen) by using the manufacturer’s protocol. cDNA was synthesized by reverse transcription by using an AMV-RT kit (Promega). The primers and conditions for RT-PCR for nAChRs were described elsewhere (14). PCR for GAPDH was used as the loading control (37).
Additionally, A549 cells were serum-starved for 24 h and stimulated with 1 μM nicotine for 36 h. Total RNA was isolated, and cDNA was synthesized and subjected to PCR by using forward and reverse primers written below for XIAP, survivin, and GAPDH, respectively. Subsequently, the fragments obtained were cloned by using a TA-cloning kit (Invitrogen), and probes were radiolabeled with [α-32P]dATP and [α-32P]dCTP by using the Prime-a-Gene labeling kit (Promega). Northern blotting was performed by using 10 μg of total RNA as described elsewhere (32, 38).
Semiquantitative RT-PCR for XIAP and survivin was performed by using sets of divergent primers (shown below), and levels were quantified as described by Mididoddi et al. (33). The primers for β-actin were described in ref. 33: XIAP (forward primer), AAGAGAAGATGACTTTTAACAG; XIAP (reverse primer), TGCTGAGTCTCCATATTGCC; survivin (forward primer), 5′-ATGGGTGCCCCGACGTTGC-3′; survivin (reverse primer), 5′-TCAATCCATGGCAGCCAGCTG-3′; GAPDH (forward primer), 5′-CAAAAGGGTCATCATCTCTGC-3′; GAPDH (reverse primer), 5′-GAGGGGCCATCCACAGTCTTC-3′.
Apoptosis Assays.
A549 and H23 cells were serum-starved for 24 h, and H1299 cells were serum-starved for 48 h to remove the effect of any exogenous growth factors. Thereafter, cells were stimulated with 1 μM nicotine in the presence or absence of 20 μM gemcitabine, 20 μM taxol, 20 μM cisplatin, or 50 μM camptothecin for 36 h. Apoptosis was measured by using a TUNEL assay kit (Promega). Parallel experiments were also set up in 90-mm tissue culture dishes, and apoptosis was ascertained by immunoblotting for PARP (Cell Signaling Technology).
siRNA Transfection and Assays.
Chemically synthesized, double-stranded siRNA for XIAP, α3nAChR, and β2nAChR was purchased from Ambion. Survivin, E2F1–siRNa, and Stat3–siRNA were obtained from Santa Cruz Biotechnology. The siRNAs were transfected at a concentration of 50 pmol each (40 pmol in the case of Stat3) or in combination in A549 cells by using Oligofectamine reagent (Invitrogen) as per the manufacturer’s instructions. A nontargeting siRNA sequence (Santa Cruz Biotechnology) was used as a control for all transfection experiments. Furthermore, a combination of two nontargeting siRNAs was used as an additional control in some of the TUNEL assays; for this purpose, a second nontargeting siRNA sequence was obtained from Qiagen. Eighteen hours after transfection, the cells were rendered quiescent for 24 h by using serum-free DMEM. Subsequently, the cells were treated with 20 μM gemcitabine, cisplatin, or taxol in the presence or absence of 1 μM nicotine for 36 h. Apoptosis was ascertained by using a TUNEL assay (Promega). The transfection experiments were also repeated by using a different set of siRNA for XIAP and survivin (Cell Signaling Technology).
Lysate Preparation and Immunoblotting.
Lysates from cells treated with different agents were prepared by Nonidet P-40 lysis as described (39). Physical interaction between proteins in vivo was analyzed by IP/Western blotting by using 200 μg of lysate and 1 μg of the appropriate antibody (sources of antibodies are listed in Supporting Methods, which is published as supporting information on the PNAS web site).
Chromatin IP Assay.
Quiescent A549 cells were stimulated with 1 μM nicotine for 24 h. A total of 2.5 × 107 cells were used per IP reaction. Cells were crosslinked with 1% formaldehyde for 20 min at room temperature. The crosslinking was terminated by addition of 0.125 M glycine. Subsequently, cells were harvested and lysates were prepared (39, 40). The lysates were immunoprecipitated with monoclonal E2F1 antibody (Santa Cruz Biotechnology), monoclonal Rb (Calbiochem), and polyclonal Stat3 antibody (Santa Cruz Biotechnology). The differential binding of E2F1, Rb, and Stat3 to the region −131 to +46 (containing putative E2F1 and Stat3 binding sites) of the survivin promoter and region −920 to −773 (a distant putative Stat3 binding site) was analyzed by PCR (39). The sequences of the PCR primers used are as follows: E2F1–Stat3 (region −131 to +46) forward primer, 5′-CGCCTCTACTCCCAGAAG-3′; E2F1–Stat3 (region −131 to +46) reverse primer, 5′-TGTAGAGATGCGGTGGTC-3′; Stat3 (region −920 to −773) forward primer, 5′-CCAAAGCAGAGGACACAC-3′; Stat3 (region −920 to −773) reverse primer, 5′-GGCCACTACCGTGATAAG-3′.
Supplementary Material
Acknowledgments
We thank Ed Seto, H. G. Wang, and W. D. Cress (H. Lee Moffitt Cancer Center) for generous gifts of reagents and Gerold Bepler and Anupama Sharma for helpful discussions. This study was supported by National Cancer Institute Grant CA63136 (to S.C.).
Abbreviations
- NSCLC
non-small cell lung cancer
- IP
immunoprecipitation
- siRNA
small interfering RNA
- nAChR
nicotinic acetylcholine receptor
- DhβE
dihydro β-erythroidine
- Stat
signal transducer and activator of transcription
- PARP
poly(ADP-ribose)polymerase
- Rb
retinoblastoma tumor suppressor protein.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Johnson D. H., Schiller J. H. Cancer Chemother. Biol. Response Modif. 2002;20:763–786. [PubMed] [Google Scholar]
- 2.Sekido Y., Fong K. M., Minna J. D. Annu. Rev. Med. 2003;54:73–87. doi: 10.1146/annurev.med.54.101601.152202. [DOI] [PubMed] [Google Scholar]
- 3.Minna J. D., Fong K., Zochbauer-Muller S., Gazdar A. F. Cancer J. 2002;8(Suppl. 1):S41–S46. [PubMed] [Google Scholar]
- 4.Wu X. H., Wang H. F., Liu Y. F., Lu X. Y., Wang J. J., Li K. Radiocarbon. 1997;39:293–297. [Google Scholar]
- 5.Li X. S., Wang H. F., Wang X. Y., Liu Y. F., Li K., Lu X. Y., Wang J. J., Liu K. X., Guo Z. Y. Radiocarbon. 1996;38:347–353. [Google Scholar]
- 6.Villablanca A. C. J. Appl. Physiol. 1998;84:2089–2098. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 7.Mai H., May W. S., Gao F., Jin Z., Deng X. J. Biol. Chem. 2003;278:1886–1891. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- 8.Sekhon H. S., Wright J. L., Churg A. Am. J. Physiol. 1994;267:L557–L563. doi: 10.1152/ajplung.1994.267.5.L557. [DOI] [PubMed] [Google Scholar]
- 9.Zhu B. Q., Heeschen C., Sievers R. E., Karliner J. S., Parmley W. W., Glantz S. A., Cooke J. P. Cancer Cell. 2003;4:191–196. doi: 10.1016/s1535-6108(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 10.Manackjee R., Minna J. D. Proc. Natl. Acad. Sci. USA. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeschen C., Jang J. J., Weis M., Pathak A., Kaji S., Hu R. S., Tsao P. S., Johnson F. L., Cooke J. P. Nat. Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 12.Heeschen C., Weis M., Aicher A., Dimmler S., Cooke J. P. J. Clin. Invest. 2002;110:527–536. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heeschen C., Weis M., Cooke J. P. J. Am. Coll. Cardiol. 2003;41:489–496. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 14.West K. A., Brognard J., Clark A. S., Linnoila I. R., Yang X., Swain S. M., Harris C., Belinsky S., Dennis P. A. J. Clin. Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West K. A., Linnoila I. R., Brognard J., Belinsky S., Harris C., Dennis P. A. Chest. 2004;125:101S–102S. doi: 10.1378/chest.125.5_suppl.101s. [DOI] [PubMed] [Google Scholar]
- 16.West K. A., Linnoila I. R., Belinsky S. A., Harris C. C., Dennis P. A. Cancer Res. 2004;64:446–451. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 17.Ye Y. N., Liu E. S., Shin V. Y., Wu W. K., Luo J. C., Cho C. H. J. Pharmacol. Exp. Ther. 2004;308:66–72. doi: 10.1124/jpet.103.058321. [DOI] [PubMed] [Google Scholar]
- 18.Schuller H. M., Plummer H. K., III, Jull B. A. Anat. Rec. 2003;270A:51–58. doi: 10.1002/ar.a.10019. [DOI] [PubMed] [Google Scholar]
- 19.Jull B. A., Plummer H. K., Schuller H. M. J. Cancer Res. Clin. Oncol. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z., Gao F., Flagg T., Deng X. J. Biol. Chem. 2004;279:23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 21.Jin Z., Gao F., Flagg T., Deng X. J. Biol. Chem. 2004;279:40209–40219. doi: 10.1074/jbc.M404056200. [DOI] [PubMed] [Google Scholar]
- 22.Heusch W. L., Maneckjee R. Carcinogenesis. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 23.Trombino S., Cesario A., Margaritora S., Granone P., Motta G., Falugi C., Russo P. Cancer Res. 2004;64:135–145. doi: 10.1158/0008-5472.can-03-1672. [DOI] [PubMed] [Google Scholar]
- 24.Xin M., Deng X. J. Biol. Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 25.Katakami N., Sugiura T., Nogami T., Yamamoto H., Negoro S., Nakano T., Okamoto N., Takada Y., Kodama K., Ariyoshi Y. Lung Cancer. 2004;43:93–100. doi: 10.1016/j.lungcan.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Bepler G. Cancer Control. 2003;10:275–276. doi: 10.1177/107327480301000401. [DOI] [PubMed] [Google Scholar]
- 27.Rosell R., Crino L., Danenberg K., Scagliotti G., Bepler G., Taron M., Alberola V., Provencio M., Camps C., De Marinis F., et al. Semin. Oncol. 2003;30:19–25. doi: 10.1016/s0093-7754(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 28.Gotti C., Clementi F. Prog. Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Lindstrom J. Mol. Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- 30.Sharma G., Vijayaraghavan S. J. Neurobiol. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]
- 31.Vaux D. L., Silke J. Biochem. Biophys. Res. Commun. 2003;304:499–504. doi: 10.1016/s0006-291x(03)00622-3. [DOI] [PubMed] [Google Scholar]
- 32.Joshi B., Ordonez-Ercan D., Dasgupta P., Chellappan S. Oncogene. 2005;24:2204–2217. doi: 10.1038/sj.onc.1208206. [DOI] [PubMed] [Google Scholar]
- 33.Mididoddi S., McGuirt J. P., Sens M. A., Todd J. H., Sens D. A. Toxicol. Lett. 1996;85:17–27. doi: 10.1016/0378-4274(96)03632-6. [DOI] [PubMed] [Google Scholar]
- 34.Dan H. C., Sun M., Kaneko S., Feldman R. I., Nicosia S. V., Wang H. G., Tsang B. K., Cheng J. Q. J. Biol. Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 35.Nam S., Buettner R., Turkson J., Kim D., Cheng J. Q., Muehlbeyer S., Hippe F., Vatter S., Merz K. H., Eisenbrand G., et al. Proc. Natl. Acad. Sci. USA. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y., Saavedra H. I., Holloway M. P., Leone G., Altura R. A. J. Biol. Chem. 2004;279:40511–40520. doi: 10.1074/jbc.M404496200. [DOI] [PubMed] [Google Scholar]
- 37.Gyory I., Wu J., Fejer G., Seto E., Wright K. L. Nat. Immunol. 2004;5:299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J., Russell D. W. Molecular Cloning: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Lab. Press; 2001. [Google Scholar]
- 39.Dasgupta P., Sun J., Wang S., Fusaro G., Betts V., Padmanabhan J., Sebti S. M., Chellappan S. Mol. Cell. Biol. 2004;24:9527–9541. doi: 10.1128/MCB.24.21.9527-9541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S., Fusaro G., Padmanabhan J., Chellappan S. P. Oncogene. 2002;21:8388–8396. doi: 10.1038/sj.onc.1205944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.